Anastomotic Rings and Inflammation Values as Biomarkers for Leakage of Stapled Circular Colorectal Anastomoses
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Collective
2.2. Inclusion and Exclusion Criteria
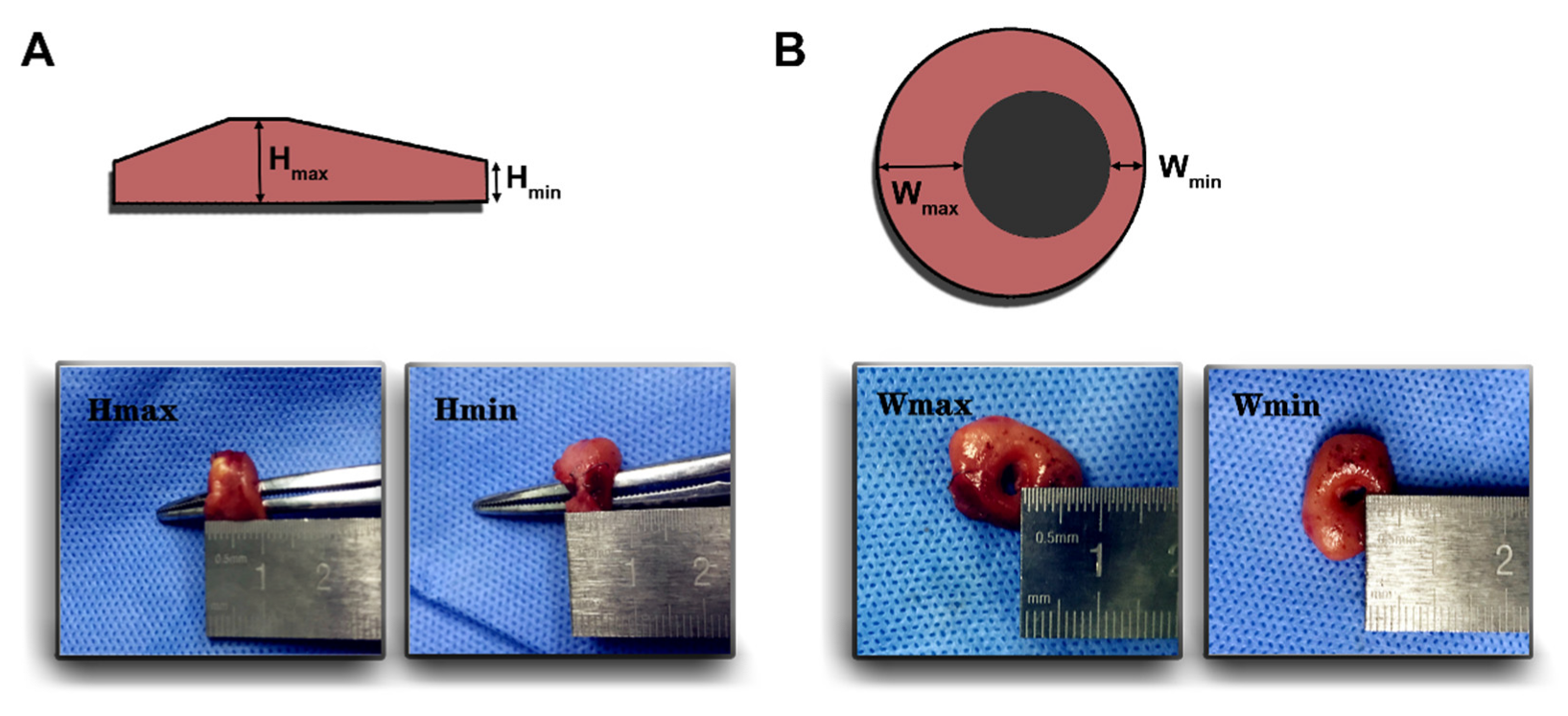
2.3. Configuration of Anastomotic Rings
2.4. Diagnostic Criteria of Anastomotic Leakage
2.5. Statistical Methods
3. Results
3.1. Patients’ Clinical Characteristics
3.2. Configuration of Anastomotic Rings
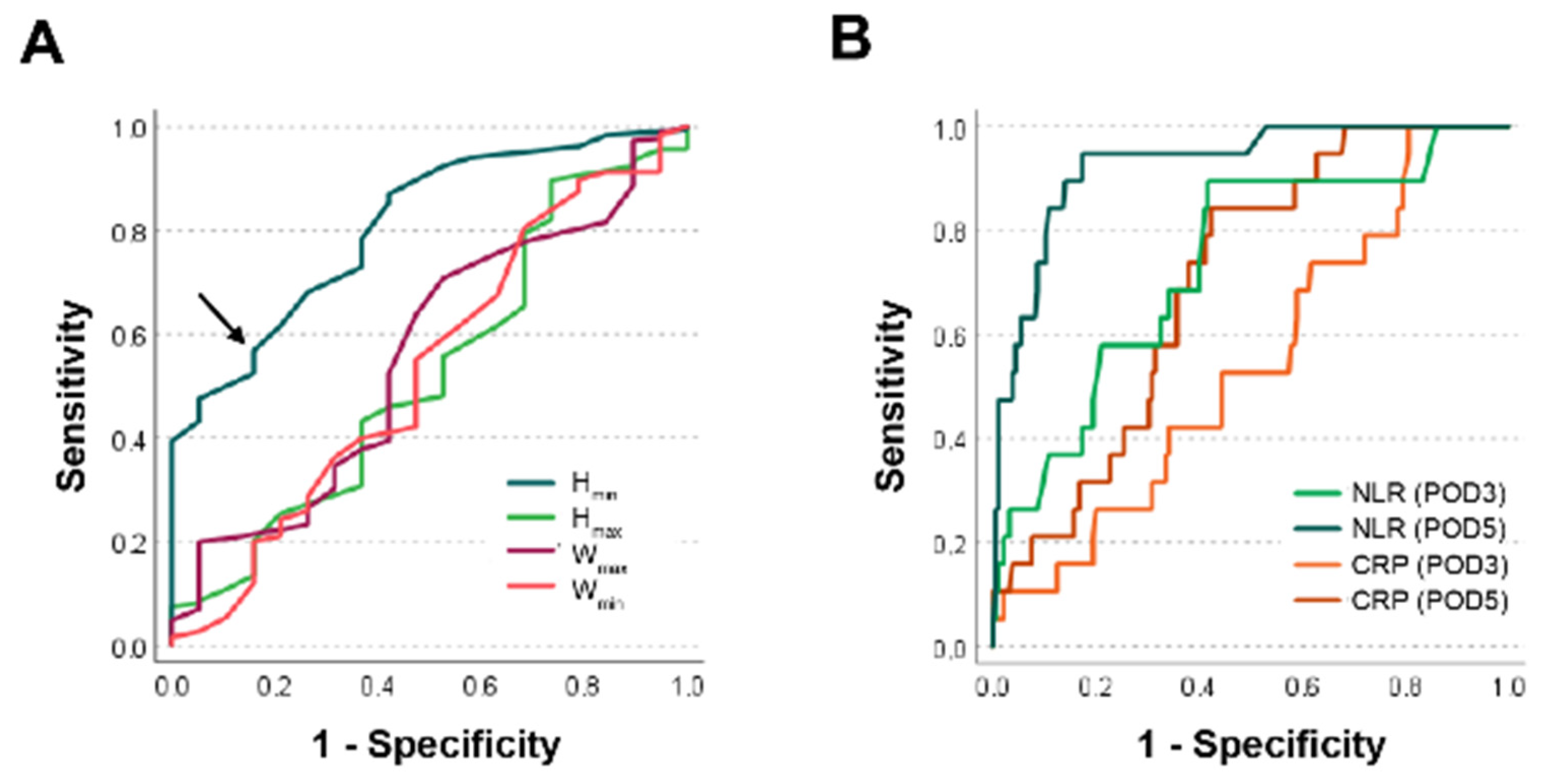
3.3. Hmin as Binary Classifier to Predict Anastomotic Leakage
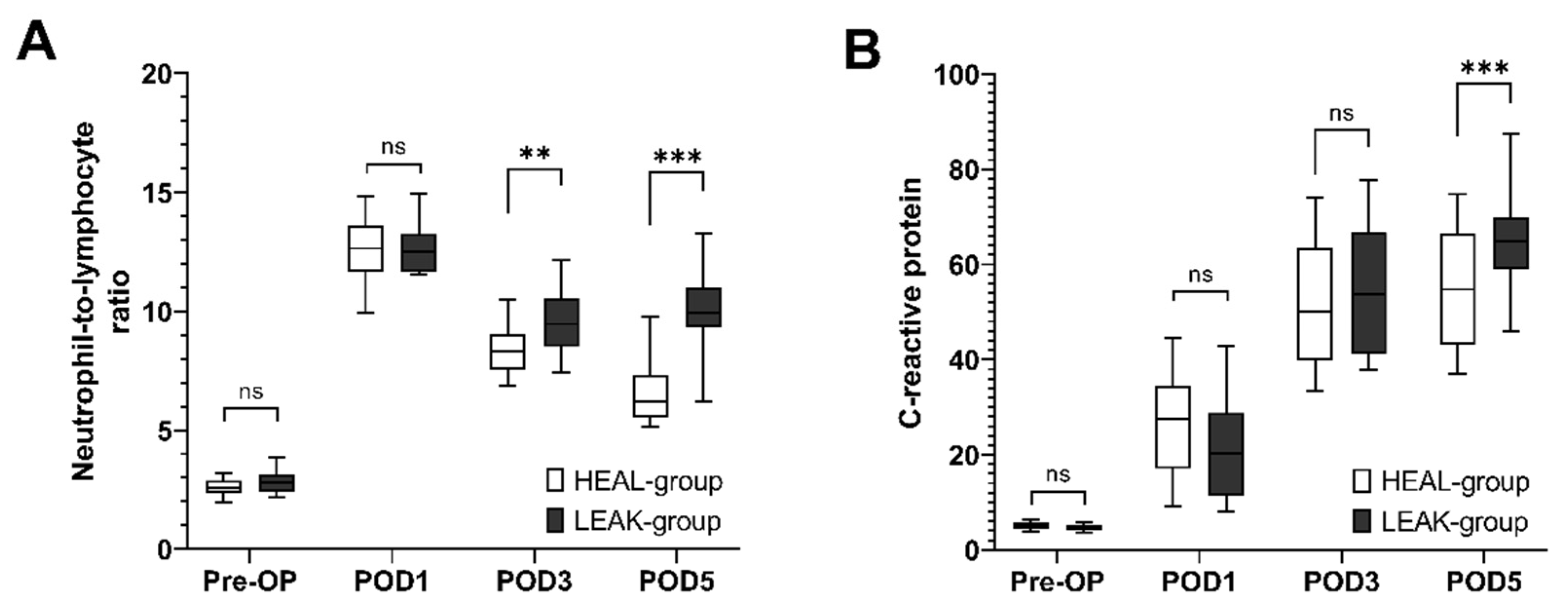
3.4. Postoperative Development of NLR and CRP Values
3.5. Accuracy of Hmin Compared to NLR and CRP as Binary Classifiers for Anastomotic Leakage
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- La Regina, D.; Di Giuseppe, M.; Lucchelli, M.; Saporito, A.; Boni, L.; Efthymiou, C.; Cafarotti, S.; Marengo, M.; Mongelli, F. Financial Impact of Anastomotic Leakage in Colorectal Surgery. J. Gastrointest. Surg. 2019, 23, 580–586. [Google Scholar] [CrossRef] [PubMed]
- Sparreboom, C.L.; Van Groningen, J.T.; Lingsma, H.F.; Wouters, M.; Menon, A.G.; Kleinrensink, G.-J.; Jeekel, J.; Lange, J.F. Lange, and group Dutch ColoRectal Audit. Different Risk Factors for Early and Late Colorectal Anastomotic Leakage in a Nationwide Audit. Dis. Colon Rectum 2018, 61, 1258–1266. [Google Scholar] [CrossRef] [PubMed]
- Gessler, B.; Eriksson, O.; Angenete, E. Angenete. Diagnosis, Treatment, and Consequences of Anastomotic Leakage in Colorectal Surgery. Int. J. Color. Dis. 2017, 32, 549–556. [Google Scholar] [CrossRef]
