Is Omalizumab Related to Ear and Labyrinth Disorders? A Disproportionality Analysis Based on a Global Pharmacovigilance Database
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Source
2.2. Data Mining and Signal Detection Criteria
2.3. Hierarchy Analysis
3. Results
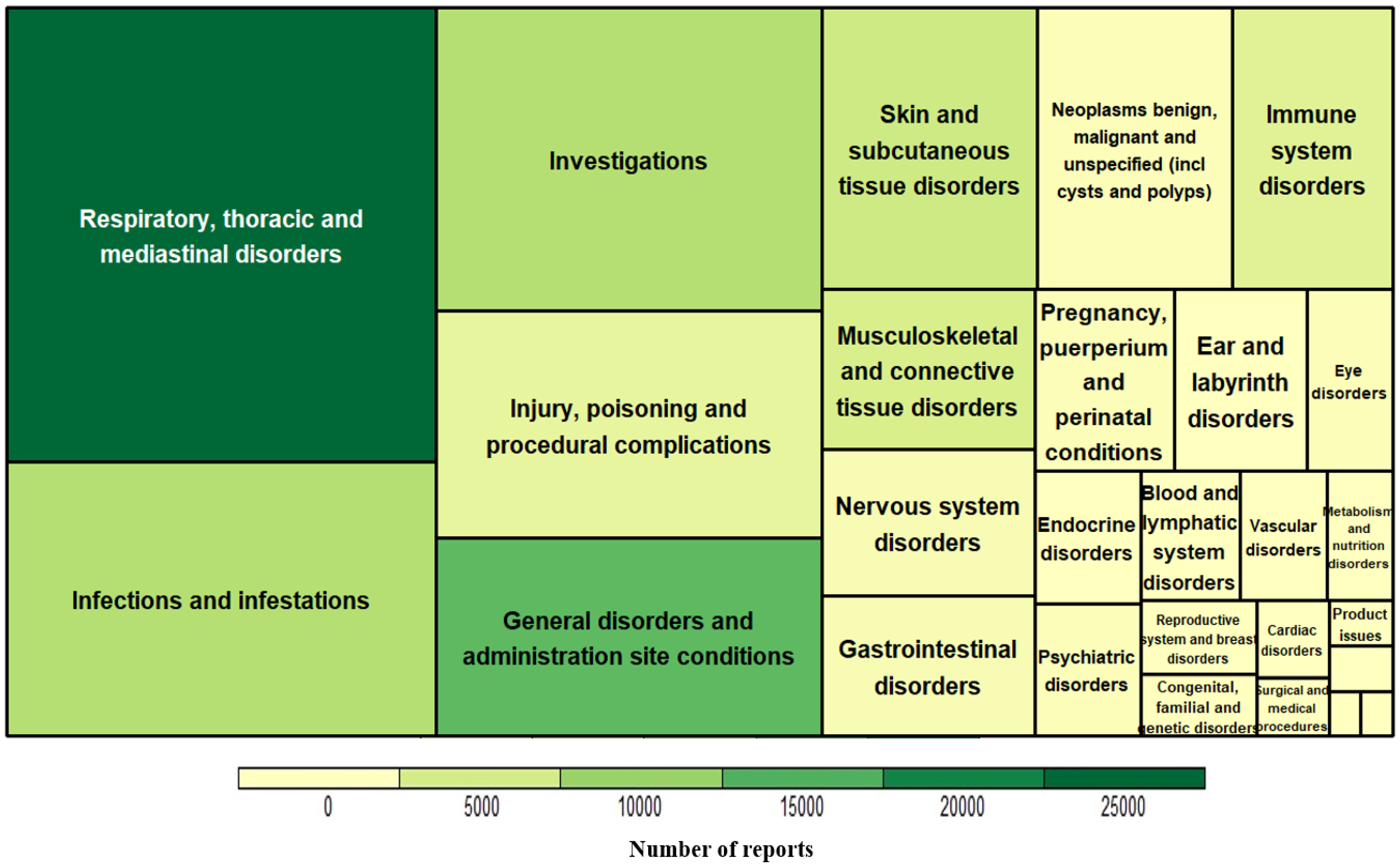
3.1. Characteristics of Omalizumab-Related AE Reports
3.2. Detected Signals of Omalizumab in Terms of System Organ Class (SOC)
3.2.1. Signals for Ear and Labyrinth Disorders
3.2.2. Eosinophil-Related Signals for Omalizumab
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Israel, E. Asthma. In Harrison’s Principles of Internal Medicine 21e; Loscalzo, J., Fauci, A., Kasper, D., Hauser, S., Longo, D., Jameson, J.L., Eds.; McGraw-Hill Education: New York, NY, USA, 2022. [Google Scholar]
- Global Strategy for Asthma Management and Prevention. 2022. Available online: https://ginasthma.org (accessed on 15 June 2022).
