Capillary Blood Gas in Children Hospitalized Due to Influenza Predicts the Risk of Lower Respiratory Tract Infection
Abstract
1. Introduction
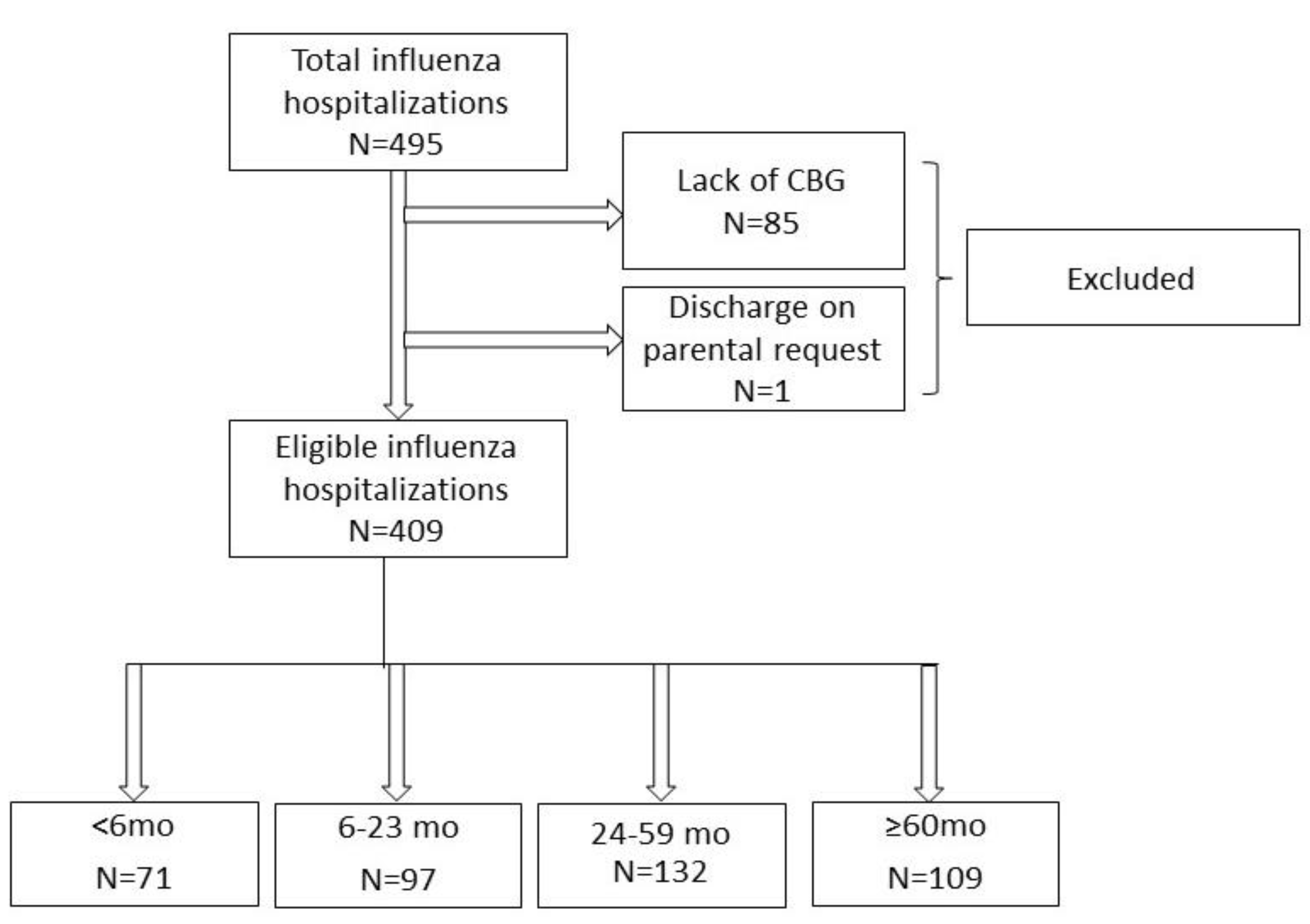
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, X.; Li, Y.; O’Brien, K.L.; Madhi, S.A.; Widdowson, M.-A.; Byass, P.; Omer, S.B.; Abbas, Q.; Ali, A.; Amu, A.; et al. Global burden of respiratory infections associated with seasonal influenza in children under 5 years in 2018: A systematic review and modelling study. Lancet Glob. Health 2020, 8, e497–e510. [Google Scholar] [CrossRef]
- Maldonado, Y.A.; O’Leary, S.T.; Banerjee, R.; Barnett, E.D.; Campbell, J.D.; Caserta, M.T.; Caserta, M.T.; Gerber, J.S.; Kourtis, A.P.; Lynfield, R.; et al. Recommendations for Prevention and Control of Influenza in Children, 2020–2021. Pediatrics 2020, 146, e2020024588. [Google Scholar] [CrossRef]
- DISEASES COI. Recommendations for Prevention and Control of Influenza in Children, 2021–2022. Pediatrics 2021, 148, e2021053745. [Google Scholar] [CrossRef] [PubMed]
- Cui, A.; Xie, Z.; Xu, J.; Hu, K.; Zhu, R.; Li, Z.; Li, Y.; Sun, L.; Xiang, X.; Xu, B.; et al. Comparative analysis of the clinical and epidemiological characteristics of human influenza virus versus human respiratory syncytial virus versus human metapneumovirus infection in nine provinces of China during 2009–2021. J. Med. Virol. 2022. [Google Scholar] [CrossRef]
- Terliesner, N.; Unterwalder, N.; Edelmann, A.; Corman, V.; Knaust, A.; Rosenfeld, L.; Gratopp, A.; Ringe, H.; Martin, L.; von Bernuth, H.; et al. Viral infections in hospitalized children in Germany during the COVID-19 pandemic: Association with non-pharmaceutical interventions. Front. Pediatr. 2022, 10, 935483. [Google Scholar] [CrossRef]
- Willis, G.; Bloomfield, L.; Berry, M.; Bulsara, C.; Chaney, G.; Cooke, H.; Maticevic, J.; Russell, K.; Zic, M.; Mak, D. The impact of a vaccine mandate and the COVID-19 pandemic on influenza vaccination uptake in Western Australian health care students. Vaccine 2022, 40, 5651–5656. [Google Scholar] [CrossRef]
- Shen, A.K.; Browne, S.; Srivastava, T.; Michel, J.J.; Tan, A.S.L.; Kornides, M.L. Factors Influencing Parental and Individual COVID-19 Vaccine Decision Making in a Pediatric Network. Vaccines 2022, 10, 1277. [Google Scholar] [CrossRef]