- Olsen, B.C.; Sakkestad, S.T.; Pfeffer, F.; Karliczek, A. Rate of Anastomotic Leakage after Rectal Anastomosis Depends on the Definition: Pelvic Abscesses Are Significant. Scand. J. Surg. 2019, 108, 241–249. [Google Scholar] [CrossRef] [PubMed]
- Foppa, C.; Ng, S.C.; Montorsi, M.; Spinelli, A. Anastomotic Leak in Colorectal Cancer Patients: New Insights and Perspectives. Eur. J. Surg. Oncol. 2020, 46, 943–954. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, K.B.; Hansen, C.H.; Krarup, P.-M.; Fransgård, T.; Madsen, M.T.; Gögenur, I. The Impact of Anastomotic Leakage on Recurrence and Long-Term Survival in Patients with Colonic Cancer: A Systematic Review and Meta-Analysis. Eur. J. Surg. Oncol. 2020, 46, 439–447. [Google Scholar] [CrossRef] [PubMed]
- Karliczek, A.; Harlaar, N.J.; Zeebregts, C.J.; Wiggers, T.; Baas, P.C.; Van Dam, G.M. Surgeons Lack Predictive Accuracy for Anastomotic Leakage in Gastrointestinal Surgery. Int. J. Color. Dis. 2009, 24, 569–576. [Google Scholar] [CrossRef] [PubMed]
- Jin, D.; Chen, L. Early Prediction of Anastomotic Leakage after Laparoscopic Rectal Surgery Using Creactive Protein. Medicine 2021, 100, e26196. [Google Scholar] [CrossRef] [PubMed]
- Platt, J.J.; Ramanathan, M.L.; Crosbie, R.A.; Anderson, J.H.; McKee, R.F.; Horgan, P.G.; McMillan, D.C. C-Reactive Protein as a Predictor of Postoperative Infective Complications after Curative Resection in Patients with Colorectal Cancer. Ann. Surg. Oncol. 2012, 19, 4168–4177. [Google Scholar] [CrossRef] [PubMed]
- Tejedor, P.; Sagias, F.; Flashman, K.; Kandala, N.L.; Khan, J. The Use of Robotic or Laparoscopic Stapler in Rectal Cancer Surgery: A Systematic Review and Meta-Analysis. J. Robot. Surg. 2020, 14, 829–833. [Google Scholar] [CrossRef]
