Orbital Tumors—Clinical, Radiologic and Histopathologic Correlation
Abstract
1. Introduction
2. Anatomy of the Orbit
3. Imaging Techniques
3.1. Intraocular Tumors
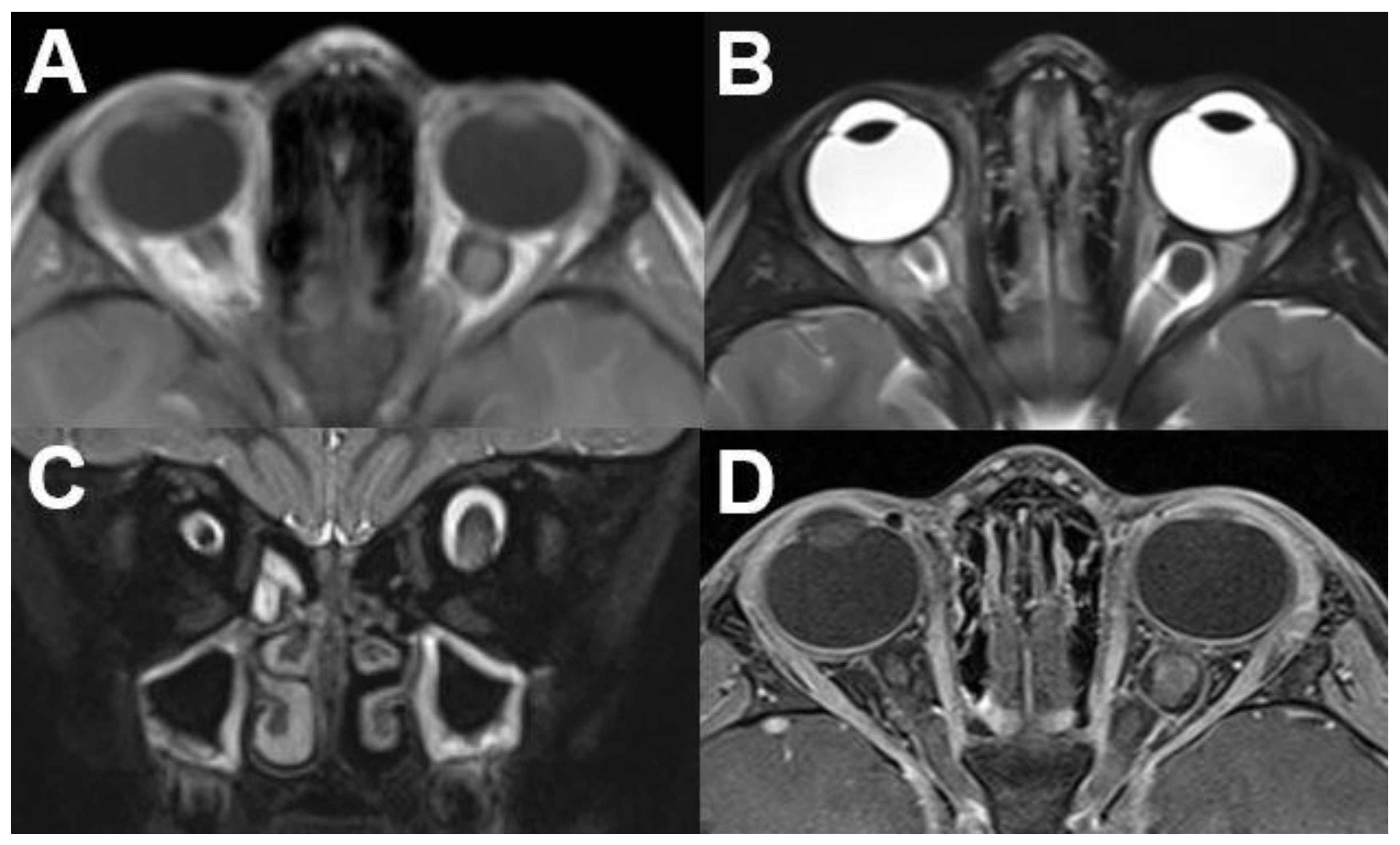
