Serum Lactate as Serum Biomarker for Cardiopulmonary Parameters within the First 24 Hours after a Spontaneous Intracerebral Hemorrhage
Abstract
1. Introduction
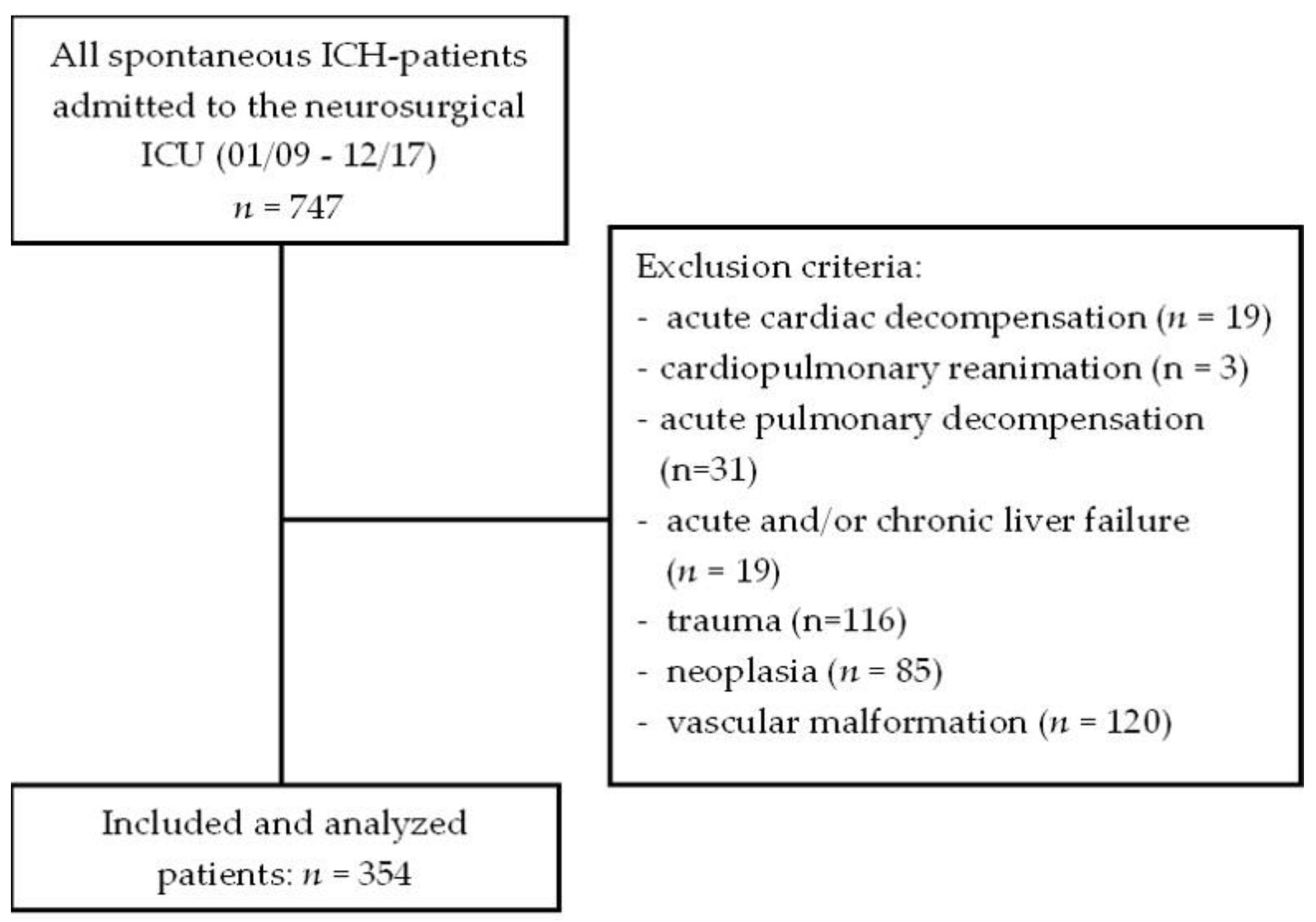
2. Materials and Methods
2.1. Study Design and Population
2.2. Baseline Data
2.3. Comorbidities and Premedication
2.4. Cardiopulmonary Parameters
2.5. Serum Biomarker
2.6. Treatment Regimen and Intensive Care Unit Management
2.7. Radiological Data
2.8. Intra-Hospital Outcome and Mortality
2.9. Statistical Analysis
3. Results
3.1. Main Characteristics
3.2. Serum Lactate
4. Discussion
4.1. Summary of the Findings
4.2. Elevated Serum Lactate Level
4.3. Cardiopulmonary Parameters
4.4. Limitations and Strengths of the Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hjalmarsson, C.; Bergfeldt, L.; Bokemark, L.; Manhem, K.; Andersson, B. Electrocardiographic Abnormalities and Elevated cTNT at Admission for Intracerebral Hemorrhage: Predictors for Survival? Ann. Noninvasive Electrocardiol. 2013, 18, 441–449. [Google Scholar] [CrossRef]
- Garrett, M.C.; Komotar, R.J.; Starke, R.M.; Doshi, D.; Otten, M.L.; Connolly, E.S. Elevated Troponin Levels are Predictive of Mortality in Surgical Intracerebral Hemorrhage Patients. Neurocritical Care 2010, 12, 199–203. [Google Scholar] [CrossRef] [PubMed]
- Caplan, L.R. Intracerebral haemorrhage. Lancet 1992, 339, 656–658. [Google Scholar] [CrossRef]
- Ahn, C.S.; Lee, S.K.; Kim, H.S.; Kong, M.H.; Song, K.Y.; Kang, D.S. Surgical outcome of spontaneous intracerebral hemorrhage in less than stuporous mental status. J. Korean Neurosurg. Soc. 2004, 35, 290–296. [Google Scholar]
- Martí–Fàbregas, J.; Belvis, R.; Guardia, E.; Cocho, D.; Munoz, J.; Marruecos, L.; Martí–Vilalta, J.L. Prognostic value of Pulsatility index in acute intra-cerebral hemorrhage. Neurology 2003, 61, 1051–1056. [Google Scholar] [CrossRef] [PubMed]
- Bender, M.; Naumann, T.; Uhl, E.; Stein, M. Early serum biomarkers for intensive care unit treatment within the first 24 hours in patients with intracerebral hemorrhage. J. Neurol. Surg. A Cent. Eur. Neurosurg. 2021, 82, 138–146. [Google Scholar] [CrossRef] [PubMed]