- Italian ColoRectal Anastomotic Leakage Study Group. Anastomotic Leakage after Elective Colorectal Surgery: A Prospective Multicentre Observational Study on Use of the Dutch Leakage Score, Serum Procalcitonin and Serum C-Reactive Protein for Diagnosis. BJS Open 2020, 4, 499–507. [Google Scholar] [CrossRef]
- Radulescu, D.; Baleanu, V.D.; Padureanu, V.; Radulescu, P.M.; Bordu, S.; Patrascu, S.; Socea, B.; Bacalbasa, N.; Surlin, M.V.; Georgescu, I.; et al. Neutrophil/Lymphocyte Ratio as Predictor of Anastomotic Leak after Gastric Cancer Surgery. Diagnostics 2020, 10, 799. [Google Scholar] [CrossRef] [PubMed]
- Al Lawati, Y.; Alkaaki, A.; Luna, J.L.R.G.; Skothos, E.; Mueller, C.; Spicer, J.; Mulder, D.; Ferri, L.; Cools-Lartigue, J. The Predictive Value of Inflammatory Biomarkers in Esophageal Anastomotic Leaks. Ann. Thorac. Surg. 2021, 112, 1790–1796. [Google Scholar] [CrossRef] [PubMed]
- Cauchy, F.; Abdalla, S.; Penna, C.; Angliviel, B.; Lambert, B.; Costaglioli, B.; Brouquet, A.; Benoist, S. Brouquet, and S. Benoist. The Small Height of an Anastomotic Colonic Doughnut Is an Independent Risk Factor of Anastomotic Leakage Following Colorectal Resection: Results of a Prospective Study on 154 Consecutive Cases. Int. J. Color. Dis. 2017, 32, 699–707. [Google Scholar] [CrossRef] [PubMed]
- Paliogiannis, P.; Deidda, S.; Maslyankov, S.; Paycheva, T.; Farag, A.; Mashhour, A.; Misiakos, E.; Papakonstantinou, D.; Mik, M.; Losinska, J.; et al. Blood Cell Count Indexes as Predictors of Anastomotic Leakage in Elective Colorectal Surgery: A Multicenter Study on 1432 Patients. World J. Surg. Oncol. 2020, 18, 89. [Google Scholar] [CrossRef] [PubMed]