- Grohskopf, L.A.; Alyanak, E.; Ferdinands, J.M.; Broder, K.R.; Blanton, L.H.; Talbot, H.K.; Fry, A.M. Prevention and Control of Seasonal Influenza with Vaccines: Recommendations of the Advisory Committee on Immunization Practices—United States, 2020–2021 Influenza Season. MMWR Recomm. Rep. 2020, 70, 1–28. [Google Scholar] [CrossRef]
- Shope, T.R.; Walker, B.H.; Aird, L.D.; Southward, L.; McCown, J.S.; Martin, J.M. Pandemic Influenza Preparedness Among Child Care Center Directors in 2008 and 2016. Pediatrics 2017, 139, e20163690C. [Google Scholar] [CrossRef]
- Gresh, L.; Kuan, G.; Sanchez, N.; Azziz-Baumgartner, E.; Ojeda, S.; Melendez, M.; Lopez, R.; Martin, E.T.; Widdowson, M.-A.; Bresee, J.; et al. Burden of Influenza and Influenza-associated Pneumonia in the First Year of Life in a Prospective Cohort Study in Managua, Nicaragua. Pediatr. Infect. Dis. J. 2016, 35, 152–156. [Google Scholar] [CrossRef]
- Sharma, L.; Rebaza, A.; Cruz, C.S.D. When “B” becomes “A”: The emerging threat of influenza B virus. Eur. Respir. J. 2019, 54, 1901325. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Qian, S.; Liu, C.; Xiao, Y.; Xu, T.; Wang, Y.; Su, H.; Chen, L.; Yuan, B.; Wang, X.; et al. Viral etiology of life-threatening pediatric pneumonia: A matched case-control study. Influ. Other Respir. Viruses 2020, 14, 452–459. [Google Scholar] [CrossRef]
- McCullers, J.A. The co-pathogenesis of influenza viruses with bacteria in the lung. Nat. Rev. Microbiol. 2014, 12, 252–262. [Google Scholar] [CrossRef] [PubMed]
- Kubale, J.; Kuan, G.; Gresh, L.; Ojeda, S.; Schiller, A.; Sanchez, N.; Lopez, R.; Azziz-Baumgartner, E.; Wraith, S.; Harris, E.; et al. Individual-level Association of Influenza Infection With Subsequent Pneumonia: A Case-control and Prospective Cohort Study. Clin. Infect. Dis. 2020, 73, e4288–e4295C. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.; Seo, E.; Yoo, R.N.; Sung, H.; Lee, J. Clinical significance of viral-bacterial codetection among young children with respiratory tract infections: Findings of RSV, influenza, adenoviral infections. Medicine 2020, 99, e18504. [Google Scholar] [CrossRef]
- Sanders, C.J.; Vogel, P.; McClaren, J.L.; Bajracharya, R.; Doherty, P.C.; Thomas, P.G. Compromised respiratory function in lethal influenza infection is characterized by the depletion of type I alveolar epithelial cells beyond threshold levels. Am. J. Physiol. Cell. Mol. Physiol. 2013, 304, L481–L488. [Google Scholar] [CrossRef]