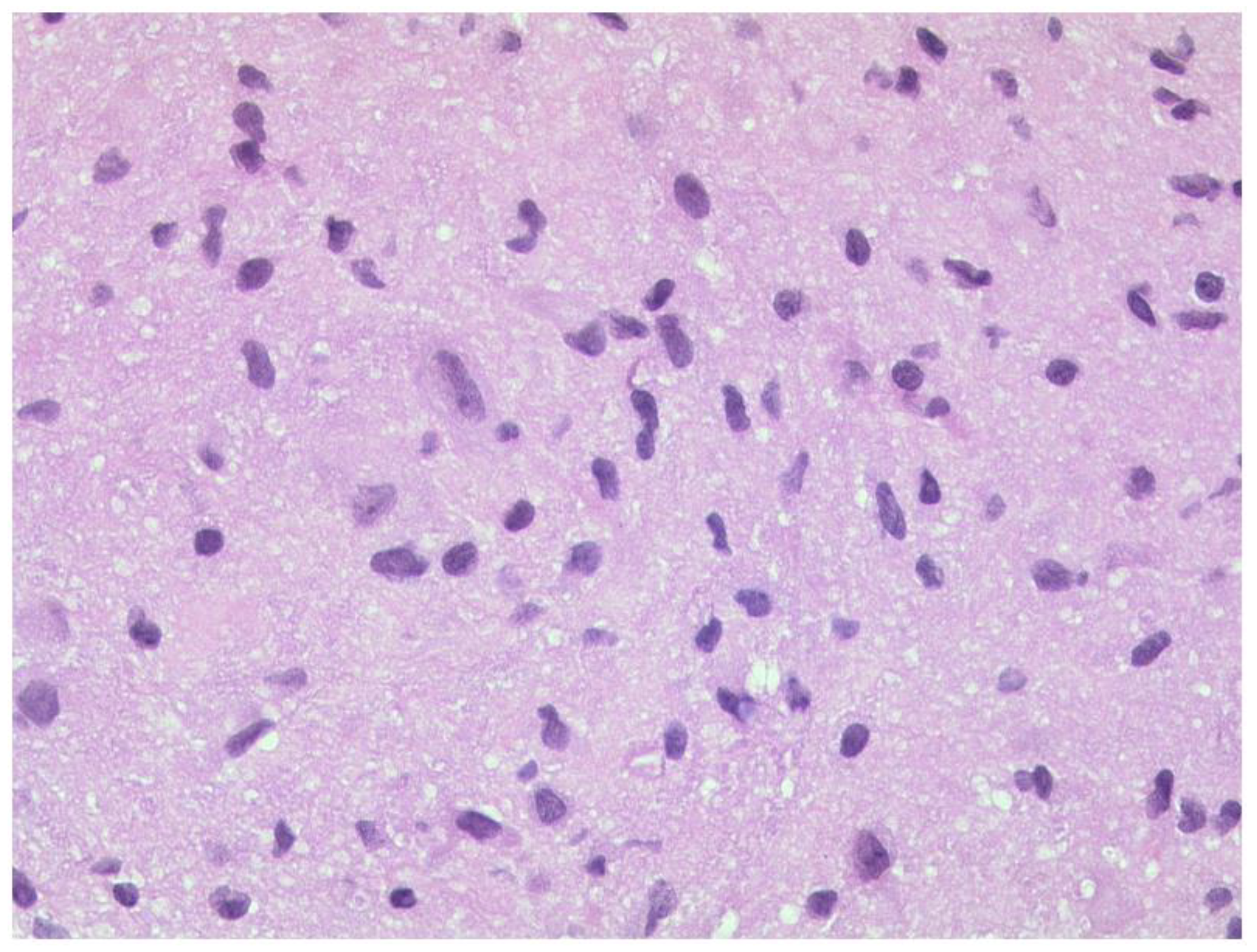
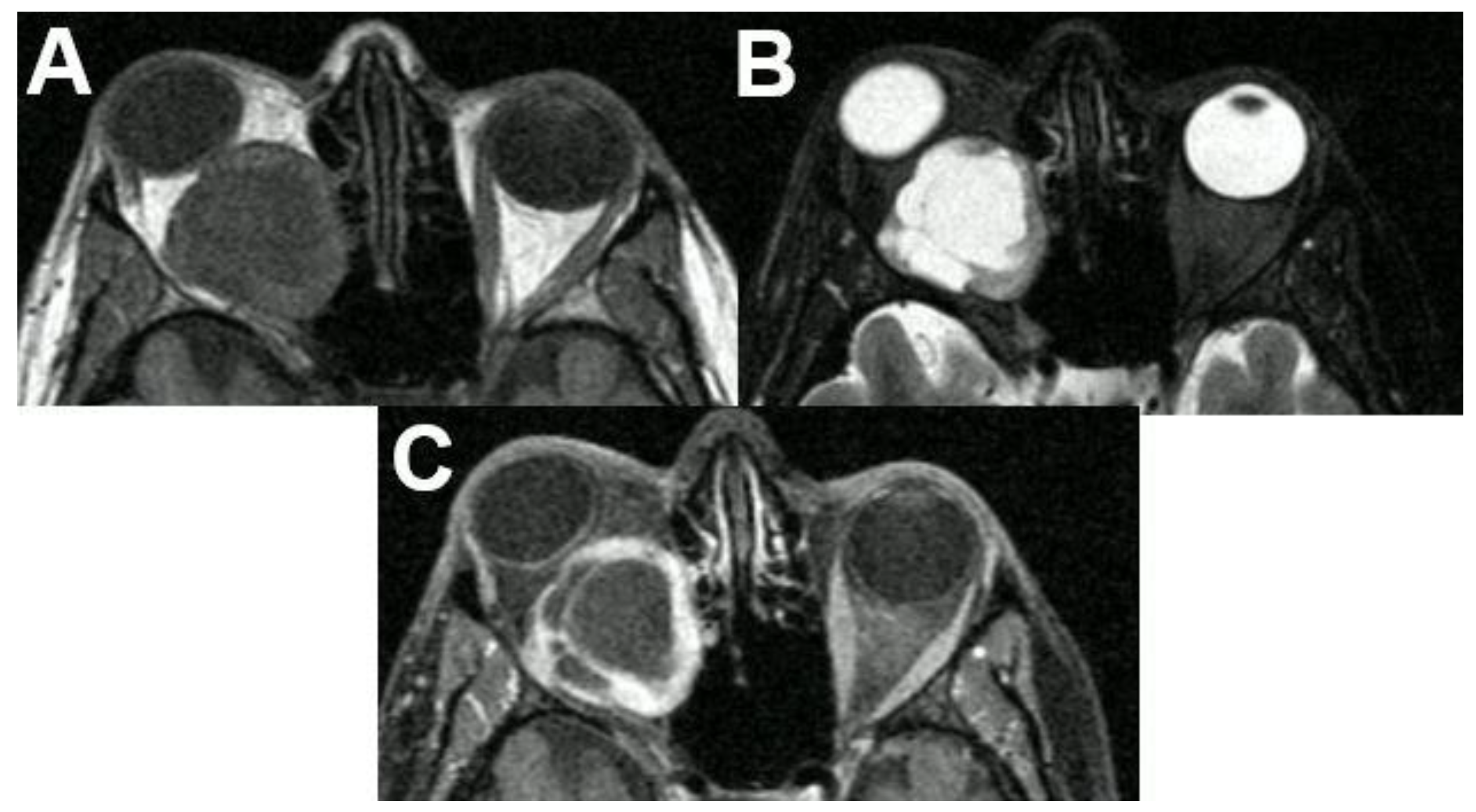
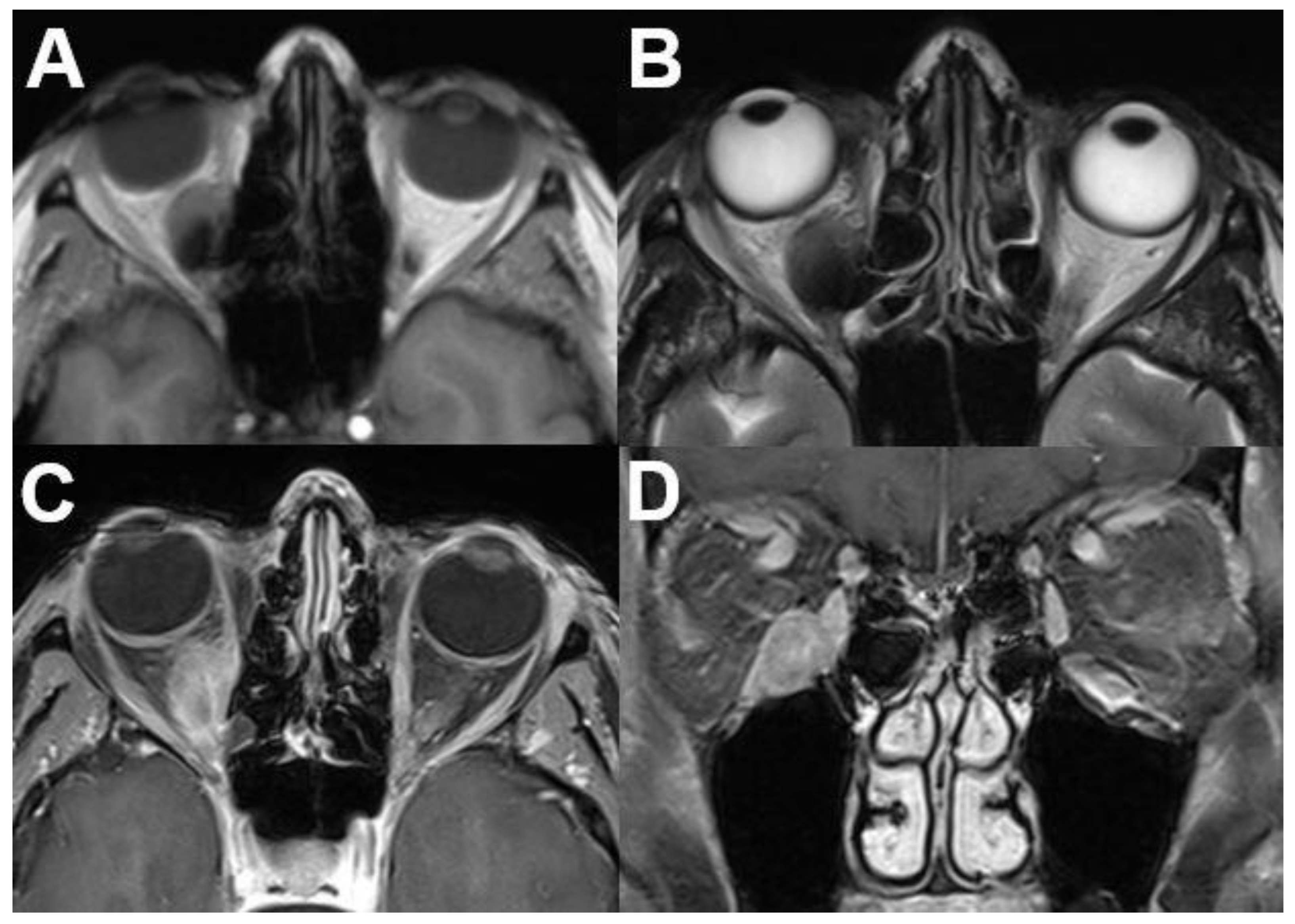