- Foerch, C.; Curdt, I.; Yan, B.; Dvorak, F.; Hermans, M.; Berkefeld, J.; Raabe, A.; Neumann-Haefelin, T.; Steinmetz, H.; Sitzer, M. Serum glial fibrillary acidic protein as a biomarker for intracerebral haemorrhage in patients with acute stroke. J. Neurol. Neurosurg. Psychiatry 2006, 77, 181–184. [Google Scholar] [CrossRef] [PubMed]
- Bender, M.; Haferkorn, K.; Friedrich, M.; Stein, M. Impact of early C-reactive protein/albumin ratio on intra-hospital mortality among patients with spontaneous intracerebral hemorrhage. J. Clin. Med. 2020, 9, 1236. [Google Scholar] [CrossRef]
- Lele, A.; Lakireddy, V.; Gorbachov, S.; Chaikittisilpa, N.; Krishnamoorthy, V.; Vavilala, M.S. A Narrative Review of Cardiovascular Abnormalities After Spontaneous Intracerebral Hemorrhage. J. Neurosurg. Anesthesiol. 2019, 31, 199–211. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, S.; Nagatani, K.; Otani, N.; Wada, K.; Mori, K. Electrocardiograph abnormalities in intracerebral hemorrhage. J. Clin. Neurosci. 2015, 22, 1959–1962. [Google Scholar] [CrossRef] [PubMed]
- Rahimi, A.R.; Katayama, M.; Mills, J. Cerebral Hemorrhage: Precipitating Event for a Tako-tsubo-like Cardiomyopathy? Clin. Cardiol. 2008, 31, 275–280. [Google Scholar] [CrossRef] [PubMed]
- Inamasu, J.; Ito, K.; Sugimoto, K.; Watanabe, E.; Kato, Y.; Hirose, Y. Cardiac wall motion abnormality associated with spontaneous intracerebral hemorrhage. Int. J. Cardiol. 2013, 168, 1667–1669. [Google Scholar] [CrossRef] [PubMed]
- Maramattom, B.V.; Weigand, S.; Reinalda, M.; Wijdicks, E.F.; Manno, E.M. Pulmonary complications after intracerebral hemorrhage. Neurocrit. Care 2006, 5, 115–119. [Google Scholar] [CrossRef]
- Andersen, L.W.; Mackenhauer, J.; Roberts, J.C.; Berg, K.M.; Cocchi, M.N.; Donnino, M.W. Etiology and therapeutic approach to elevated lactate levels. Mayo Clin. Proc. 2013, 88, 1127–1140. [Google Scholar] [CrossRef] [PubMed]
- Mikkelsen, M.E.; Miltiades, A.N.; Gaieski, D.F.; Goyal, M.; Fuchs, B.D.; Shah, C.V.; Bellamy, S.L.; Christie, J.D. Serum lactate is associated with mortality in severe sepsis independent of organ failure and shock. Crit. Care Med. 2009, 37, 1670–1677. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, N.I.; Howell, M.D.; Talmor, D.; Nathanson, L.A.; Lisbon, A.; Wolfe, R.E.; Weiss, J.W. Serum lactate as a predictor of mortality in emergency department patients with infection. Ann. Emerg. Med. 2005, 45, 524–528. [Google Scholar] [CrossRef] [PubMed]