- Niethamer, T.K.; Stabler, C.T.; Leach, J.P.; Zepp, J.A.; Morley, M.P.; Babu, A.; Zhou, S.; Morrisey, E.E. Defining the role of pulmonary endothelial cell heterogeneity in the response to acute lung injury. Elife 2020, 9, e53072. [Google Scholar] [CrossRef]
- LeMessurier, K.S.; Tiwary, M.; Morin, N.P.; Samarasinghe, A.E. Respiratory Barrier as a Safeguard and Regulator of Defense Against Influenza A Virus and Streptococcus pneumoniae. Front. Immunol. 2020, 11, 3. [Google Scholar] [CrossRef]
- McNally, B.; Ye, F.; Willette, M.; Flaño, E. Local Blockade of Epithelial PDL-1 in the Airways Enhances T Cell Function and Viral Clearance during Influenza Virus Infection. J. Virol. 2013, 87, 12916–12924. [Google Scholar] [CrossRef]
- Schneider, C.; Nobs, S.P.; Heer, A.K.; Kurrer, M.; Klinke, G.; Van Rooijen, N.; Vogel, J.; Kopf, M. Alveolar Macrophages Are Essential for Protection from Respiratory Failure and Associated Morbidity following Influenza Virus Infection. PLoS Pathog. 2014, 10, e1004053. [Google Scholar] [CrossRef]
- Peltola, V.; Ziegler, T.; Ruuskanen, O. Influenza A and B Virus Infections in Children. Clin. Infect. Dis. 2003, 36, 299–305. [Google Scholar] [CrossRef] [PubMed]
- Yildizdas, D.; Yapıcıoğlu, H.; Yilmaz, H.L.; Sertdemir, Y. Correlation of simultaneously obtained capillary, venous, and arterial blood gases of patients in a paediatric intensive care unit. Arch. Dis. Child. 2004, 89, 176–180. [Google Scholar] [CrossRef] [PubMed]
- Kirubakaran, C.; Gnananayagam, J.E.J.; Sundaravalli, E.K. Comparison of blood gas values in arterial and venous blood. Indian J. Pediatr. 2003, 70, 781–785. [Google Scholar] [CrossRef] [PubMed]
- Escalante-Kanashiro, R.; Tantaleán-Da-Fieno, J. Capillary blood gases in a pediatric intensive care unit. Crit. Care Med. 2000, 28, 224–226. [Google Scholar] [CrossRef] [PubMed]
- Harrison, A.M.; Lynch, J.M.; Dean, J.M.; Witte, M.K. Comparison of simultaneously obtained arterial and capillary blood gases in pediatric intensive care unit patients. Crit. Care Med. 1997, 25, 1904–1908. [Google Scholar] [CrossRef] [PubMed]
- CDC. Available online: https://www.cdc.gov/flu/professionals/antivirals/summary-clinicians.htm (accessed on 30 August 2022).