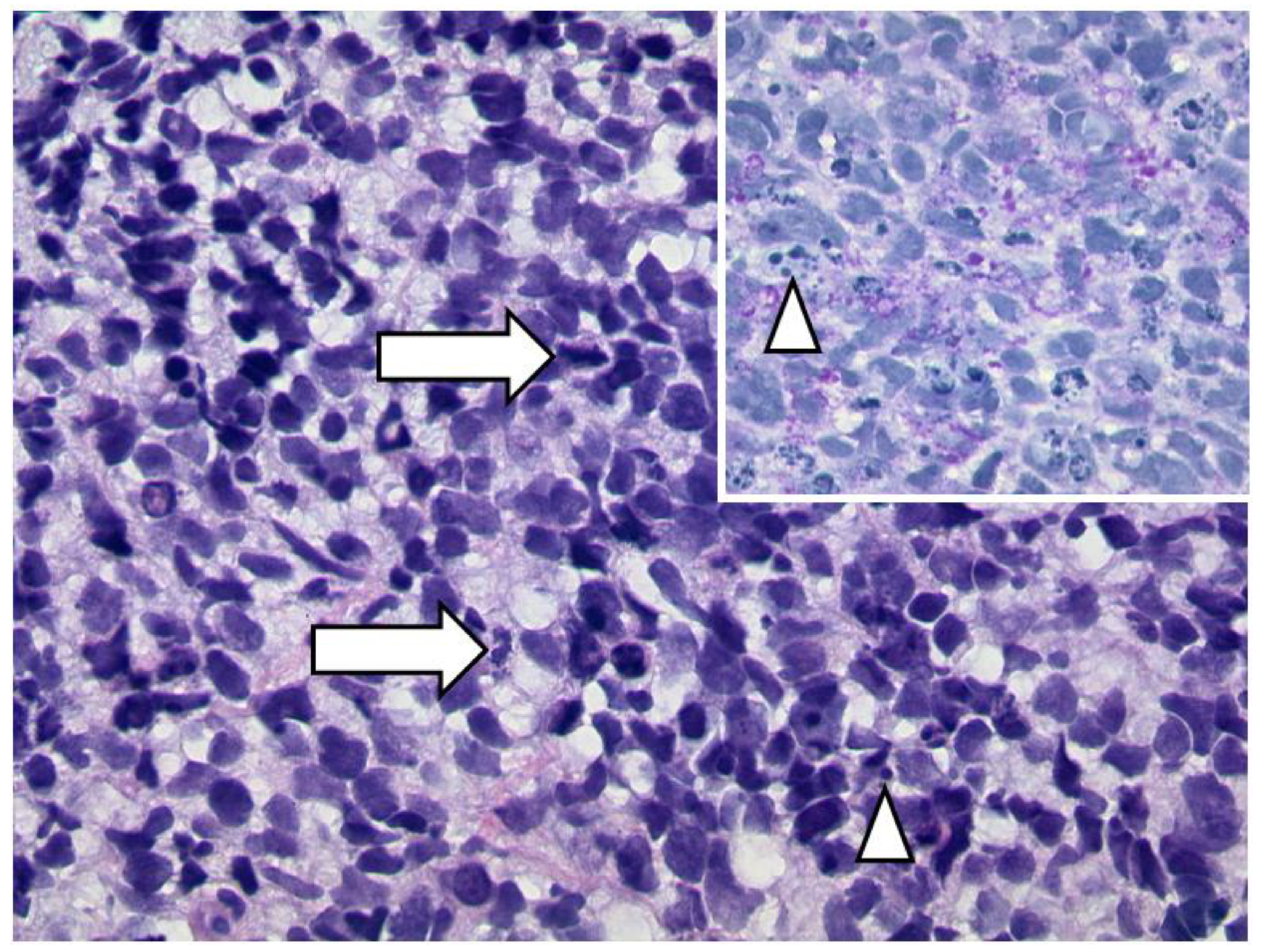
3.1.1. Retinoblastoma
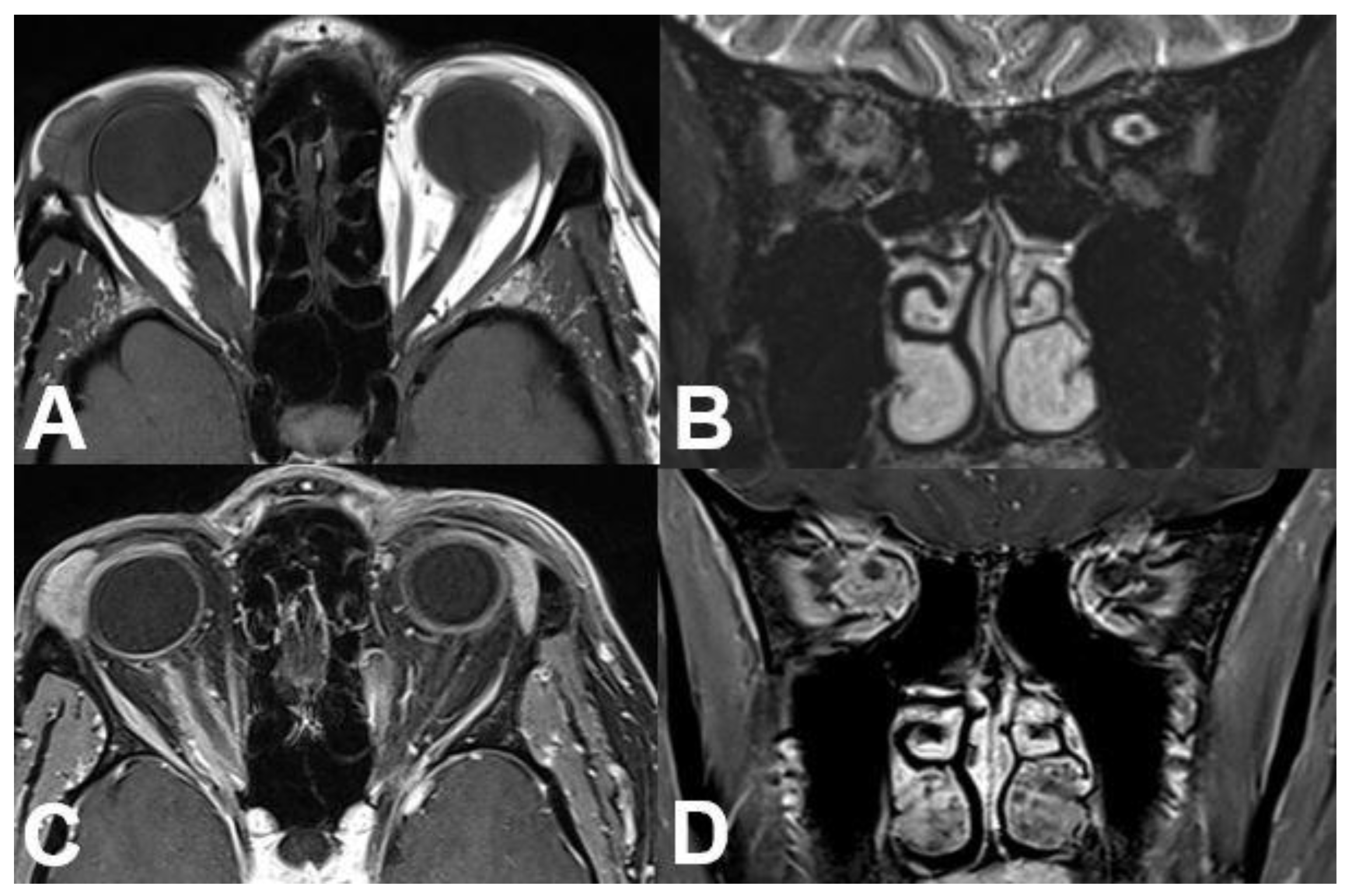
