Psychiatric Illness or Immune Dysfunction—Brain Perfusion Imaging Providing the Answer in a Case of Anti-NMDAR Encephalitis
Abstract
1. Introduction
2. Case Presentation
2.1. Methods
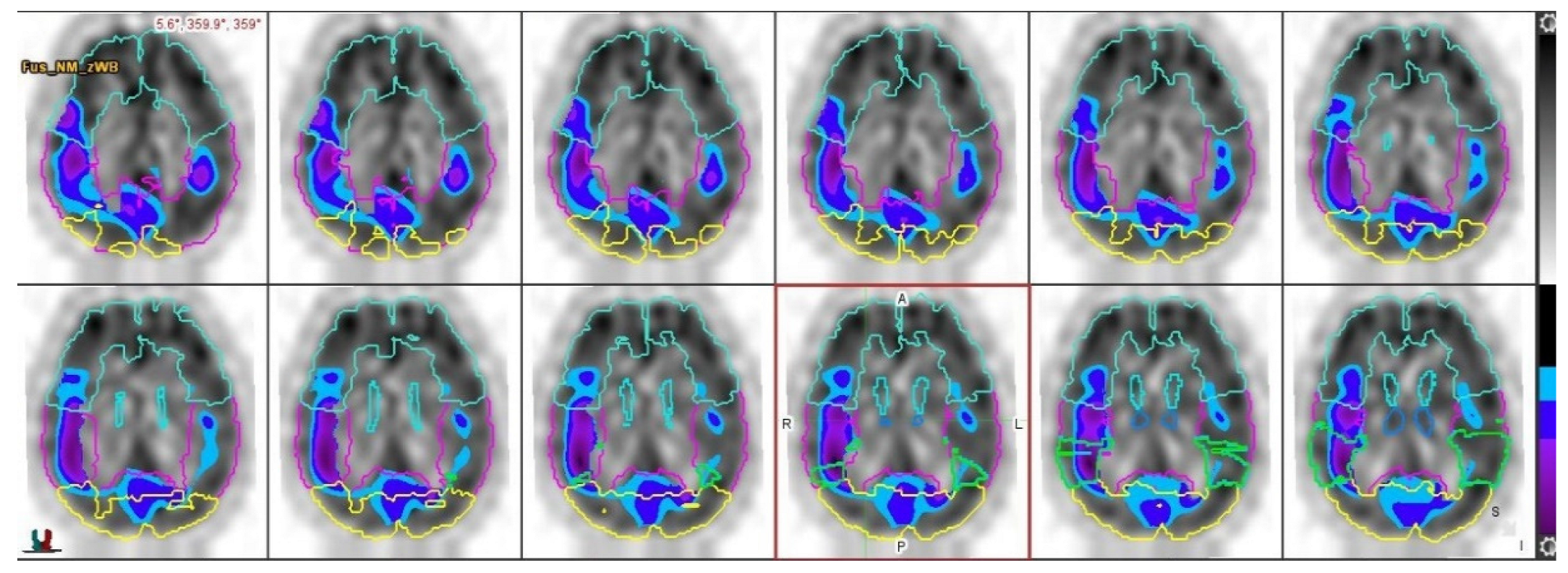
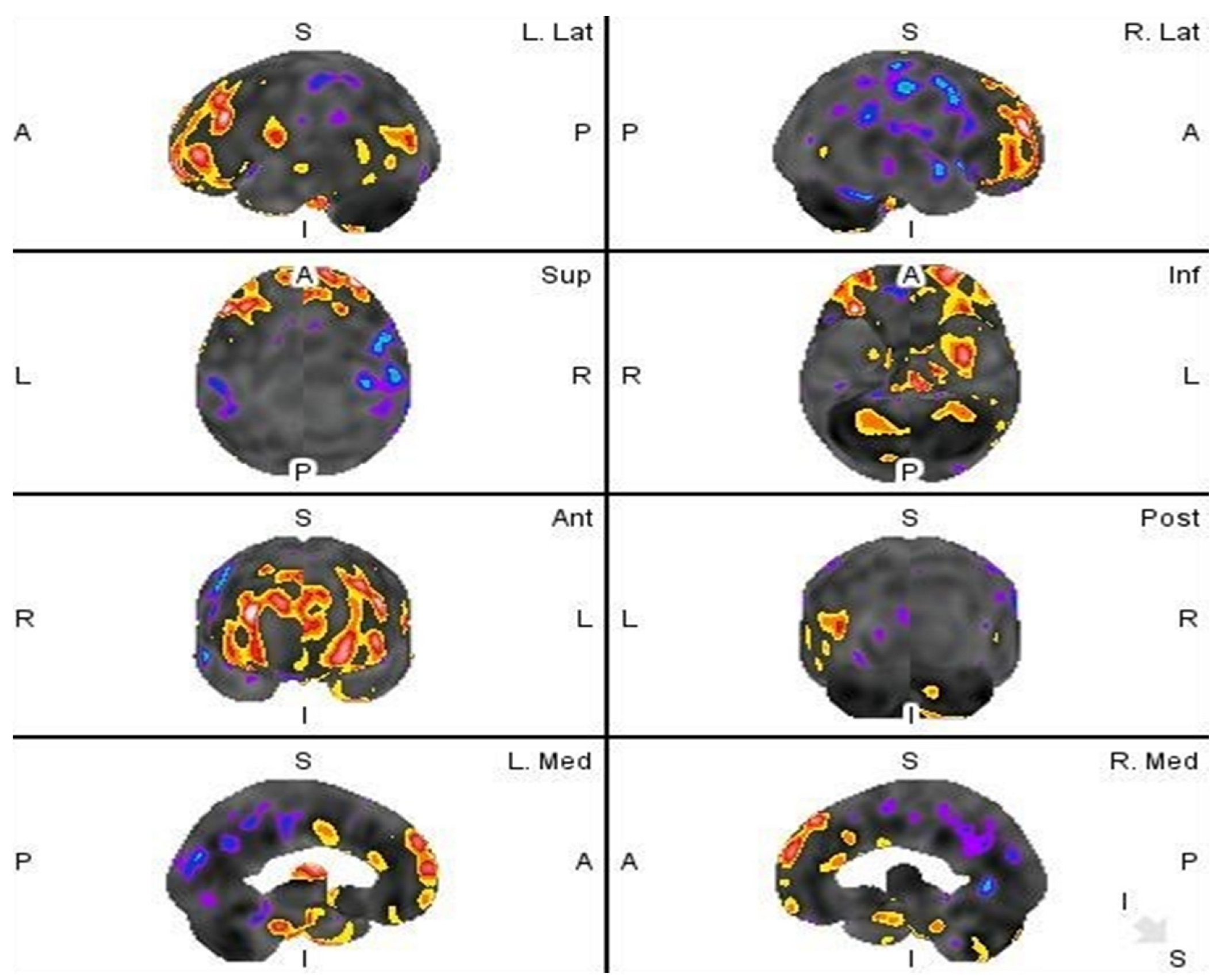
2.2. Results
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dalmau, J.; Gleichman, A.J.; Hughes, E.G.; Rossi, J.E.; Peng, X.; Lai, M.; Dessain, S.K.; Rosenfeld, M.R.; Balice-Gordon, R.; Lynch, D.R. Anti-NMDA-receptor encephalitis: Case series and analysis of the effects of antibodies. Lancet Neurol. 2008, 7, 1091–1098. [Google Scholar] [CrossRef]
- Agarwal, R.; Gupta, V. Anti-NMDA Receptor Encephalitis in Children. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Dalmau, J.; Graus, F. Antibody-Mediated Encephalitis. N. Engl. J. Med. 2018, 378, 840–851. [Google Scholar] [CrossRef]
- Kayser, M.S.; Titulaer, M.J.; Gresa-Arribas, N.; Dalmau, J. Frequency and characteristics of isolated psychiatric episodes in anti–N-methyl-d-aspartate receptor encephalitis. JAMA Neurol. 2013, 70, 1133–1139. [Google Scholar] [CrossRef]
- Vitaliani, R.; Mason, W.; Ances, B.; Zwerdling, T.; Jiang, Z.; Dalmau, J. Paraneoplastic encephalitis, psychiatric symptoms, and hypoventilation in ovarian teratoma. Ann. Neurol. 2005, 58, 594–604. [Google Scholar] [CrossRef] [PubMed]
- González-Valcárcel, J.; Rosenfeld, M.R.; Dalmau, J. Diagnóstico diferencial en la encefalitis por anticuerpos contra el receptor NMDA [Differential diagnosis of encephalitis due to anti-NMDA receptor antibodies]. Neurologia 2010, 25, 409–413. [Google Scholar] [CrossRef] [PubMed]
- Iizuka, T.; Sakai, F. Anti-nMDA receptor encephalitis--clinical manifestations and pathophysiology. Brain Nerve. 2008, 60, 1047–1060. (In Japanese) [Google Scholar] [PubMed]
- Masdeu, J.C.; Dalmau, J.; Berman, K.F. NMDA Receptor Internalization by Autoantibodies: A Reversible Mechanism Underlying Psychosis? Trends Neurosci. 2016, 39, 300–310. [Google Scholar] [CrossRef]
- Kruse, J.L.; Lapid, M.I.; Lennon, V.A.; Klein, C.J.; Toole, O.O.; Pittock, S.J.; Strand, E.A.; Frye, M.A.; McKeon, A. Psychiatric Autoimmunity: N-Methyl-D-Aspartate Receptor IgG and Beyond. Psychosomatics 2015, 56, 227–241. [Google Scholar] [CrossRef]