- Hryniewicz, W. Rekomendacje Postępowania w Pozaszpitalnych Zakażeniach Układu Oddechowego; NarodCowy Instytut Leków: Warszawa, Poland, 2016. [Google Scholar]
- Wrotek, A.; Kobiałka, M.; Jackowska, T. Capillary Blood Gas Predicts Risk of Intensive Care in Children with Bronchiolitis. Children 2021, 8, 719. [Google Scholar] [CrossRef]
- Vo, A.T.; Liu, D.R.; Schmidt, A.R.; Festekjian, A. Capillary blood gas in infants with bronchiolitis: Can end-tidal capnography replace it? Am. J. Emerg. Med. 2021, 45, 144–148. [Google Scholar] [CrossRef]
- Zhang, J.; Zhao, Y.; Chen, Y. Laboratory findings in patients with avian-origin influenza A (H7N9) virus infections. J. Med. Virol. 2013, 86, 895–898. [Google Scholar] [CrossRef]
- Goritzka, M.; Makris, S.; Kausar, F.; Durant, L.; Pereira, C.; Kumagai, Y.; Culley, F.; Mack, M.; Akira, S.; Johansson, C. Alveolar macrophage–derived type I interferons orchestrate innate immunity to RSV through recruitment of antiviral monocytesC. J. Exp. Med. 2015, 212, 699–714. [Google Scholar] [CrossRef]
- Choi, J.; Callaway, Z.; Kim, H.-B.; Fujisawa, T.; Kim, C.-K. The role of TNF-α in eosinophilic inflammation associated with RSV bronchiolitis. Pediatr. Allergy Immunol. 2010, 21, 474–479. [Google Scholar] [CrossRef]


| LRTI | Without LRTI | ||||||
|---|---|---|---|---|---|---|---|
| <6 mo | Mean/Median with SD or IQR | Mean/Median with SD or IQR | p | ||||
| age [months] | 1 | 0 | 4 | 2 | 1 | 4 | 0.703 |
| duration of signs/syndromes [days] | 2 | 1 | 4 | 1 | 1 | 2 | 0.007 |
| the highest fever [Celsius degrees] | 39.0 | 38.3 | 40.0 | 38.4 | 38.1 | 39.0 | 0.047 |
| LOS [days] | 10 | 5 | 13 | 5 | 4 | 7 | 0.000 |
| breath rate [per minute] | 50 | 36 | 60 | 40 | 30 | 40 | 0.005 |
| heart rate [per minute] | 141.24 | 21.62 | (SD) | 135.43 | 17.59 | (SD) | 0.262 * |
| CRP [mg/L] | 2.20 | 0.57 | 11.40 | 1.25 | 1.00 | 4.04 | 0.393 |
| PCT [ng/mL] | 0.14 | 0.10 | 0.24 | 0.15 | 0.12 | 0.21 | 0.582 |
| WBC [*10^3/μL] | 9.90 | 7.40 | 12.70 | 6.90 | 4.66 | 9.70 | 0.015 |
| ANC [*10^3/μL] | 2.25 | 1.48 | 3.49 | 1.99 | 1.21 | 2.84 | 0.092 |
| pH | 7.42 | 0.04 | (SD) | 7.45 | 0.05 | (SD) | 0.025 * |
| pCO2 [mmHg] | 37.57 | 5.24 | (SD) | 32.07 | 6.56 | (SD) | 0.001 * |
| pO2 [mmHg] | 52.40 | 47.40 | 60.30 | 60.85 | 52.85 | 67.15 | 0.018 |