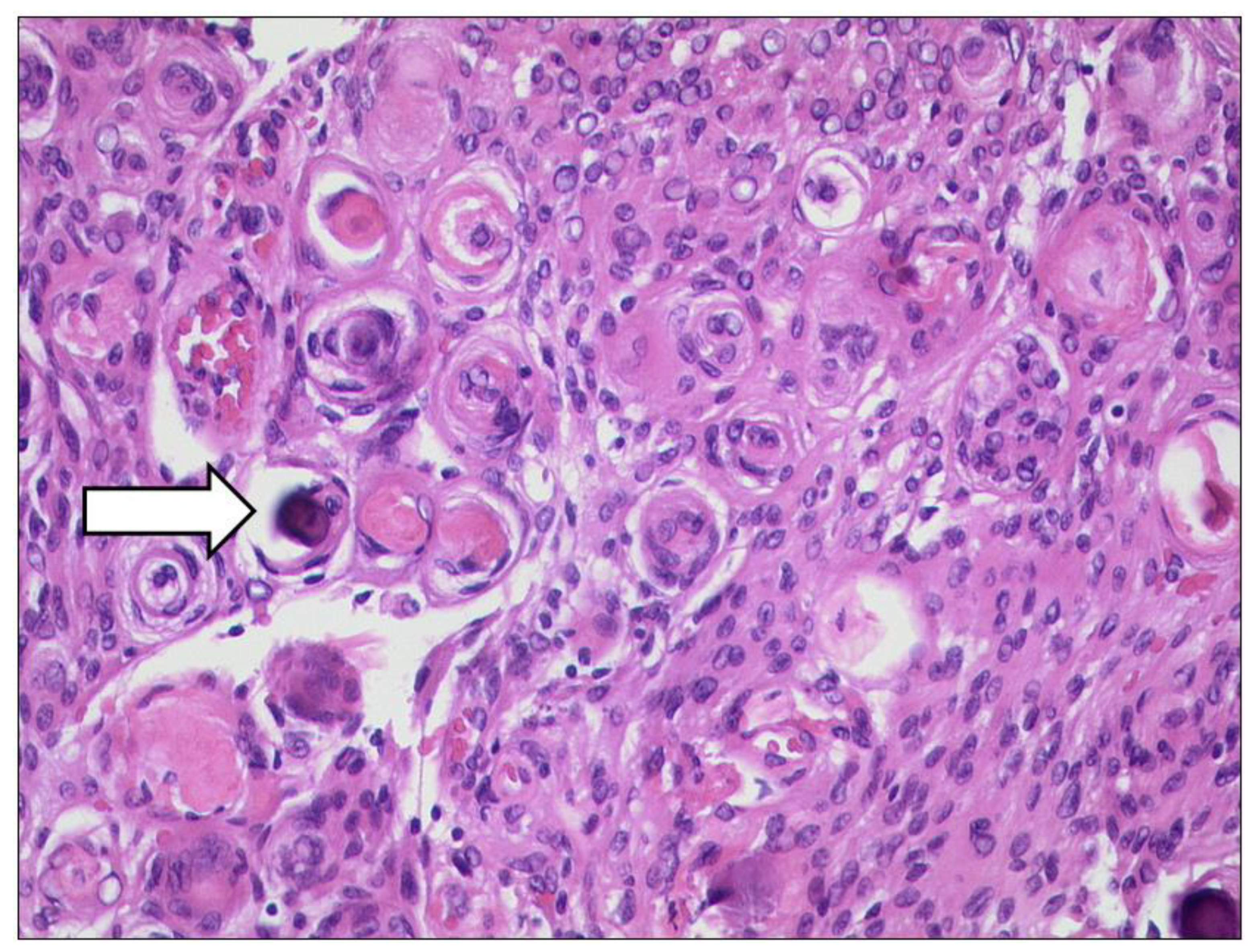
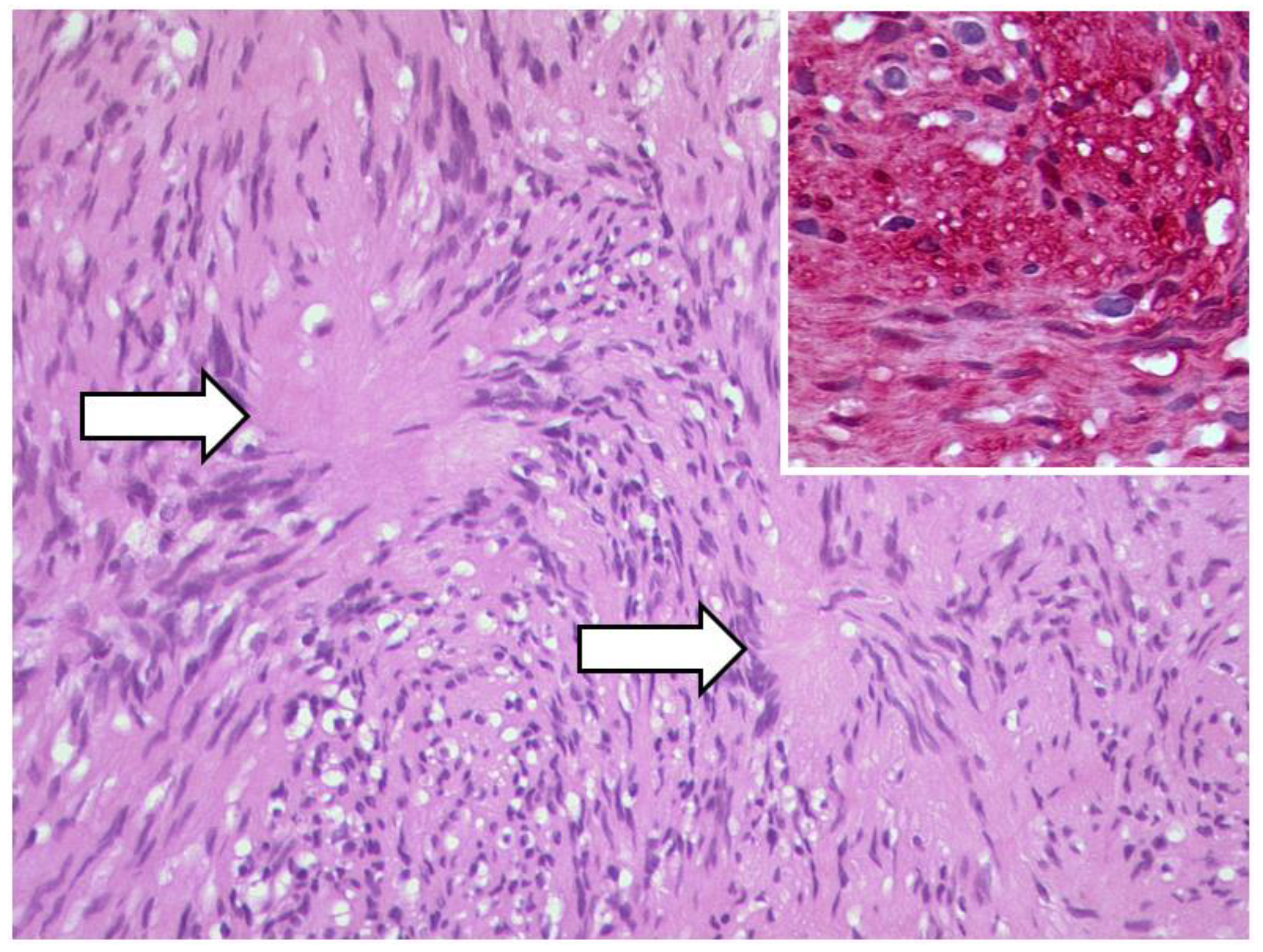
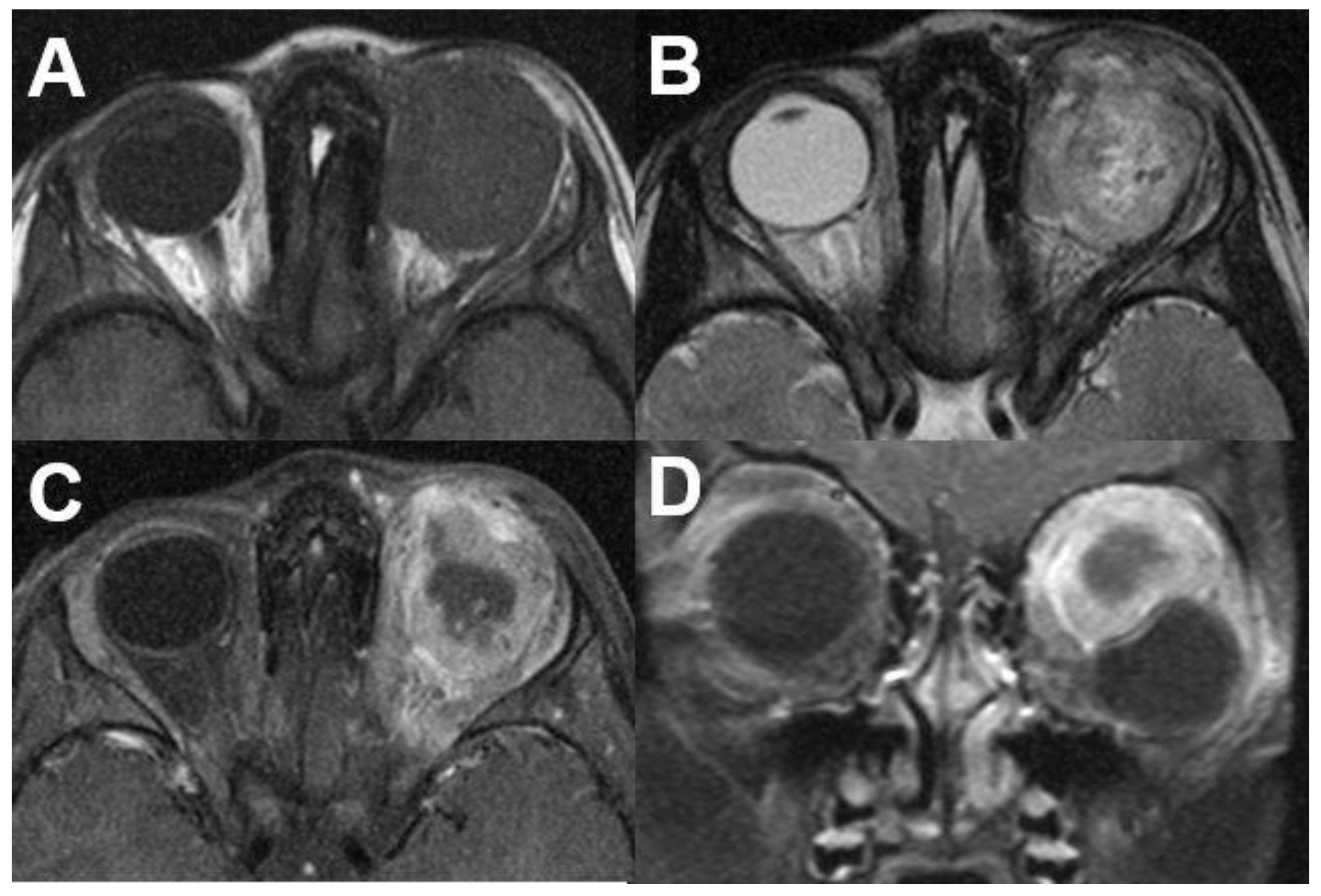