- Walker, P.A.; Kunjuraman, B.; Bartolo, D.C.C. Neutrophil-to-Lymphocyte Ratio Predicts Anastomotic Dehiscence. ANZ J. Surg. 2018. [CrossRef] [PubMed]
- Kubo, H.; Murayama, Y.; Arita, T.; Kuriu, Y.; Nakanishi, M.; Otsuji, E. The Prognostic Value of Preoperative Neutrophil-to-Lymphocyte Ratio in Colorectal Cancer. World J. Surg. 2016, 40, 2796–2802. [Google Scholar] [CrossRef] [PubMed]
- Cook, E.J.; Walsh, S.R.; Farooq, N.; Alberts, J.C.; Justin, T.A.; Keeling, N.J. Post-Operative Neutrophil-Lymphocyte Ratio Predicts Complications Following Colorectal Surgery. Int. J. Surg. 2007, 5, 27–30. [Google Scholar] [CrossRef] [PubMed]
- Mik, M.; Dziki, L.; Berut, M.; Trzcinski, R.; Dziki, A. Neutrophil to Lymphocyte Ratio and C-Reactive Protein as Two Predictive Tools of Anastomotic Leak in Colorectal Cancer Open Surgery. Dig. Surg. 2018, 35, 77–84. [Google Scholar] [CrossRef] [PubMed]
| Items | Total (n = 204) | Groups | p-Value | |
|---|---|---|---|---|
| LEAK (n = 19) | HEAL (n = 185) | |||
| Gender (m/f) | 121/83 | 12/7 | 109/76 | 0.72 |
| Age | 63.2 ± 11.6 | 66.2 ± 11.8 | 62.9 ± 11.5 | 0.24 |
| ASA Score | 2.2 ± 0.4 | 2.1 ± 0.3 | 2.2 ± 0.4 | 0.24 |
| BMI (kg/m2) | 22.8 ± 2.1 | 22.5 ± 1.9 | 22.9 ± 2.1 | 0.46 |
| OP time (min) | 161.4 ± 31.1 | 161.1 ± 33.5 | 161.4 ± 30.9 | 0.96 |
| Blood loss (mL) | 175.8 ± 38.1 | 165.8 ± 44.5 | 176.8 ± 37.3 | 0.23 |
| UICC/AJCC stage (II/III) | 143/61 | 13/6 | 130/55 | 0.87 |
| Distance to anal verge (>10 cm/<10 cm) | 114/90 | 10/9 | 104/81 | 0.76 |
| Hmin (mm) | 5.1 ± 0.8 | 4.42 ± 0.45 | 5.21 ± 0.78 | <0.001 |