| SatO2 | 88.21 | 4.04 | (SD) | 91.65 | 4.29 | (SD) | 0.002 * |
| 6–23 mo | mean/median with SD or IQR | mean/median with SD or IQR | p | ||||
| age [months] | 12 | 9 | 18 | 13 | 9 | 17 | 0.657 |
| duration of signs/syndromes [days] | 4 | 1 | 5 | 1 | 1 | 4 | 0.053 |
| the highest fever [Celsius degrees] | 39.0 | 38.6 | 39.5 | 39.3 | 38.7 | 40.0 | 0.064 |
| LOS [days] | 8 | 6 | 11 | 5 | 4 | 7 | 0.000 |
| breath rate [per minute] | 30 | 25 | 40 | 28 | 25 | 30 | 0.121 |
| heart rate [per minute] | 120 | 115 | 140 | 120 | 110 | 130 | 0.345 |
| CRP [mg/L] | 6.33 | 2.72 | 20.72 | 4.28 | 1.20 | 14.15 | 0.216 |
| PCT [ng/mL] | 0.35 | 0.18 | 1.03 | 0.20 | 0.13 | 0.54 | 0.026 |
| WBC [*10^3/μL] | 11.95 | 8.55 | 16.77 | 9.05 | 6.71 | 10.86 | 0.001 |
| ANC [*10^3/μL] | 4.78 | 2.55 | 8.92 | 4.16 | 2.12 | 6.06 | 0.071 |
| pH | 7.43 | 0.04 | (SD) | 7.44 | 0.05 | (SD) | 0.046 * |
| pCO2 [mmHg] | 32.75 | 29.15 | 35.05 | 30.40 | 27.00 | 33.50 | 0.069 |
| pO2 [mmHg] | 65.80 | 59.20 | 70.95 | 71.20 | 65.00 | 76.40 | 0.006 |
| SatO2 | 93.45 | 91.35 | 94.55 | 95.10 | 93.30 | 96.10 | 0.000 |
| 24–59 mo | mean/median with SD or IQR | mean/median with SD or IQR | p | ||||
| age [months] | 37 | 29 | 48 | 39 | 31 | 50 | 0.878 |
| duration of signs/syndromes [days] | 4 | 3 | 6 | 3 | 1 | 5 | 0.020 |
| the highest fever [Celsius degrees] | 39.2 | 39.0 | 40.0 | 39.5 | 39.0 | 40.0 | 0.789 |
| LOS [days] | 7 | 5 | 9 | 4 | 3 | 6 | 0.000 |
| breath rate [per minute] | 28 | 24 | 30 | 24 | 22 | 26 | 0.029 |
| heart rate [per minute] | 120 | 100 | 130 | 109 | 100 | 120 | 0.137 |
| CRP [mg/L] | 7.70 | 3.46 | 16.64 | 5.75 | 1.00 | 17.21 | 0.086 |
| PCT [ng/mL] | 0.21 | 0.13 | 0.80 | 0.17 | 0.10 | 0.36 | 0.135 |
| WBC [*10^3/μL] | 8.09 | 5.81 | 9.95 | 7.52 | 5.42 | 10.47 | 0.270 |
| ANC [*10^3/μL] | 4.32 | 2.31 | 7.26 | 3.95 | 2.19 | 6.69 | 0.389 |
| pH | 7.43 | 0.04 | (SD) | 7.43 | 0.05 | (SD) | 0.974 * |
| pCO2 [mmHg] | 32.60 | 4.94 | (SD) | 33.40 | 5.65 | (SD) | 0.847 * |
| pO2 [mmHg] | 65.90 | 60.00 | 74.85 | 68.95 | 63.80 | 77.20 | 0.055 |
| SatO2 | 93.70 | 91.20 | 95.40 | 94.25 | 92.60 | 95.90 | 0.054 |
| >60 mo | mean/median with SD or IQR | mean/median with SD or IQR | p | ||||
| age [months] | 74 | 64 | 95 | 84 | 70 | 107 | 0.169 |
| duration of signs/syndromes [days] | 5 | 2 | 6 | 3 | 1 | 4 | 0.010 |