3.1.2. Uveal Melanoma
3.2. Intraconal Tumors
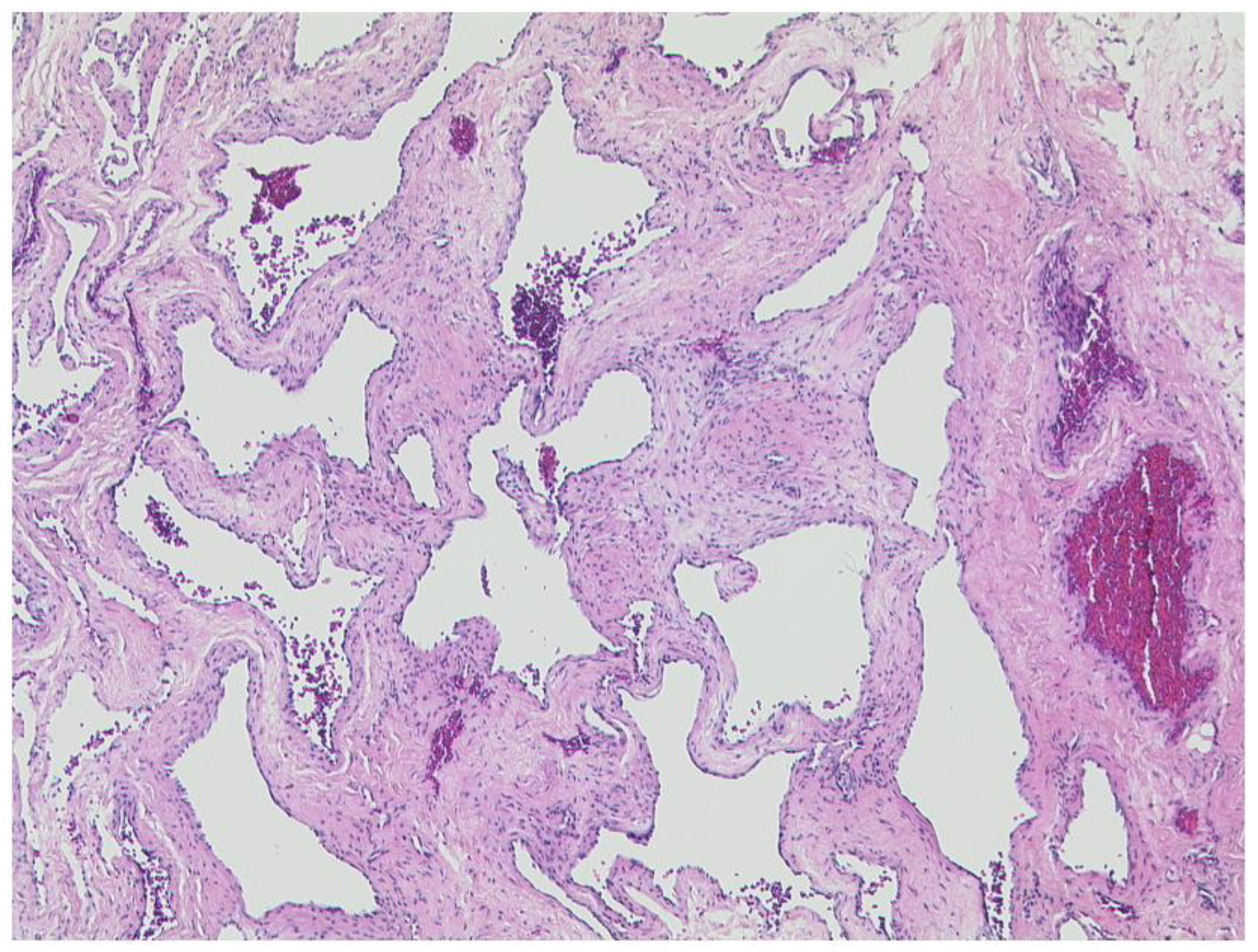