- Fritscher, L.; Chapman, K.R. Omalizumab for asthma: Pharmacology and clinical profile. Expert Rev. Respir. Med. 2009, 3, 119–127. [Google Scholar] [CrossRef]
- Chung, K.F.; Wenzel, S.E.; Brozek, J.L.; Bush, A.; Castro, M.; Sterk, P.J.; Adcock, I.M.; Bateman, E.D.; Bel, E.H.; Bleecker, E.R.; et al. International ERS/ATS guidelines on definition, evaluation and treatment of severe asthma. Eur. Respir. J. 2014, 43, 343–373. [Google Scholar] [CrossRef] [PubMed]
- Hyland, M.E.; Whalley, B.; Jones, R.C.; Masoli, M. A qualitative study of the impact of severe asthma and its treatment showing that treatment burden is neglected in existing asthma assessment scales. Qual. Life Res. 2015, 24, 631–639. [Google Scholar] [CrossRef]
- Papaioannou, A.I.; Mplizou, M.; Porpodis, K.; Fouka, E.; Zervas, E.; Samitas, K.; Markatos, M.; Bakakos, P.; Papiris, S.; Gaga, M.; et al. Long-term efficacy and safety of omalizumab in patients with allergic asthma: A real-life study. Allergy Asthma Proc. 2021, 42, 235–242. [Google Scholar] [CrossRef]
- McGregor, M.C.; Krings, J.G.; Nair, P.; Castro, M. Role of Biologics in Asthma. Am. J. Respir. Crit. Care Med. 2019, 199, 433–445. [Google Scholar] [CrossRef]
- Al-Quteimat, O.; Laila, A. Valproate Interaction with Carbapenems: Review and Recommendations. Hosp. Pharm. 2020, 55, 181–187. [Google Scholar] [CrossRef] [PubMed]
- Gordillo, R.; Spitzer, A. The nephrotic syndrome. Pediatr. Rev. 2009, 30, 94–104. [Google Scholar] [CrossRef] [PubMed]
- Incorvaia, C.; Mauro, M.; Makri, E.; Leo, G.; Ridolo, E. Two decades with omalizumab: What we still have to learn. Biologics 2018, 12, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Agache, I.; Akdis, C.A.; Akdis, M.; Canonica, G.W.; Casale, T.; Chivato, T.; Corren, J.; Chu, D.K.; Del Giacco, S.; Eiwegger, T.; et al. EAACI Biologicals Guidelines—Recommendations for severe asthma. Allergy 2021, 76, 14–44. [Google Scholar] [CrossRef] [PubMed]
- Bagnasco, D.; Caminati, M.; Ferrando, M.; Aloè, T.; Testino, E.; Canonica, G.W.; Passalacqua, G. Anti-IL-5 and IL-5Ra: Efficacy and Safety of New Therapeutic Strategies in Severe Uncontrolled Asthma. BioMed Res. Int. 2018, 2018, 5698212. [Google Scholar] [CrossRef] [PubMed]
- Bagnasco, D.; Ferrando, M.; Varricchi, G.; Passalacqua, G.; Canonica, G.W. A Critical Evaluation of Anti-IL-13 and Anti-IL-4 Strategies in Severe Asthma. Int. Arch. Allergy Immunol. 2016, 170, 122–131. [Google Scholar] [CrossRef] [PubMed]
- Abbas, F.; Georas, S.; Cai, X.; Khurana, S. Asthma biologics: Real-world effectiveness, impact of switching biologics, and predictors of response. Ann. Allergy Asthma Immunol. 2021, 127, 655–660. [Google Scholar] [CrossRef]