| the highest fever [Celsius degrees] | 39.5 | 39.0 | 40.0 | 39.5 | 39.0 | 40.0 | 0.958 |
| LOS [days] | 6 | 4 | 8 | 4 | 3 | 5 | 0.001 |
| breath rate [per minute] | 22 | 20 | 24 | 22 | 20 | 24 | 0.696 |
| heart rate [per minute] | 100 | 90 | 110 | 91 | 85 | 100 | 0.150 |
| CRP [mg/L] | 6.83 | 1.90 | 20.70 | 6.99 | 3.19 | 15.05 | 0.841 |
| PCT [ng/mL] | 0.22 | 0.10 | 0.45 | 0.14 | 0.08 | 0.26 | 0.104 |
| WBC [*10^3/μL] | 5.94 | 4.70 | 8.89 | 6.18 | 4.43 | 8.51 | 0.941 |
| ANC [*10^3/μL] | 3.56 | 2.09 | 6.89 | 3.52 | 2.28 | 5.42 | 0.961 |
| pH | 7.44 | 7.41 | 7.44 | 7.42 | 7.41 | 7.45 | 0.658 |
| pCO2 [mmHg] | 35.30 | 30.90 | 39.90 | 35.80 | 32.20 | 38.60 | 0.773 |
| pO2 [mmHg] | 67.60 | 59.70 | 74.30 | 69.70 | 63.70 | 75.00 | 0.288 |
| SatO2 | 94.10 | 89.80 | 95.30 | 94.45 | 92.70 | 95.60 | 0.287 |
| 0–6 mo | |||||||||
| LRTI | AUC | 95%CI | p | cut off | Sensitivity 95%CI | Specificity 95%CI | PPV 95%CI | NPV 95%CI | |
| pH | 0.651 | 0.520 | 0.783 | 0.024 | 7.442 | 73.91% | 54.17% | 43.59% | 81.25% |
| 51.59–89.77% | 39.17–68.63% | 34.31–53.35% | 67.50–90.04% | ||||||
| pCO2 | 0.749 | 0.633 | 0.865 | 0.000 | 36.10 | 69.57% | 72.92% | 55.17% | 83.33% |
| 47.08–86.79% | 58.15–84.72% | 41.84–67.80% | 72.47–90.47% | ||||||
| pO2 | 0.727 | 0.604 | 0.849 | 0.000 | 58.00 | 73.91% | 60.42% | 47.22% | 82.86% |
| 51.59% -89.77% | 45.27–74.23% | 36.89–57.79% | 70.07–90.89% | ||||||
| SatO2 | 0.740 | 0.620 | 0.861 | 0.000 | 93.00 | 91.30% | 50.00% | 46.67% | 92.31% |
| 71.96–98.93% | 35.23–64.77% | 39.10–54.39% | 75.60–97.89% | ||||||
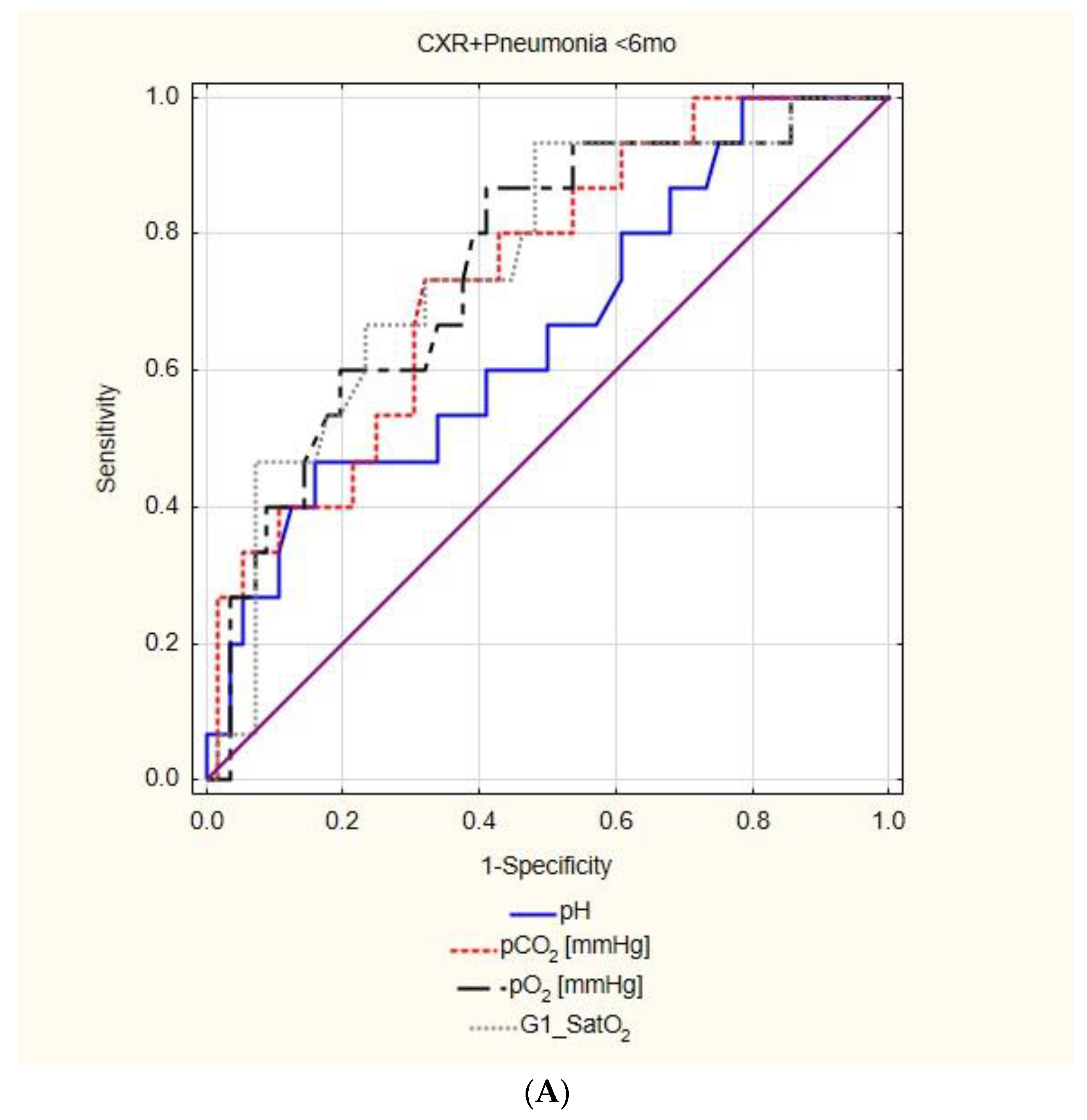
| CXR+ pneumonia | AUC | 95%CI | p | cut off | Sensitivity 95%CI | Specificity 95%CI | PPV 95%CI | NPV 95%CI | |
| pH | insignificant | ||||||||
| pCO2 | 0.740 | 0.607 | 0.873 | 0.000 | 36.10 | 73.33% | 67.86% | 37.93% | 90.48% |
| 44.90–92.21% | 54.04–79.71% | 27.28–49.88% | 80.11–95.73% | ||||||
| pO2 | 0.754 | 0.619 | 0.888 | 0.000 | 58.00 | 86.67% | 58.93% | 36.11% | 94.29% |
| 59.54–98.34% | 44.98–71.90% | 28.05–45.03% | 81.68–98.39% | ||||||
| SatO2 | 0.756 | 0.621 | 0.891 | 0.000 | 91.60 | 93.33% | 51.79% | 34.15% | 96.67% |
| 68.05–99.83% | 38.03–65.34% | 27.69–41.25% | 81.11–99.49% | ||||||
| 6–23 mo | |||||||||
| LRTI | AUC | 95%CI | p | cut off | Sensitivity 95%CI | Specificity 95%CI | PPV 95%CI | NPV 95%CI | |