| Hmax (mm) | 8.7 ± 1.0 | 8.57 ± 1.03 | 8.71 ± 1.03 | 0.58 |
| Wmin (mm) | 4.9 ± 0.7 | 4.86 ± 0.78 | 4.94 ± 0.72 | 0.68 |
| Wmax (mm) | 8.6 ± 0.9 | 8.46 ± 0.81 | 8.65 ± 0.90 | 0.37 |
| NLR preop | 2.6 ± 0.4 | 2.8 ± 0.5 | 2.6 ± 0.4 | 0.10 |
| NLR at POD1 | 12.6 ± 1.4 | 12.7 ± 1.0 | 12.5 ± 1.4 | 0.69 |
| NLR at POD3 | 8.5 ± 1.2 | 9.54 ± 1.31 | 8.44 ± 1.12 | 0.002 |
| NLR at POD5 | 7.0 ± 1.8 | 10.03 ± 1.55 | 6.65 ± 1.45 | <0.001 |
| CRP preop (mg/L) | 5.0 ± 0.8 | 4.7 ± 0.7 | 5.0 ± 0.8 | 0.06 |
| CRP at POD1 (mg/L) | 26.0 ± 11.0 | 22.3 ± 11.2 | 26.3 ± 11.0 | 0.15 |
| CRP at POD3 (mg/L) | 52.1 ± 13.3 | 53.7 ± 13.0 | 52.0 ± 13.3 | 0.57 |
| CRP at POD5 (mg/L) | 56.0 ± 12.7 | 64.90 ± 10.43 | 55.17 ± 12.61 | <0.001 |
| Items | Mean ± SD (in mm) | p-Value | |
|---|---|---|---|
| CDH25A (n = 69) | CDH29A (n = 135) | ||
| Hmin | 5.21 ± 0.82 | 5.09 ± 0.77 | 0.30 |
| Hmax | 8.76 ± 0.96 | 8.66 ± 1.07 | 0.50 |
| Wmin | 4.99 ± 0.77 | 4.90 ± 0.70 | 0.37 |
| Wmax | 8.73 ± 1.04 | 8.59 ± 0.80 | 0.28 |
| Cut-Off | Total (n) | Groups | Leakage Rate | |
|---|---|---|---|---|
| LEAK (n) | HEAL (n) | |||
| >4.95 mm | 108 | 3 | 105 | 2.8% 1 |
| <4.95 mm | 96 | 16 | 80 | 16.7% 1 |
| Total | 204 | 19 | 185 | 9.3% |
| Items | Total (n = 204) | Cut-Off Value (4.95 mm) | p-Value | |
|---|---|---|---|---|
| Above (n = 108) | Below (n = 96) | |||
| Gender (m/f) | 121/83 | 63/45 | 58/38 | 0.76 |
| Age | 63.2 ± 11.6 | 62.5 ± 11.6 | 63.96 ± 11.5 | 0.38 |
| ASA Score | 2.2 ± 0.4 | 2.2 ± 0.4 | 2.2 ± 0.4 | 0.79 |
| BMI (kg/m2) | 22.8 ± 2.1 | 22.9 ± 2.1 | 22.8 ± 2.1 | 0.74 |
| OP time (min) | 161.4 ± 31.1 | 161.0 ± 33.0 | 161.8 ± 29.0 | 0.86 |
| Blood loss (mL) | 175.8 ± 38.1 | 178.9 ± 37.4 | 172.3 ± 38.7 | 0.22 |