- Florance, N.R.; Davis, R.L.; Lam, C.; Szperka, C.; Zhou, L.; Ahmad, S.; Campen, C.J.; Moss, H.; Peter, N.; Gleichman, A.J.; et al. Anti-N-methyl-D-aspartate receptor (NMDAR) encephalitis in children and adolescents. Ann. Neurol. 2009, 66, 11–18. [Google Scholar] [CrossRef]
- Kiernan, C.M.; Solórzano, C.C. Pheochromocytoma and Paraganglioma: Diagnosis, Genetics, and Treatment. Surg. Oncol. Clin. North Am. 2016, 25, 119–138. [Google Scholar] [CrossRef]
- Gunawardane, P.T.K.; Grossman, A. Phaeochromocytoma and Paraganglioma. Adv. Exp. Med. Biol. 2017, 956, 239–259. [Google Scholar] [CrossRef] [PubMed]
- Pacak, K.; Wimalawansa, S.J. Pheochromocytoma and paraganglioma. Endocr. Pract. 2015, 21, 406–412. [Google Scholar] [CrossRef] [PubMed]
- Farrugia, F.; Charalampopoulos, A. Pheochromocytoma. Endocr. Regul. 2019, 53, 191–212. [Google Scholar] [CrossRef]
- Nosadini, M.; Mohammad, S.S.; Corazza, F.; Ruga, E.M.; Kothur, K.; Perilongo, G.; Frigo, A.C.; Toldo, I.; Dale, R.C.; Sartori, S. Herpes simplex virus-induced anti-N-methyl-d-aspartate receptor encephalitis: A systematic literature review with analysis of 43 cases. Dev. Med. Child Neurol. 2017, 59, 796–805. [Google Scholar] [CrossRef] [PubMed]
- Steiner, J.; Walter, M.; Glanz, W.; Sarnyai, Z.; Bernstein, H.-G.; Vielhaber, S.; Kästner, A.; Skalej, M.; Jordan, W.; Schiltz, K.; et al. Increased prevalence of diverse N-methyl-D-aspartate glutamate receptor antibodies in patients with an initial diagnosis of schizophrenia: Specific relevance of IgG NR1a antibodies for distinction from N-methyl-D-aspartate glutamate receptor encephalitis. JAMA Psychiatry 2013, 70, 271–278. [Google Scholar] [CrossRef]
- Zandi, M.S.; Irani, S.R.; Lang, B.; Waters, P.; Jones, P.B.; McKenna, P.; Coles, A.J.; Vincent, A.; Lennox, B.R. Disease-relevant autoantibodies in first episode schizophrenia. J. Neurol. 2011, 258, 686–688. [Google Scholar] [CrossRef]
- Peery, H.E.; Day, G.S.; Doja, A.; Xia, C.; Fritzler, M.J.; Foster, W.G. Anti-NMDA receptor encephalitis in children: The disorder, its diagnosis, and treatment. Handb. Clin. Neurol. 2013, 112, 1229–1233. [Google Scholar] [CrossRef]