3.2.1. Venous Varices
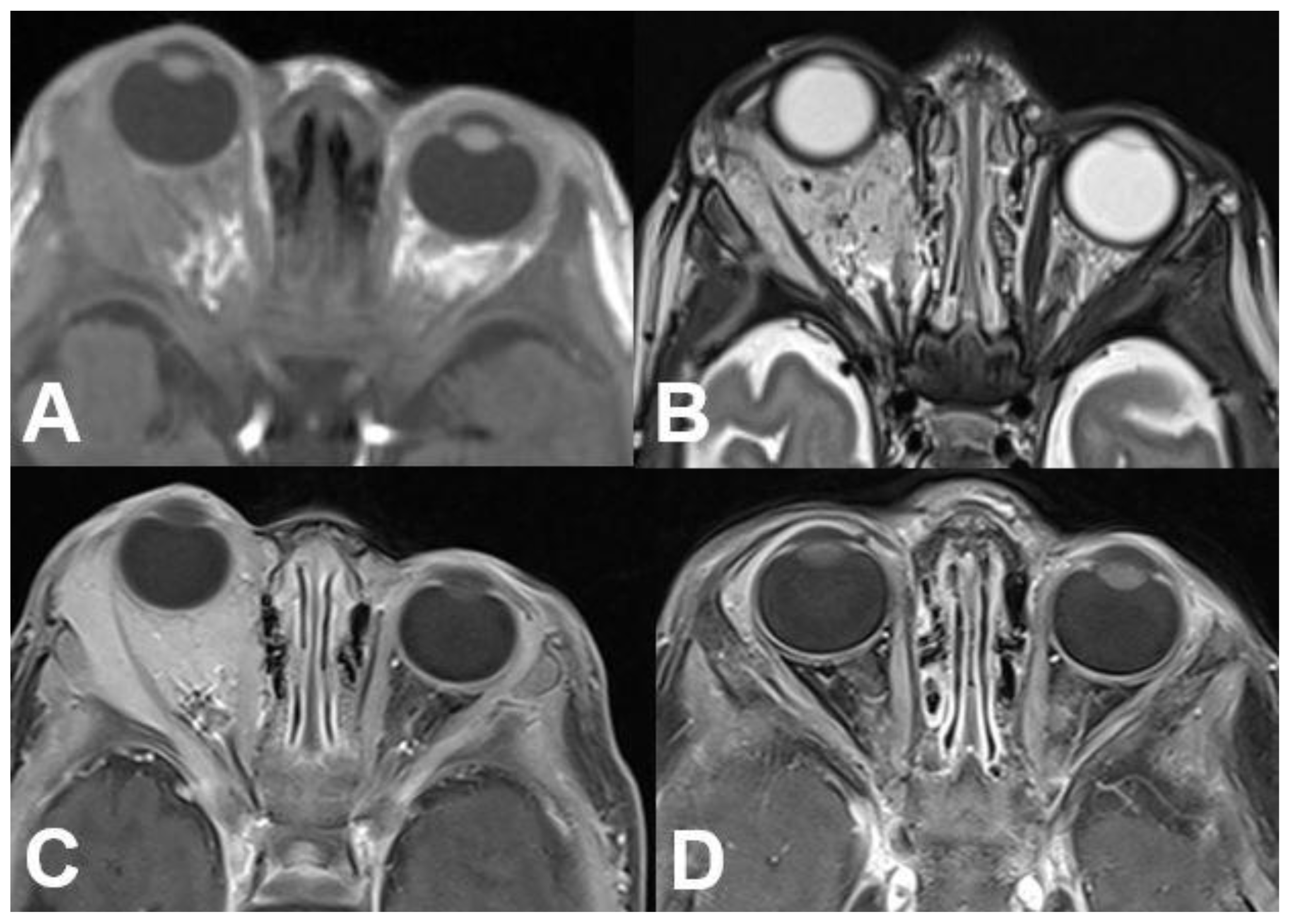
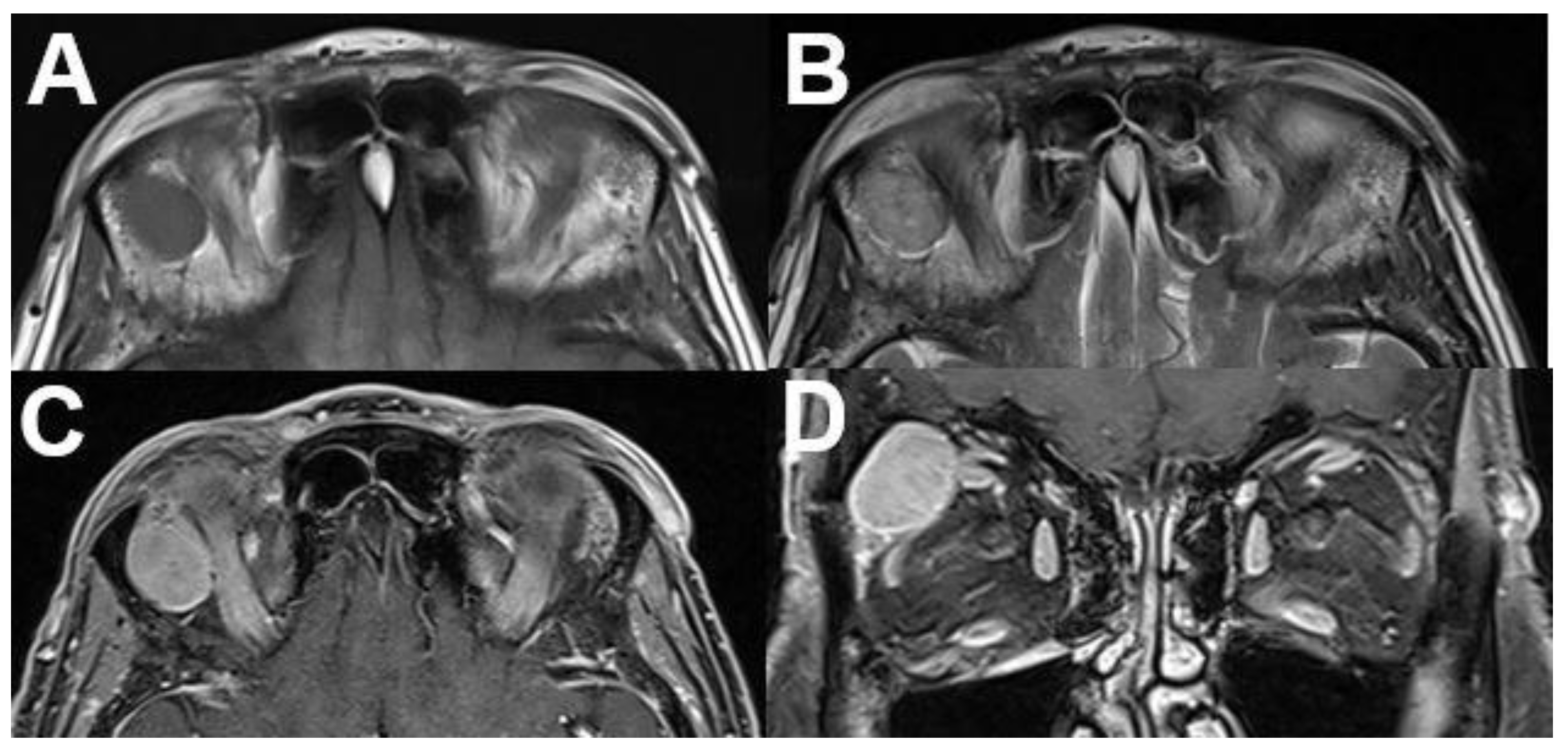