- Salvi, S.S.; Babu, K.S. Treatment of allergic asthma with monoclonal anti-IgE antibody. N. Engl. J. Med. 2000, 342, 1292–1293. [Google Scholar] [PubMed]
- Milgrom, H.; Berger, W.; Nayak, A.; Gupta, N.; Pollard, S.; McAlary, M.; Taylor, A.F.; Rohane, P. Treatment of childhood asthma with anti-immunoglobulin E antibody (omalizumab). Pediatrics 2001, 108, E36. [Google Scholar] [CrossRef] [PubMed]
- Busse, W.; Corren, J.; Lanier, B.Q.; McAlary, M.; Fowler-Taylor, A.; Cioppa, G.D.; van As, A.; Gupta, N. Omalizumab, anti-IgE recombinant humanized monoclonal antibody, for the treatment of severe allergic asthma. J. Allergy Clin. Immunol. 2001, 108, 184–190. [Google Scholar] [CrossRef] [PubMed]
- Akaba, T.; Kondo, M.; Kobayashi, F.; Honda, N.; Muramatsu, S.; Yagi, O.; Takeyama, K.; Seo, Y.; Nonaka, M.; Tagaya, E. Characteristics of patients with severe asthma who experienced treatment failure with omalizumab. Pulm. Pharmacol. Ther. 2021, 68, 102032. [Google Scholar] [CrossRef] [PubMed]
- Iino, Y. Role of IgE in eosinophilic otitis media. Allergol. Int. 2010, 59, 233–238. [Google Scholar] [CrossRef] [PubMed]
- Seo, Y.; Nonaka, M.; Pawankar, R. Eosinophilic otitis media and comorbid asthma. Curr. Opin. Allergy Clin. Immunol. 2020, 20, 9–13. [Google Scholar] [CrossRef] [PubMed]
- Iino, Y. Eosinophilic otitis media: A new middle ear disease entity. Curr. Allergy Asthma Rep. 2008, 8, 525–530. [Google Scholar] [CrossRef] [PubMed]
- Giezen, T.J.; Mantel-Teeuwisse, A.K.; Meyboom, R.H.; Straus, S.M.; Leufkens, H.G.; Egberts, T.C. Mapping the safety profile of biologicals: A disproportionality analysis using the WHO adverse drug reaction database, VigiBase. Drug Saf. 2010, 33, 865–878. [Google Scholar] [CrossRef]
- Stricker, B.H.; Psaty, B.M. Detection, verification, and quantification of adverse drug reactions. BMJ 2004, 329, 44–47. [Google Scholar] [CrossRef] [PubMed]
- Mota, D.; Rama, T.A.; Severo, M.; Moreira, A. Potential cancer risk with omalizumab? A disproportionality analysis of the WHO’s VigiBase pharmacovigilance database. Allergy 2021, 76, 3209–3211. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Wang, Z.; Cui, L.; Xu, Y.; Guan, K.; Zhao, B. Anaphylactic risk related to omalizumab, benralizumab, reslizumab, mepolizumab, and dupilumab. Clin. Transl. Allergy 2021, 11, e12038. [Google Scholar] [CrossRef]
- Lazarou, J.; Pomeranz, B.H.; Corey, P.N. Incidence of adverse drug reactions in hospitalized patients: A meta-analysis of prospective studies. JAMA 1998, 279, 1200–1205. [Google Scholar] [CrossRef] [PubMed]
- USFDA, Code of Federal Regulation Title 21 (21CFR) 312.32. Volume 5. Available online: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRsearch.cfm?CFRPart=312 (accessed on 11 July 2022).