| pH | 0.622 | 0.510 | 0.734 | 0.033 | 7.455 | 82.50% | 47.37% | 52.38% | 79.41% |
| 67.22–92.66% | 33.98–61.03% | 45.28–59.39% | 65.10–88.86% | ||||||
| pCO2 | insignificant | ||||||||
| pO2 | 0.666 | 0.556 | 0.776 | 0.003 | 68.60 | 72.50% | 59.65% | 55.77% | 75.56% |
| 56.11–85.40% | 45.82–72.44% | 46.58–64.58% | 64.15–84.23% | ||||||
| SatO2 | 0.714 | 0.609 | 0.818 | 0.000 | 94.00 | 72.50% | 64.91% | 59.18% | 77.08% |
| 56.11–85.40% | 51.13–77.09% | 49.25–68.42% | 66.26–85.21% | ||||||
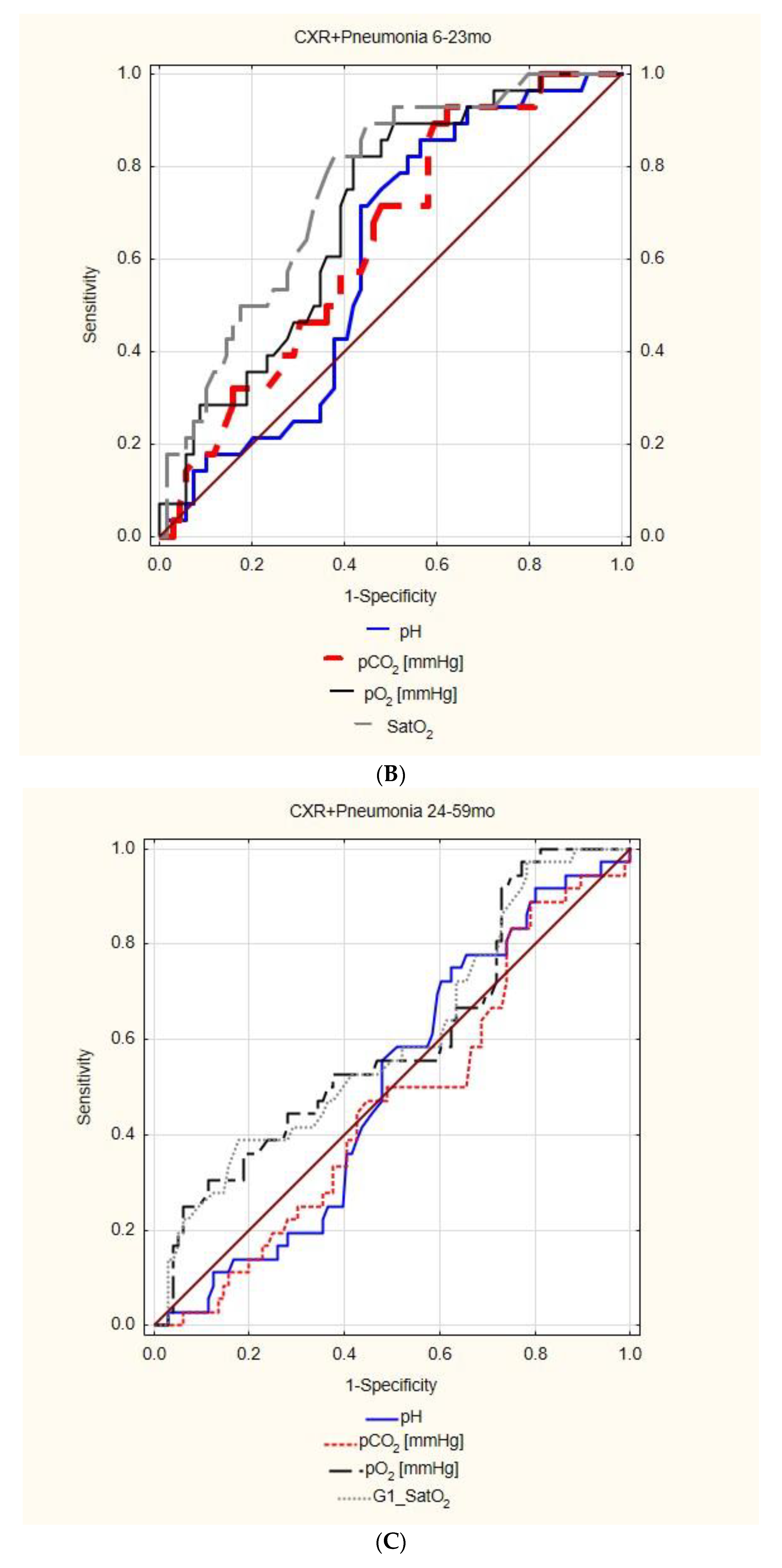
| CXR+ pneumonia | AUC | 95%CI | p | cut off | Sensitivity 95%CI | Specificity 95%CI | PPV 95%CI | NPV 95%CI | |
| pH | insignificant | ||||||||
| pCO2 | 0.640 | 0.527 | 0.754 | 0.016 | 28.00 | 92.86% | 37.68% | 37.68% | 92.86% |
| 76.50–99.12% | 26.29–50.17% | 32.89–42.73% | 76.77–98.08% | ||||||
| pO2 | 0.695 | 0.588 | 0.803 | 0.000 | 68.60 | 82.14% | 57.97% | 44.23% | 88.89% |
| 63.11–93.94% | 45.48–69.76% | 36.39–52.37% | 77.90–94.78% | ||||||
| SatO2 | 0.755 | 0.655 | 0.855 | 0.000 | 94.00 | 82.14% | 62.32% | 46.94% | 89.58% |
| 63.11–93.94% | 49.83–73.71% | 38.42–55.64% | 79.19–95.11% | ||||||
| 24–59 mo | |||||||||
| LRTI | AUC | 95%CI | p | cut off | Sensitivity 95%CI | Specificity 95%CI | PPV 95%CI | NPV 95%CI | |
| pH | insignificant | ||||||||
| pCO2 | insignificant | ||||||||
| pO2 | 0.601 | 0.501 | 0.702 | 0.048 | 76.60 | 91.30% | 29.07% | 40.78% | 86.21% |
| 79.21–97.58% | 19.78–39.86% | 36.93–44.74% | 69.84–94.40% | ||||||
| SatO2 | 0.602 | 0.502 | 0.702 | 0.045 | 96.20 | 95.65% | 23.26% | 40.00% | 90.91% |
| 85.16–99.47% | 14.82–33.61% | 36.89–43.20% | 70.97–97.61% | ||||||
| CXR+ pneumonia | AUC | 95%CI | p | cut off | Sensitivity 95%CI | Specificity 95%CI | PPV 95%CI | NPV 95%CI | |
| pH | insignificant | ||||||||
| pCO2 | |||||||||
| pO2 | |||||||||
| SatO2 | |||||||||
| 0–6 mo | |||||||||||
| CXR | LOS | ||||||||||
| AUC | 95%CI | p | cut off | AUC | 95%CI | p | cut off | ||||
| pH | insignificant | pH | insignificant | ||||||||
| pCO2 | 0.663 | 0.519 | 0.808 | 0.027 | 36.1 | pCO2 | 0.636 | 0.503 | 0.768 | 0.045 | 36.0 |
| pO2 | 0.703 | 0.573 | 0.833 | 0.002 | 52.3 | pO2 | 0.726 | 0.608 | 0.843 | 0.000 | 52.3 |
| SatO2 | 0.676 | 0.533 | 0.819 | 0.016 | 91.6 | SatO2 | 0.712 | 0.591 | 0.833 | 0.001 | 88.2 |
| 6–23 mo | |||||||||||