- Gresa-Arribas, N.; Titulaer, M.J.; Torrents, A.; Aguilar, E.; McCracken, L.; Leypoldt, F.; Gleichman, A.J.; Balice-Gordon, R.; Rosenfeld, M.R.; Lynch, D.; et al. Antibody titres at diagnosis and during follow-up of anti-NMDA receptor encephalitis: A retrospective study. Lancet Neurol. 2014, 13, 167–177, Correction in Lancet Neurol. 2014, 13, 135. [Google Scholar] [CrossRef]
- Finke, C.; Kopp, U.A.; Prüss, H.; Dalmau, J.; Wandinger, K.P.; Ploner, C.J. Cognitive deficits following anti-NMDA receptor encephalitis. J. Neurol. Neurosurg. Psychiatry 2012, 83, 195–198. [Google Scholar] [CrossRef]
- Kelley, B.P.; Patel, S.C.; Marin, H.L.; Corrigan, J.J.; Mitsias, P.D.; Griffith, B. Autoimmune Encephalitis: Pathophysiology and Imaging Review of an Overlooked Diagnosis. AJNR Am. J. Neuroradiol. 2017, 38, 1070–1078. [Google Scholar] [CrossRef]
- Titulaer, M.J.; McCracken, L.; Gabilondo, I.; Armangue, T.; Glaser, C.; Iizuka, T.; Honig, L.S.; Benseler, S.M.; Kawachi, I.; Martinez-Hernandez, E.; et al. Treatment and prognostic factors for long-term outcome in patients with anti-NMDA receptor encephalitis: An observational cohort study. Lancet Neurol. 2013, 12, 157–165. [Google Scholar] [CrossRef]
- Long, Q.; Lv, Z.; Zhao, J.; Shi, K.; Li, C.; Fan, B.; Zheng, J. Cerebral gray matter volume changes in patients with anti-N-methyl-D-aspartate receptor encephalitis: A voxel-based morphometry study. Front. Neurol. 2022, 13, 892242. [Google Scholar] [CrossRef] [PubMed]
- Kerik-Rotenberg, N.; Diaz-Meneses, I.; Hernandez-Ramirez, R.; Muñoz-Casillas, R.; Reynoso-Mejia, C.A.; Flores-Rivera, J.; Espinola-Nadurille, M.; Ramirez-Bermudez, J.; Aguilar-Palomeque, C. A Metabolic Brain Pattern Associated With Anti-N-Methyl-D-Aspartate Receptor Encephalitis. Psychosomatics 2020, 61, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.C.; Tseng, J.R.; Wu, C.L.; Su, F.-C.; Weng, W.-C.; Hsu, C.-C.; Chang, K.-H.; Wu, C.-F.; Hsiao, I.-T.; Lin, C.-P. Different FDG-PET metabolic patterns of anti-AMPAR and anti-NMDAR encephalitis: Case report and literature review. Brain Behav. 2020, 10, e01540. [Google Scholar] [CrossRef]