- Cocchi, M.N.; Miller, J.; Hunziker, S.; Carney, E.; Salciccioli, J.; Farris, S.; Joyce, N.; Zimetbaum, P.; Howell, M.D.; Donnino, M.W. The association of lactate and vasopressor need for mortality prediction in survivors of cardiac arrest. Minerva Anestesiol. 2011, 77, 1063–1071. [Google Scholar] [PubMed]
- Aslar, A.K.; Kuzu, M.A.; Elhan, A.H.; Tanik, A.; Hengirmen, S. Admission lactate level and the APACHE II score are the most useful predictors of prognosis following torso trauma. Injury 2004, 35, 746–752. [Google Scholar] [CrossRef] [PubMed]
- Newman, T.S.; Magnuson, T.H.; Ahrendt, S.; A Smith-Meek, M.; Bender, J.S. The changing face of mesenteric infarction. Am. Surg. 1998, 64, 611–616. [Google Scholar] [PubMed]
- Almenoff, P.L.; Leavy, J.; Weil, M.H.; Goldberg, N.B.; Vega, D.; Rackow, E.C. Prolongation of the half-life of lactate after maximal exercise in patients with hepatic dysfunction. Crit. Care Med. 1989, 17, 870–873. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, F.; Schenk, L.M.; Schneider, M.; Bernstock, J.D.; Bode, C.; Borger, V.; Gessler, F.; Güresir, E.; Hadjiathanasiou, A.; Hamed, M.; et al. Predictive relevance of baseline lactate and glucose levels in patients with spontaneous deep-seated intracerebral hemorrhage. Brain Sci. 2021, 11, 633. [Google Scholar] [CrossRef]
- Teasdale, G.; Jennett, B. Assessment of coma and impaired consciousness. A practical scale. Lancet 1974, 2, 81–84. [Google Scholar] [CrossRef]
- Le Gall, J.R.; Lemeshow, S.; Saulnier, F. A new Simplified Acute Physiology Score (SAPS II) based on a European/North American multicenter study. JAMA 1993, 270, 2957–2963. [Google Scholar] [CrossRef] [PubMed]
- Evans, W.A., Jr. An encephalographic ratio for estimating ventricular enlargement and cerebral atrophy. Arch. Neurol. Psychiatry 1942, 47, 931–937. [Google Scholar] [CrossRef]
- A Graeb, D.; Robertson, W.D.; Lapointe, J.S.; A Nugent, R.; Harrison, P.B. Computed tomographic diagnosis of intraventricular hemorrhage. Etiology and prognosis. Radiology 1982, 143, 91–96. [Google Scholar] [CrossRef]