| CXR | LOS | ||||||||||
| AUC | 95%CI | p | cut off | AUC | 95%CI | p | cut off | ||||
| pH | insignificant | pH | insignificant | ||||||||
| pCO2 | 0.622 | 0.510 | 0.734 | 0.033 | 28.5 | pCO2 | insignificant | ||||
| pO2 | 0.693 | 0.587 | 0.799 | 0.000 | 68.6 | pO2 | 0.633 | 0.517 | 0.750 | 0.025 | 68.1 |
| SatO2 | 0.751 | 0.652 | 0.849 | 0.000 | 94.6 | SatO2 | 0.659 | 0.544 | 0.775 | 0.007 | 92.9 |
| 24–59 mo | |||||||||||
| CXR | LOS | ||||||||||
| AUC | 95%CI | p | cut off | AUC | 95%CI | p | cut off | ||||
| pH | insignificant | pH | insignificant | ||||||||
| pCO2 | pCO2 | 0.390 | 0.288 | 0.493 | 0.036 | ||||||
| pO2 | pO2 | insignificant | |||||||||
| SatO2 | SatO2 | ||||||||||
| Negative CXR | Odds Reduction of CXR Performance | |||||||
|---|---|---|---|---|---|---|---|---|
| Cut-Off | OR | 95%CI | p | % | 95%CI | |||
| SatO2 < 6 mo | 91.6% | 0.1644 | 0.0781 | 0.3461 | <0.01 | 83.56 | 65.39 | 92.19 |
| SatO2 6–23 mo | 94% | 0.1486 | 0.0793 | 0.2785 | <0.01 | 85.14 | 72.15 | 92.07 |
| SatO2 0–23 mo | 0.1585 | 0.0986 | 0.2550 | <0.01 | 84.15 | 74.5 | 90.14 | |
| pO2 < 6 mo | 58 mmHg | 0.1283 | 0.0603 | 0.2734 | <0.01 | 87.17 | 72.66 | 93.97 |
| pO2 6–23 mo | 68.6 mmHg | 0.1731 | 0.0933 | 0.3210 | <0.01 | 82.69 | 67.9 | 90.67 |
| pO2 0–23 mo | 0.1542 | 0.0958 | 0.2483 | <0.01 | 84.58 | 75.17 | 90.42 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wrotek, A.; Jackowska, T. Capillary Blood Gas in Children Hospitalized Due to Influenza Predicts the Risk of Lower Respiratory Tract Infection. Diagnostics 2022, 12, 2412. https://doi.org/10.3390/diagnostics12102412
Wrotek A, Jackowska T. Capillary Blood Gas in Children Hospitalized Due to Influenza Predicts the Risk of Lower Respiratory Tract Infection. Diagnostics. 2022; 12(10):2412. https://doi.org/10.3390/diagnostics12102412
Chicago/Turabian StyleWrotek, August, and Teresa Jackowska. 2022. "Capillary Blood Gas in Children Hospitalized Due to Influenza Predicts the Risk of Lower Respiratory Tract Infection" Diagnostics 12, no. 10: 2412. https://doi.org/10.3390/diagnostics12102412
APA StyleWrotek, A., & Jackowska, T. (2022). Capillary Blood Gas in Children Hospitalized Due to Influenza Predicts the Risk of Lower Respiratory Tract Infection. Diagnostics, 12(10), 2412. https://doi.org/10.3390/diagnostics12102412