- Ito, K.; Shimano, Y.; Imabayashi, E.; Nakata, Y.; Omachi, Y.; Sato, N.; Arima, K.; Matsuda, H. Concordance between (99m)Tc-ECD SPECT and 18F-FDG PET interpretations in patients with cognitive disorders diagnosed according to NIA-AA criteria. Int. J. Geriatr. Psychiatry 2014, 29, 1079–1086. [Google Scholar] [CrossRef] [PubMed]
- Iizuka, T.; Sakai, F.; Ide, T.; Monzen, T.; Yoshii, S.; Iigaya, M.; Suzuki, K.; Lynch, D.R.; Suzuki, N.; Hata, T.; et al. Anti-NMDA receptor encephalitis in Japan: Long-term outcome without tumor removal. Neurology 2008, 70, 504–511. [Google Scholar] [CrossRef]
- World Medical Association. World Medical Association Declaration of Helsinki: Ethical principles for medical research involving human subject. JAMA 2013, 310, 2191–2194. [Google Scholar] [CrossRef]
- Boellaard, R. Standards for PET image acquisition and quantitative data analysis. J. Nucl. Med. 2009, 50 (Suppl. S1), 11S–20S. [Google Scholar] [CrossRef]
- Borghammer, P.; Jonsdottir, K.Y.; Cumming, P.; Ostergaard, K.; Vang, K.; Ashkanian, M.; Vafaee, M.; Iversen, P.; Gjedde, A. Normalization in PET group comparison studies—The importance of a valid reference region. Neuroimage 2008, 40, 529–540. [Google Scholar] [CrossRef]
- Marcoux, A.; Burgos, N.; Bertrand, A.; Teichmann, M.; Routier, A.; Wen, J.; Samper-González, J.; Bottani, S.; Durrleman, S.; Habert, M.-O.; et al. An Automated Pipeline for the Analysis of PET Data on the Cortical Surface. Front. Neuroinform. 2018, 12, 94. [Google Scholar] [CrossRef]
- Soonawala, D.; Amin, T.; Ebmeier, K.P.; Steele, J.D.; Dougall, N.; Besta, J.; Mignecoc, O.; Nobili, F.; Scheidhauere, K. Statistical parametric mapping of (99m)Tc-HMPAO-SPECT images for the diagnosis of Alzheimer’s disease: Normalizing to cerebellar tracer uptake. Neuroimage 2002, 17, 1193–1202. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, H.; Hashida, H.; Takahashi, Y. Dystonic Seizures and Intense Hyperperfusion of the Basal Ganglia in a Patient with Anti-N-Methyl-D-Aspartate Receptor Encephalitis. Case Rep Neurol. 2017, 9, 272–276. [Google Scholar] [CrossRef] [PubMed]