- Van Swieten, J.C.; Koudstaal, P.J.; Visser, M.C.; Schouten, H.J.; van Gijn, J. Interobserver agreement for the assessment of handicap in stroke patients. Stroke 1988, 19, 604–607. [Google Scholar] [CrossRef]
- Consoli, A.; Nurjhan, N.; Reilly, J.J., Jr.; Bier, D.M.; Gerich, J.E. Contribution of liver and skeletal muscle to alanine and lactate metabolism in humans. Am. J. Physiol. 1990, 259, E677–E684. [Google Scholar] [CrossRef]
- Van Hall, G. Lactate kinetics in human tissues at rest and during exercise. Acta Physiol. 2010, 199, 499–508. [Google Scholar] [CrossRef]
- Connor, H.; Woods, H.F.; Ledingham, J.G.; Murray, J.D. A model of L(+)-lactate metabolism in normal man. Ann. Nutr. Metab. 1982, 26, 254–263. [Google Scholar] [CrossRef]
- Behrouz, R.; Hafeez, S.; Miller, C.M. Admission leukocytosis in intracerebral hemorrhage: Associated factors and prognostic implications. Neurocrit. Care 2015, 23, 370–373. [Google Scholar] [CrossRef]
- Juraschek, S.P.; Bower, J.K.; Selvin, E.; Subash Shantha, G.P.; Hoogeveen, R.C.; Ballantyne, C.M.; Young, J.H. Plasma lactate and incident hypertension in the atherosclerosis risk in communities study. Am. J. Hypertens. 2015, 28, 216–224. [Google Scholar] [CrossRef] [PubMed]
- Oh, T.K.; Song, I.A.; Bae, H.J.; Jeon, Y.T. Serum lactate level upon admission to the neuro-intensive care unit and 90-day mortality: A retrospective study. J. Clin. Neurosci. 2019, 70, 173–177. [Google Scholar] [CrossRef]
- Qureshi, A.I. Acute hypertensive response in patients with stroke: Pathophysiology and management. Circulation 2008, 118, 176–187. [Google Scholar] [CrossRef] [PubMed]
- Takeda, R.; Ogura, T.; Ooigawa, H.; Fushihara, G.; Yoshikawa, S.I.; Okada, D.; Araki, R.; Kurita, H. A practical prediction model for early hematoma expansion in spontaneous deep ganglionic intracerebral hemorrhage. Clin. Neurol. Neurosurg. 2013, 115, 1028–1031. [Google Scholar] [CrossRef] [PubMed]
- Cottis, R.; Magee, N.; Higgins, D.J. Haemodynamic monitoring with pulse-induced contour cardiac output (PiCCO) in critical care. Intensiv. Care Nurs. 2003, 19, 301–307. [Google Scholar] [CrossRef]