- Hou, Y.; Ye, X.; Wu, G.; Cheng, G.; Du, X.; He, J. A comparison of disproportionality analysis methods in national adverse drug reaction databases of China. Expert Opin. Drug Saf. 2014, 13, 853–857. [Google Scholar] [CrossRef] [PubMed]
- Evans, S.J.; Waller, P.C.; Davis, S. Use of proportional reporting ratios (PRRs) for signal generation from spontaneous adverse drug reaction reports. Pharmacoepidemiol. Drug Saf. 2001, 10, 483–486. [Google Scholar] [CrossRef] [PubMed]
- Rothman, K.J.; Lanes, S.; Sacks, S.T. The reporting odds ratio and its advantages over the proportional reporting ratio. Pharmacoepidemiol. Drug Saf. 2004, 13, 519–523. [Google Scholar] [CrossRef] [PubMed]
- Mozzicato, P. MedDRA: An Overview of the Medical Dictionary for Regulatory Activities. Pharm. Med. 2009, 23, 65–75. [Google Scholar] [CrossRef]
- Edwards, I.R.; Aronson, J.K. Adverse drug reactions: Definitions, diagnosis, and management. Lancet 2000, 356, 1255–1259. [Google Scholar] [CrossRef]
- Chen, T.; Ashman, P.E.; Bojrab, D.I., 2nd; Johnson, A.P.; Hong, R.S.; Benson, B.; Svider, P.F. Diagnosis and management of eosinophilic otitis media: A systematic review. Acta Oto-Laryngol. 2021, 141, 579–587. [Google Scholar] [CrossRef] [PubMed]
- Lara-Sanchez, H.; Vallejo, L.A. Eosinophilic Otitis Media. N. Engl. J. Med. 2017, 376, e10. [Google Scholar] [CrossRef] [PubMed]
- Haarman, M.G.; van Hunsel, F.; de Vries, T.W. Adverse drug reactions of montelukast in children and adults. Pharmacol. Res. Perspect. 2017, 5, e00341. [Google Scholar] [CrossRef]
- Bleecker, E.R.; Menzies-Gow, A.N.; Price, D.B.; Bourdin, A.; Sweet, S.; Martin, A.L.; Alacqua, M.; Tran, T.N. Systematic Literature Review of Systemic Corticosteroid Use for Asthma Management. Am. J. Respir. Crit. Care Med. 2020, 201, 276–293. [Google Scholar] [CrossRef]
- Cain, D.W.; Cidlowski, J.A. Immune regulation by glucocorticoids. Nat. Rev. Immunol. 2017, 17, 233–247. [Google Scholar] [CrossRef] [PubMed]
- Suzaki, I.; Kimura, Y.; Tanaka, A.; Hirano, K.; Ishibashi, A.; Mizuyoshi, T.; Ando, I.; Kitajima, T.; Watanabe, S.; Hinohira, Y.; et al. Successful treatment of eosinophilic otitis media associated with severe bronchial asthma with an anti-IL-5 monoclonal antibody, mepolizumab. Auris Nasus Larynx 2019, 46, 141–146. [Google Scholar] [CrossRef] [PubMed]
- Kagoshima, H.; Hori, R.; Kojima, T.; Okanoue, Y.; Taguchi, A.; Yamamoto, H.; Hasebe, K.; Shoji, K. Successful treatment of eosinophilic chronic rhinosinusitis and eosinophilic otitis media using the anti-IL-5 receptor monoclonal antibody benralizumab: A case report. Respir. Med. Case Rep. 2020, 30, 101135. [Google Scholar] [CrossRef] [PubMed]
- Okude, A.; Tagaya, E.; Kondo, M.; Nonaka, M.; Tamaoki, J. A Case of Severe Asthma with Eosinophilic Otitis Media Successfully Treated with Anti-IgE Monoclonal Antibody Omalizumab. Case Rep. Pulmonol. 2012, 2012, 340525. [Google Scholar] [CrossRef] [PubMed]