- Suárez, J.P.; Domínguez, M.L.; Gómez, M.A.; Portilla, J.C.; Gómez, M.; Casado, I. Brain perfusion SPECT with 99mTc-HMPAO in the diagnosis and follow-up of patients with anti-NMDA receptor encephalitis. SPECT cerebral de perfusión con 99mTc-HMPAO en el diagnóstico y seguimiento de la encefalitis con anticuerpos contra el receptor NMDA. Neurologia 2018, 33, 622–623. (In English) [Google Scholar] [CrossRef]
- Igarashi, A.; Okumura, A.; Kitamura, Y.; Jinbo, K.; Akatsuka, S.; Tanuma, N.; Shimizu, T.; Hayashi, M. Acute limbic encephalitis with focal hyperperfusion on single photon emission computed tomography. Brain Dev. 2013, 35, 181–184. [Google Scholar] [CrossRef] [PubMed]
- Kimura, N.; Kumamoto, T.; Takahashi, Y. Brain perfusion SPECT in limbic encephalitis associated with autoantibody against the glutamate receptor epsilon 2. Clin. Neurol. Neurosurg. 2014, 118, 44–48. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, S.; Koide, Y.; Fujiwara, M.; Nakazawa, K.; Takahashi, Y.; Hara, H. Subacute encephalitis associated with anti-glutamate receptor antibodies: Serial studies of MRI, 1H-MRS and SPECT. Rinsho Shinkeigaku 2008, 48, 196–201. (In Japanese) [Google Scholar] [CrossRef] [PubMed][Green Version]

| Region | Z-Score | Z-Score | LZ-Score | LZ-Score | RZ-Score | RZ-Score | L-R%diff | L-R%diff | L-R% Diff Z-Score | L-R% Diff Z-Score |
|---|---|---|---|---|---|---|---|---|---|---|
| B | C | B | C | B | C | B | C | B | C | |
| Parietal lobe | −1.82 | −0.69 | −0.99 | −0.22 | −2.4 | −1.16 | 4.7 | 4.7 | 2.19 | 2.19 |
| Superior parietal lobe | −0.59 | −0.15 | 0 | 0.25 | −1.1 | −0.62 | 6.12 | 6.12 | 1.78 | 1.78 |
| medial temporal lobe | −0.03 | 0.44 | 0.94 | 1.05 | −0.82 | −0.14 | 3.66 | 3.66 | 1.37 | 1.37 |
| Thalamus | −0.34 | 0.62 | 1.13 | 1.17 | −0.62 | −0.3 | 5.54 | 5.54 | 1.81 | 1.81 |
| Occipital lobe | −0.26 | 0.23 | 0.44 | 0.61 | −0.84 | −0.1 | 6.17 | 6.17 | 1.61 | 1.61 |
| Temporal lobe | 0.55 | 0.62 | 2.37 | 1.43 | −1.46 | −0.21 | 5.62 | 5.62 | 2.64 | 2.64 |
| Lateral temporal lobe | 0.57 | 0.63 | 1.97 | 1.3 | −0.88 | −0.8 | 5.62 | 5.62 | 2.07 | 2.07 |
| cerebellar hemisphere | −0.53 | X | 0.3 | X | −1.18 | X | 3.04 | X | 2.25 | X |
| frontal lobe | 3.97 | 1.44 | 4.2 | 2.07 | 1.45 | 0.85 | 3.62 | 3.62 | 2.35 | 2.35 |