| UICC Stage (II/III) | 143/61 | 79/29 | 64/32 | 0.31 |
| Distance to anal verge (> 10 cm/< 10 cm) | 114/90 | 64/44 | 50/46 | 0.30 |
| Classifier | ROC-AUC |
|---|---|
| Hmin | 0.81 |
| Hmax | 0.53 |
| Wmin | 0.53 |
| Wmax | 0.56 |
| NLR at POD3 | 0.74 |
| NLR at POD5 | 0.93 |
| CRP at POD3 | 0.54 |
| CRP at POD5 | 0.70 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, F.; Qiao, S.; Yao, N.; Li, C.; Weber, M.-C.; Jefferies, B.; Friess, H.; Reischl, S.; Neumann, P.-A. Anastomotic Rings and Inflammation Values as Biomarkers for Leakage of Stapled Circular Colorectal Anastomoses. Diagnostics 2022, 12, 2902. https://doi.org/10.3390/diagnostics12122902
Zhang F, Qiao S, Yao N, Li C, Weber M-C, Jefferies B, Friess H, Reischl S, Neumann P-A. Anastomotic Rings and Inflammation Values as Biomarkers for Leakage of Stapled Circular Colorectal Anastomoses. Diagnostics. 2022; 12(12):2902. https://doi.org/10.3390/diagnostics12122902
Chicago/Turabian StyleZhang, Feng, Song Qiao, Ning Yao, Chunqiao Li, Marie-Christin Weber, Benedict Jefferies, Helmut Friess, Stefan Reischl, and Philipp-Alexander Neumann. 2022. "Anastomotic Rings and Inflammation Values as Biomarkers for Leakage of Stapled Circular Colorectal Anastomoses" Diagnostics 12, no. 12: 2902. https://doi.org/10.3390/diagnostics12122902
APA StyleZhang, F., Qiao, S., Yao, N., Li, C., Weber, M.-C., Jefferies, B., Friess, H., Reischl, S., & Neumann, P.-A. (2022). Anastomotic Rings and Inflammation Values as Biomarkers for Leakage of Stapled Circular Colorectal Anastomoses. Diagnostics, 12(12), 2902. https://doi.org/10.3390/diagnostics12122902