- Roboz, G.J.; Rafii, S. Interleukin-5 and the regulation of eosinophil production. Curr. Opin. Hematol. 1999, 6, 164–168. [Google Scholar] [CrossRef] [PubMed]
- Shomali, W.; Gotlib, J. Eosinophils and Their Disorders. In Williams Hematology, 10e; Kaushansky, K., Prchal, J.T., Burns, L.J., Lichtman, M.A., Levi, M., Linch, D.C., Eds.; McGraw-Hill Education: New York, NY, USA, 2021. [Google Scholar]
- Hanania, N.A.; Corren, J.; Holweg, C.; Haselkorn, T.; Yang, M.; Lyon, R.C.; Iqbal, A.; Casale, T.B. Effects of Omalizumab on Blood Eosinophil Numbers in Patients with Allergic Asthma. J. Allergy Clin. Immunol. 2019, 143 (Suppl. 2), AB95. [Google Scholar] [CrossRef]
- Wynn, T.A. IL-13 effector functions. Annu. Rev. Immunol. 2003, 21, 425–456. [Google Scholar] [CrossRef] [PubMed]
- Iino, Y.; Tomioka-Matsutani, S.; Matsubara, A.; Nakagawa, T.; Nonaka, M. Diagnostic criteria of eosinophilic otitis media, a newly recognized middle ear disease. Auris Nasus Larynx 2011, 38, 456–461. [Google Scholar] [CrossRef] [PubMed]
- Junttila, I.S. Tuning the Cytokine Responses: An Update on Interleukin (IL)-4 and IL-13 Receptor Complexes. Front. Immunol. 2018, 9, 888. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.M.; Wilson, T.M.; Metcalfe, D.D. The mast cell and allergic diseases: Role in pathogenesis and implications for therapy. Clin. Exp. Allergy 2008, 38, 4–18. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Duraisamy, K.; Chow, B.K. Unlocking the Non-IgE-Mediated Pseudo-Allergic Reaction Puzzle with Mas-Related G-Protein Coupled Receptor Member X2 (MRGPRX2). Cells 2021, 10, 1033. [Google Scholar] [CrossRef] [PubMed]
- Mendoza, R.P.; Anderson, C.C.; Fudge, D.H.; Roede, J.R.; Brown, J.M. Metabolic Consequences of IgE- and Non-IgE-Mediated Mast Cell Degranulation. J. Immunol. 2021, 207, 2637–2648. [Google Scholar] [CrossRef] [PubMed]
- Tete, S.; Saggini, A.; Maccauro, G.; Rosati, M.; Conti, F.; Cianchetti, E.; Tripodi, D.; Toniato, E.; Fulcheri, M.; Salini, V.; et al. Interleukin-9 and mast cells. J. Biol. Regul. Homeost. Agents 2012, 26, 319–326. [Google Scholar]
- Shields, R.L.; Whether, W.R.; Zioncheck, K.; O’Connell, L.; Fendly, B.; Presta, L.G.; Thomas, D.; Saban, R.; Jardieu, P. Inhibition of allergic reactions with antibodies to IgE. Int. Arch. Allergy Immunol. 1995, 107, 308–312. [Google Scholar] [CrossRef]
- Celebi Sozener, Z.; Gorgulu, B.; Mungan, D.; Sin, B.A.; Misirligil, Z.; Aydin, O.; Bavbek, S. Omalizumab in the treatment of eosinophilic granulomatosis with polyangiitis (EGPA): Single-center experience in 18 cases. World Allergy Organ. J. 2018, 11, 39. [Google Scholar] [CrossRef]
- Loizou, D.; Enav, B.; Komlodi-Pasztor, E.; Hider, P.; Kim-Chang, J.; Noonan, L.; Taber, T.; Kaushal, S.; Limgala, R.; Brown, M.; et al. A pilot study of omalizumab in eosinophilic esophagitis. PLoS ONE 2015, 10, e0113483. [Google Scholar] [CrossRef]