| Visual Analysis/Hypoperfusion Areas | MiM Software Quantification/Whole Brain Normalization | MiM Software Quantification/Cerebellum Normalization |
|---|---|---|
| Parietal lobe (right) | Confirmed, right parietal lobe hyperperfusion | Significant statistical difference in perfusion between L and R, but without true right parietal hypoperfusion |
| Temporal lobe (right) | Significant statistical difference in perfusion between L and R, but without true right temporal hypoperfusion | Significant statistical difference in perfusion between L and R, but without true right temporal hypoperfusion |
| Thalamus (right) | Significant statistical difference in perfusion between L and R, but without true right thalamus hypoperfusion | Significant statistical difference in perfusion between L and R, but without true right thalamus hypoperfusion |
| Frontal lobe (right) | Bilateral frontal lobe hyperperfusion, primarily left frontal lobe | Left frontal lobe hyperperfusion |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Šiško Markoš, I.; Blažeković, I.; Peitl, V.; Jukić, T.; Supanc, V.; Karlović, D.; Fröbe, A. Psychiatric Illness or Immune Dysfunction—Brain Perfusion Imaging Providing the Answer in a Case of Anti-NMDAR Encephalitis. Diagnostics 2022, 12, 2377. https://doi.org/10.3390/diagnostics12102377
Šiško Markoš I, Blažeković I, Peitl V, Jukić T, Supanc V, Karlović D, Fröbe A. Psychiatric Illness or Immune Dysfunction—Brain Perfusion Imaging Providing the Answer in a Case of Anti-NMDAR Encephalitis. Diagnostics. 2022; 12(10):2377. https://doi.org/10.3390/diagnostics12102377
Chicago/Turabian StyleŠiško Markoš, Ines, Ivan Blažeković, Vjekoslav Peitl, Tomislav Jukić, Višnja Supanc, Dalibor Karlović, and Ana Fröbe. 2022. "Psychiatric Illness or Immune Dysfunction—Brain Perfusion Imaging Providing the Answer in a Case of Anti-NMDAR Encephalitis" Diagnostics 12, no. 10: 2377. https://doi.org/10.3390/diagnostics12102377
APA StyleŠiško Markoš, I., Blažeković, I., Peitl, V., Jukić, T., Supanc, V., Karlović, D., & Fröbe, A. (2022). Psychiatric Illness or Immune Dysfunction—Brain Perfusion Imaging Providing the Answer in a Case of Anti-NMDAR Encephalitis. Diagnostics, 12(10), 2377. https://doi.org/10.3390/diagnostics12102377