| Parameter | Results |
|---|---|
| Baseline data | |
| Age, years, mean (±SD) * | 68.6 (13) |
| Women, n (%) * | 161 (45.5) |
| Men, n (%) * | 193 (54.5) |
| Body Mass Index, kg/m2, median (IQR) * | 26.1 (24.2–29.3) |
| Glasgow Coma Scale score, median (IQR) * | 8 (3–12) |
| APACHE II score, median (IQR) * | 14 (11–19) |
| Hospital stay, median (IQR) *** | 16.5 (4.8–27) |
| Comorbidities | |
| Chronic arterial hypertension, n (%) * | 208 (58.8) |
| Chronic obstructive pulmonary diseases, n (%) * | 15 (4.2) |
| Cardiac arrhythmia, n (%) * | 70 (19.8) |
| Coronary artery disease, n (%) * | 44 (12.4) |
| Heart failure, n (%) * | 20 (5.6) |
| Chronic renal insufficiency, n (%) * | 21 (5.9) |
| Diabetes mellitus, n (%) * | 56 (15.8) |
| Premedication | |
| Antihypertensive drugs, n (%) * | 164 (46.3) |
| Antiobstructive drugs, n (%) * | 5 (1.4) |
| Antidiabetic drugs, n (%) * | 36 (10.2) |
| Antiplatelet agents, n (%) * | 50 (14.1) |
| New oral anticoagulants, n (%) * | 13 (3.7) |
| Vitamin K antagonist, n (%) * | 73 (20.6) |
| Cardiopulmonary parameters | |
| Norepinephrine application rate, µg/kg/min, mean (±SD) ** | 0.03 (0.04) |
| Requiring norepinephrine, n (%) ** | 153 (43.2) |
| Systolic blood pressure, mmHg, median (IQR) ** | 137 (129–145.3) |
| Heart rate, beats per minute, median (IQR) ** | 75 (64–87) |
| Inspiratory oxygen fraction, mean (±SD) ** | 34.8 (13.4) |
| Endotracheal intubation, n (%) ** | 211 (59.6) |
| PEEP level, median (IQR) ** | 7 (6–9) |
| Arterial oxygen partial pressure (mmHg), median (IQR) ** | 109 (98–123) |
| Body temperature, centigrade, median (IQR) * | 36.3 (35.5–36.9) |
| Serum biomarkers | |
| White blood cells, giga/L, mean (±SD) * | 10.9 (4.4) |
| Hemoglobin, g/dL, mean (±SD) * | 13.1 (2) |
| Hematocrit, l/L, mean (±SD) * | 0.39 (0.05) |
| Cholinesterase, U/L, mean (±SD) * | 7809.4 (2282.3) |
| Blood glucose, mg/dL, mean (±SD) * | 163 (59.3) |
| Serum lactate, mmol/L, mean (±SD) * | 1.7 (1.5) |
| Troponin I (n = 177), µg/dL, mean (±SD) * | 0.31 (2.6) |
| Elevated Troponin I, n (%) *,a | 49 (27.7) |
| Cortisol, µg/dL, mean (±SD) * | 27.7 (19.4) |
| C-reactive protein, mg/L, mean (±SD) * | 22 (39.9) |
| Albumin, g/L, mean (±SD) * | 38.2 (5.4) |