- Sperry, S.L.; Woosley, J.T.; Shaheen, N.J.; Dellon, E.S. Influence of race and gender on the presentation of eosinophilic esophagitis. Am. J. Gastroenterol. 2012, 107, 215–221. [Google Scholar] [CrossRef]
- Iino, Y.; Hara, M.; Hasegawa, M.; Matsuzawa, S.; Shinnabe, A.; Kanazawa, H.; Yoshida, N. Effect of omalizumab on biomarkers in middle ear effusion in patients with eosinophilic otitis media. Acta Otolaryngol. 2014, 134, 366–372. [Google Scholar] [CrossRef] [PubMed]
| Number of Reports | Specific AEs | All other AEs |
|---|---|---|
| Target drug | A | B |
| All other drugs | C | D |
| Indices | Formula | Criteria |
|---|---|---|
| PRR | [A/(A + B)]/[C/(C + D)] | PRR ≥ 2 |
| ROR | (A/B)/(C/D) | ROR ≥ 2 |
| IC | IC = log2P(AE, Drug)/P(AE)P(Drug) | Under a limit of 95% IC ≥ 0 |
| Demographics | No. of Reports of Omalizumab (%) [N = 32,618] | No. of Reports of Omalizumab Related to Ear and Labyrinth Disorders (%) [N = 586] |
|---|---|---|
| Continent | ||
| Africa | 142 (0.44%) | 1 (0.17%) |
| Americas | 25,516 (78.23%) | 512 (87.37%) |
| Asia | 1321 (4.05%) | 5 (0.85%) |
| Europe | 5399 (16.55%) | 67 (11.43%) |
| Oceania | 240 (0.74%) | 1 (0.17%) |
| Age group | ||
| 0–27 days | 37 (0.11%) | 1 (0.17%) |
| 28 days to 23 months | 36 (0.11%) | 0 (0%) |
| 2–11 years | 425 (1.3%) | 3 (0.51%) |
| 12–17 years | 1068 (3.27%) | 20 (0.341%) |
| 18–44 years | 5712 (17.51%) | 134 (22.87%) |
| 45–65 years | 7192 (22.05%) | 192 (32.76%) |
| 65–74 years | 1963 (6.02%) | 47 (8.02%) |
| ≥75 years | 838 (2.57%) | 20 (3.41%) |
| Unknown | 15,347 (47.05%) | 169 (28.84%) |
| Gender | ||
| Male | 8880 (27.22%) | 183 (31.23%) |
| Female | 21,078 (64.62%) | 393 (67.06%) |
| Unknown | 460 (8.16%) | 10 (1.71%) |
| Serious | ||
| Yes | 16,592 (50.87%) | 393 (67.06%) |
| No | 15,330 (47.00%) | 186 (31.74%) |
| Unknown | 696 (2.13%) | 7 (1.19%) |
| Notifier | ||
| Physician | 14,137 (43.34%) | 230 (39.25%) |
| Pharmacist | 1057 (3.24%) | 7 (1.19%) |
| Other Health Professional | 6383 (19.57%) | 121 (20.65%) |
| Consumer/Non-Health Professional | 9773 (29.96%) | 195 (33.28%) |
| Lawyer | 8 (0.02%) | 1 (0.17%) |
| Unknown | 1260 (3.86) | 32 (5.46%) |
| Agents | Total Number of Reports | Number of Signals for Ear and Labyrinth Disorders | Number of Reports for Ear and Labyrinth Disorder Signals |
|---|---|---|---|
| Omalizumab | 32,618 | 17 | 394 |
| Mepolizumab | 7344 | 3 | 35 |
| Benralizumab | 2387 | 0 | 0 |
| Reslizumab | 315 | 0 | 0 |
| Dupilumab | 20,559 | 0 | 0 |
| AE | No. of Reports (%) | PRR | ROR | IC025 |
|---|---|---|---|---|
| Omalizumab | ||||
| Ear pain | 152 (38.58%) | 6.27 | 6.30 | 2.37 |
| Ear discomfort | 76 (19.29%) | 7.10 | 7.12 | 2.42 |
| Otorrhoea | 25 (6.35%) | 17.17 | 17.18 | 3.06 |
| Ear pruritus | 23 (5.84%) | 8.22 | 8.23 | 2.17 |