| Creatinine, mg/dL, mean (±SD) * | 0.9 (0.6) |
| Prothrombin time, %, mean (±SD) * | 83.7 (26.2) |
| Partial thromboplastin time, seconds, mean (±SD) * | 32.5 (11.4) |
| Antithrombin III, %/NORM, mean (±SD) * | 88.7 (15.9) |
| Fibrinogen, g/L, mean (±SD) * | 3.3 (1.1) |
| Treatment regime | |
| Medical treatment, n (%) *** | 151 (42.7) |
| Additional Surgical Treatment, n (%) *** | 203 (57.3) |
| Radiological data | |
| Localization | |
| Supratentorial, lobar, n (%) * | 122 (34.4) |
| Supratentorial, deep, n (%) * | 180 (50.8) |
| Infratentorial, n (%) * | 52 (14.7) |
| ICH volume, cm3, mean (±SD) | 52.3 (42.2) |
| IVH, n (%) * | 248 (70.1) |
| Hydrocephalus, n (%) * | 158 (44.6) |
| Intra-hospital outcome | |
| mRS score, median (IQR) **** | 5 (4–6) |
| mRS 0, n (%) **** | 0 |
| mRS 1, n (%) **** | 21 (5.9) |
| mRS 2, n (%) **** | 24 (6.8) |
| mRS 3, n (%) **** | 25 (7.1) |
| mRS 4, n (%) **** | 73 (20.6) |
| mRS 5, n (%) **** | 100 (28.2) |
| mRS 6, n (%) **** | 111 (31.4) |
| Favorable outcome, n (%) **** | 143 (40.4) |
| Unfavorable outcome, n (%) **** | 211 (59.6) |
| Parameter | Lactate-Positive (n = 115) | Lactate-Negative (n = 239) | p-Value |
|---|---|---|---|
| Baseline Data | |||
| Age, years, mean (±SD) * | 67.4 (12.5) | 69.1 (13.4) | 0.26 |
| Women, n (%) * | 47 (40.9) | 114 (47.7) | 0.23 |
| Men, n (%) * | 68 (59.1) | 125 (52.3) | |
| Body Mass Index, kg/m2, median (IQR) * | 26.1 (24.2–29.4) | 26.1 (23.9–28.7) | 0.6 |
| Glasgow Coma Scale score, median (IQR) * | 7 (3–10) | 9 (4–13) | <0.0001 |
| APACHE II score, median (IQR) * | 16 (13–19) | 14 (10–18) | 0.002 |
| Hospital stay, median (IQR) *** | 18 (4–29) | 19 (5–26) | 0.78 |
| Comorbidities | |||
| Chronic arterial hypertension, n (%) * | 57 (49.6) | 151 (63.2) | 0.02 |
| Chronic obstructive pulmonary diseases, n (%) * | 3 (2.6) | 12 (5) | 0.29 |
| Cardiac arrhythmia, n (%) * | 20 (17.4) | 50 (20.9) | 0.44 |
| Coronary artery disease, n (%) * | 12 (10.4) | 32 (13.4 | 0.43 |
| Heart failure, n (%) * | 9 (7.8) | 11 (4.6) | 0.22 |
| Chronic renal insufficiency, n (%) * | 5 (4.3) | 16 (6.7) | 0.38 |
| Diabetes mellitus, n (%) * | 22 (19.1) | 34 (14.2) | 0.24 |
| Premedication | |||
| Antihypertensive drugs, n (%) * | 38 (33) | 126 (52.7) | 0.0005 |
| Antiobstructive drugs, n (%) * | 1 (9) | 4 (1.7) | 0.55 |
| Antidiabetic drugs, n (%) * | 15 (13) | 21 (8.8) | 0.22 |
| Antiplatelet agents, n (%) * | 11 (9.6) | 39 (16.3) | 0.09 |
| New oral anticoagulants, n (%) * | 5 (4.3) | 8 (3.3) | 0.64 |
| Vitamin K antagonist, n (%) * | 20 (17.4) | 53 (22.2) | 0.3 |
| Cardiopulmonary parameter | |||
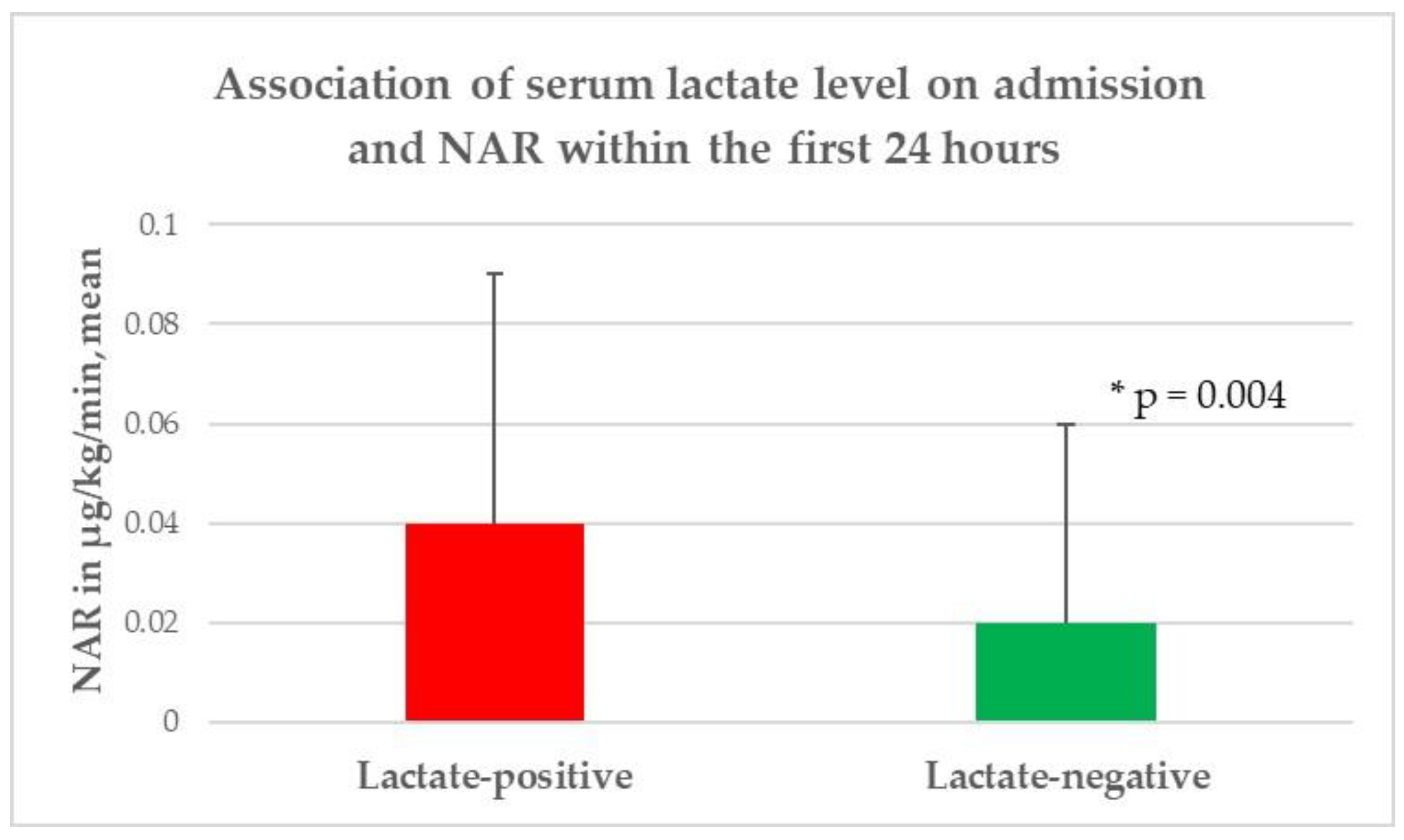
| Norepinephrine application rate, µg/kg/min, mean (±SD) ** | 0.04 (0.05) | 0.02 (0.04) | 0.004 |
| Requiring norepinephrine, n (%) ** | 58 (50.4) | 95 (39.7) | 0.06 |
| Systolic blood pressure, mmHg, median (IQR) ** | 138 (127–145) | 137 (129–146) | 0.66 |
| Heart rate, beats per minute, median (IQR) ** | 75 (63–87) | 75 (64–87) | 0.86 |
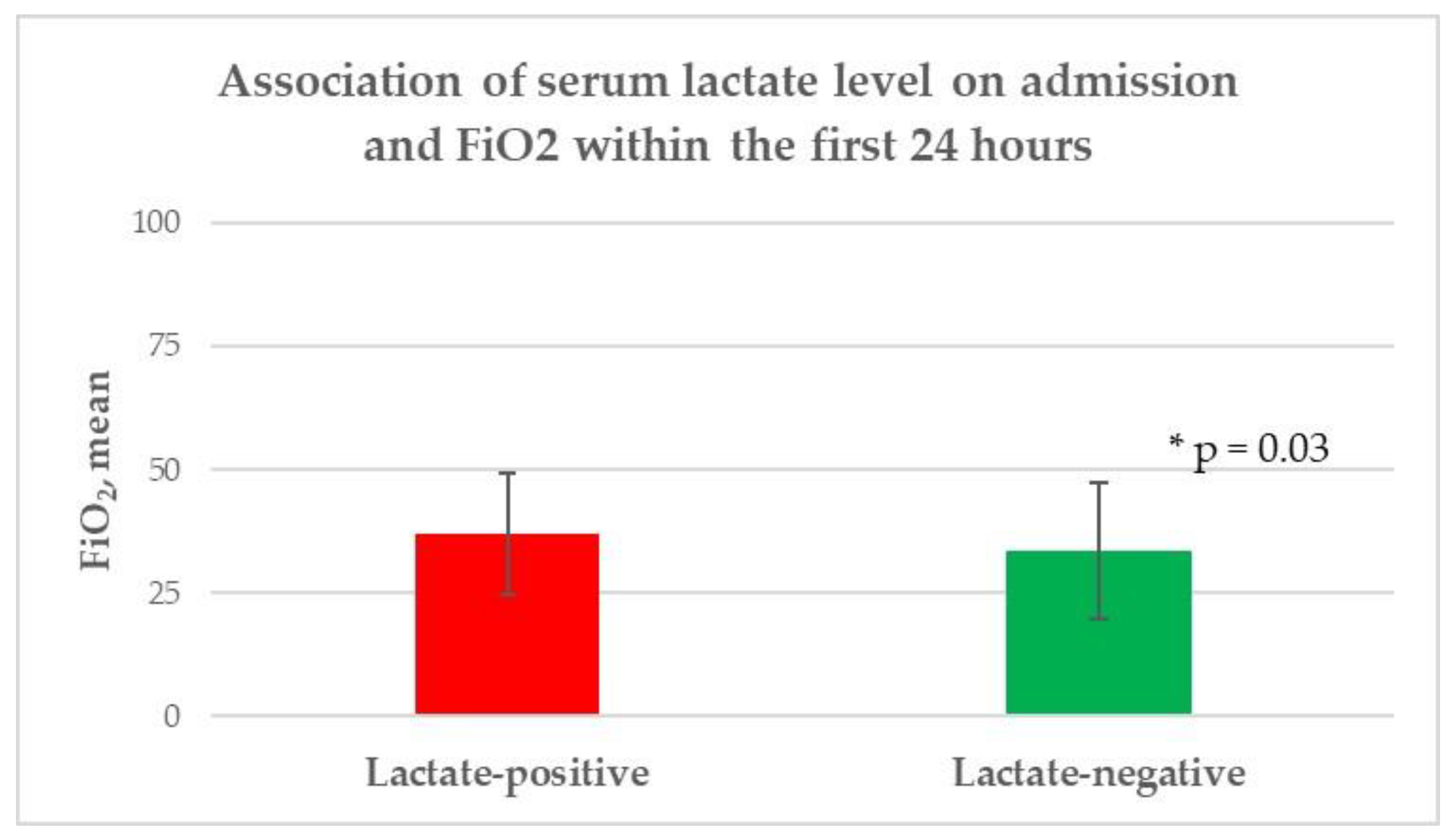
| Inspiratory oxygen fraction, mean (±SD) ** | 37 (12.2) | 33.7 (13.8) | 0.03 |
| Endotracheal intubation, n (%) ** | 82 (71.3) | 129 (54) | 0.002 |
| PEEP level, median (IQR) ** | 7 (6–9) | 8 (6–9.8) | 0.71 |
| Arterial oxygen partial pressure (mmHg), median (IQR) ** | 109 (99–126) | 108 (98–123) | 0.13 |
| Body temperature, centigrade, median (IQR) * | 36.3 (35.5–36.8) | 36.3 (35.4–37) | 0.33 |
| Serum biomarkers | |||
| White blood cells, giga/L, mean (±SD) * | 12.1 (5) | 10.4 (4) | 0.001 |
| Hemoglobin, g/dL, mean (±SD) * | 13.5 (2) | 12.9 (2) | 0.003 |
| Hematocrit, %, mean (±SD) * | 0.39 (0.05) | 0.38 (0.05) | 0.02 |
| Cholinesterase, U/L, mean (±SD) * | 8151.9 (2421) | 7644.6 (2198.6) | 0.05 |
| Blood glucose, mg/dL, mean (±SD) * | 192.3 (72.8) | 150 (45.7) | <0.0001 |
| Troponin I (n = 177), µg/dL, mean (±SD) * | 0.56 (4.1) | 0.27 (0.4) | 0.3 |
| Elevated Troponin I, n (%) *,a | 21 (18.3) | 28 (11.7) | 0.72 |
| Cortisol, µg/dL, mean (±SD) * | 29.7 (20.9) | 26.9 (18.6) | 0.24 |
| C-reactive protein, mg/L, mean (±SD) * | 19.8 (39.5) | 23.1 (39.2) | 0.46 |
| Albumin, g/L, mean (±SD) * | 39.5 (5.2) | 37.6 (5.4) | 0.002 |
| Creatinine, mg/dL, mean (±SD) * | 0.9 (0.5) | 1 (0.7) | 0.39 |
| Prothrombin time, %, mean (±SD) * | 83.4 (26.9) | 83.9 (26) | 0.87 |
| Partial thromboplastin time, seconds, mean (±SD) * | 33.7 (15.8) | 31.9 (8.6) | 0.17 |