| Ear congestion | 21 (5.33%) | 8.55 | 8.56 | 2.17 |
| Ear swelling | 17 (4.31%) | 7.17 | 7.17 | 1.83 |
| Ear disorder | 16 (4.06%) | 2.30 | 2.31 | 0.35 |
| Tympanic membrane perforation | 12 (3.05%) | 7.77 | 7.78 | 1.67 |
| Deafness unilateral | 11 (2.79%) | 2.19 | 2.19 | 0.08 |
| Vertigo positional | 8 (2.03%) | 4.59 | 4.59 | 0.75 |
| Middle ear effusion | 8 (2.03%) | 3.90 | 3.90 | 0.56 |
| Meniere’s disease | 7 (1.78%) | 4.01 | 4.01 | 0.47 |
| Cerumen impaction | 5 (1.27%) | 4.71 | 4.71 | 0.28 |
| Tympanic membrane disorder | 4 (1.02%) | 10.72 | 10.72 | 0.62 |
| Tympanic membrane scarring | 3 (0.76%) | 162.74 | 162.76 | 0.69 |
| Eustachian tube obstruction | 3 (0.76%) | 17.75 | 17.76 | 0.33 |
| Eustachian tube disorder | 3 (0.76%) | 13.11 | 13.11 | 0.21 |
| Mepolizumab | ||||
| Ear pain | 19 (54.2%) | 3.47 | 3.47 | 0.98 |
| Ear disorder | 8 (22.9%) | 5.13 | 5.14 | 0.88 |
| Ear discomfort | 8 (22.9%) | 3.30 | 3.31 | 0.37 |
| AE | No. of Reports | PRR | ROR | IC025 |
|---|---|---|---|---|
| Omalizumab | ||||
| Eosinophilic granulomatosis with polyangiitis | 124 | 81.54 | 81.85 | 5.82 |
| Eosinophilia | 77 | 2.35 | 2.36 | 0.89 |
| Eosinophil count increase | 76 | 12.29 | 12.31 | 3.15 |
| Eosinophilic pneumonia | 14 | 6.00 | 6.00 | 2.35 |
| Eosinophilic oesophagitis | 11 | 27.75 | 27.76 | 3.66 |
| Hypereosinophilic syndrome | 8 | 41.66 | 41.67 | 2.43 |
| Eosinophilic pneumonia chronic | 5 | 50.86 | 50.87 | 3.18 |
| Eosinophil count abnormal | 3 | 14.47 | 14.47 | 0.25 |
| Mepolizumab | ||||
| Eosinophilic bronchitis | 4 | 726.14 | 726.54 | 1.41 |
| Eosinophilic oesophagitis | 4 | 43.84 | 43.87 | 1.19 |
| Benralizumab | ||||
| Eosinophil count abnormal | 10 | 775.56 | 779.19 | 4.48 |
| Dupilumab | ||||
| Eosinophil count abnormal | 5 | 38.68 | 38.68 | 1.59 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, H.T.; Park, S.; Jung, Y.W.; Choi, S.A. Is Omalizumab Related to Ear and Labyrinth Disorders? A Disproportionality Analysis Based on a Global Pharmacovigilance Database. Diagnostics 2022, 12, 2434. https://doi.org/10.3390/diagnostics12102434
Park HT, Park S, Jung YW, Choi SA. Is Omalizumab Related to Ear and Labyrinth Disorders? A Disproportionality Analysis Based on a Global Pharmacovigilance Database. Diagnostics. 2022; 12(10):2434. https://doi.org/10.3390/diagnostics12102434
Chicago/Turabian StylePark, Hyeon Tae, Sunny Park, Yong Woo Jung, and Soo An Choi. 2022. "Is Omalizumab Related to Ear and Labyrinth Disorders? A Disproportionality Analysis Based on a Global Pharmacovigilance Database" Diagnostics 12, no. 10: 2434. https://doi.org/10.3390/diagnostics12102434
APA StylePark, H. T., Park, S., Jung, Y. W., & Choi, S. A. (2022). Is Omalizumab Related to Ear and Labyrinth Disorders? A Disproportionality Analysis Based on a Global Pharmacovigilance Database. Diagnostics, 12(10), 2434. https://doi.org/10.3390/diagnostics12102434