| Antithrombin III, %/NORM, mean (±SD) * | 87.7 (16.8) | 89.3 (15.4) | 0.5 |
| Fibrinogen, g/L, mean (±SD) * | 3.1 (1.1) | 3.4 (1.1) | 0.74 |
| Treatment regime | |||
| Medical treatment, n (%) *** | 42 (36.5) | 109 (45.6) | 0.11 |
| Additional Surgical Treatment, n (%) *** | 73 (63.5) | 130 (54.4) | |
| Radiological data | |||
| Localization | |||
| Supratentorial, lobar, n (%) * | 37 (33.2) | 85 (35.6) | 0.53 |
| Supratentorial, deep, n (%) * | 56 (48.7) | 124 (51.9) | 0.57 |
| Infratentorial, n (%) * | 22 (19.1) | 30 (12.6) | 0.1 |
| ICH volume, cm3, mean (±SD) | 57.5 (44.5) | 49.7 (40.9) | 0.11 |
| IVH, n (%) * | 84 (73) | 164 (68.6) | 0.4 |
| Hydrocephalus, n (%) * | 56 (48.7) | 102 (42.7) | 0.29 |
| Intra-hospital outcome | |||
| mRS score, median (IQR) **** | 5 (4–6) | 5 (4–6) | 0.06 |
| Survivor, n (%) | 71 (61.7) | 172 (72) | 0.52 |
| Non-survivor, n (%) | 44 (38.3) | 67 (28) | |
| Favorable outcome, n (%) **** | 42 (36.5) | 101 (42.3) | 0.3 |
| Unfavorable outcome, n (%) **** | 73 (63.5) | 138 (57.7) | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bender, M.; Haferkorn, K.; Nagl, J.; Uhl, E.; Stein, M. Serum Lactate as Serum Biomarker for Cardiopulmonary Parameters within the First 24 Hours after a Spontaneous Intracerebral Hemorrhage. Diagnostics 2022, 12, 2414. https://doi.org/10.3390/diagnostics12102414
Bender M, Haferkorn K, Nagl J, Uhl E, Stein M. Serum Lactate as Serum Biomarker for Cardiopulmonary Parameters within the First 24 Hours after a Spontaneous Intracerebral Hemorrhage. Diagnostics. 2022; 12(10):2414. https://doi.org/10.3390/diagnostics12102414
Chicago/Turabian StyleBender, Michael, Kristin Haferkorn, Jasmin Nagl, Eberhard Uhl, and Marco Stein. 2022. "Serum Lactate as Serum Biomarker for Cardiopulmonary Parameters within the First 24 Hours after a Spontaneous Intracerebral Hemorrhage" Diagnostics 12, no. 10: 2414. https://doi.org/10.3390/diagnostics12102414
APA StyleBender, M., Haferkorn, K., Nagl, J., Uhl, E., & Stein, M. (2022). Serum Lactate as Serum Biomarker for Cardiopulmonary Parameters within the First 24 Hours after a Spontaneous Intracerebral Hemorrhage. Diagnostics, 12(10), 2414. https://doi.org/10.3390/diagnostics12102414