3.2.2. Cavernous Hemangioma
3.3. Extraconal Tumors
3.3.1. Capillary Hemangioma
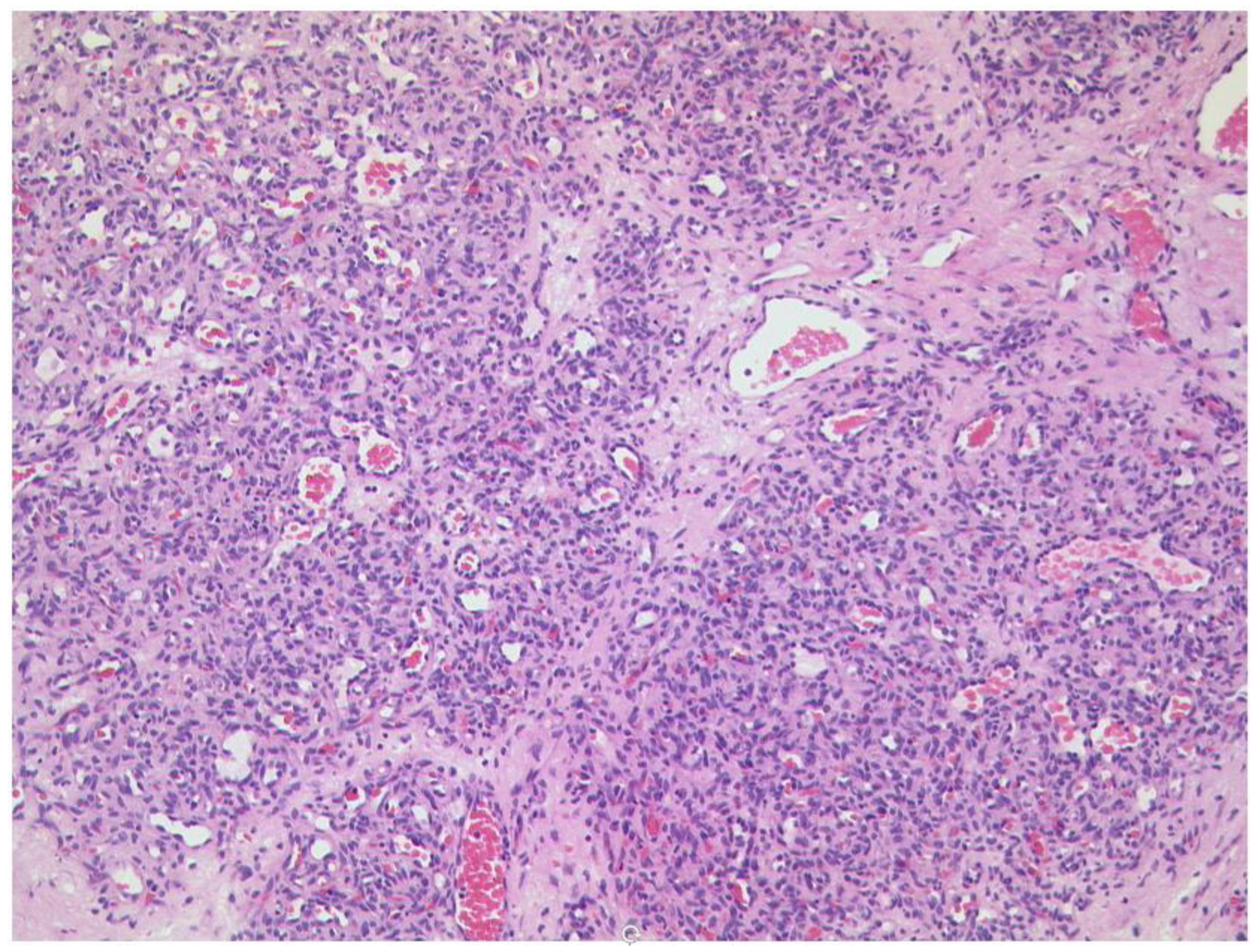
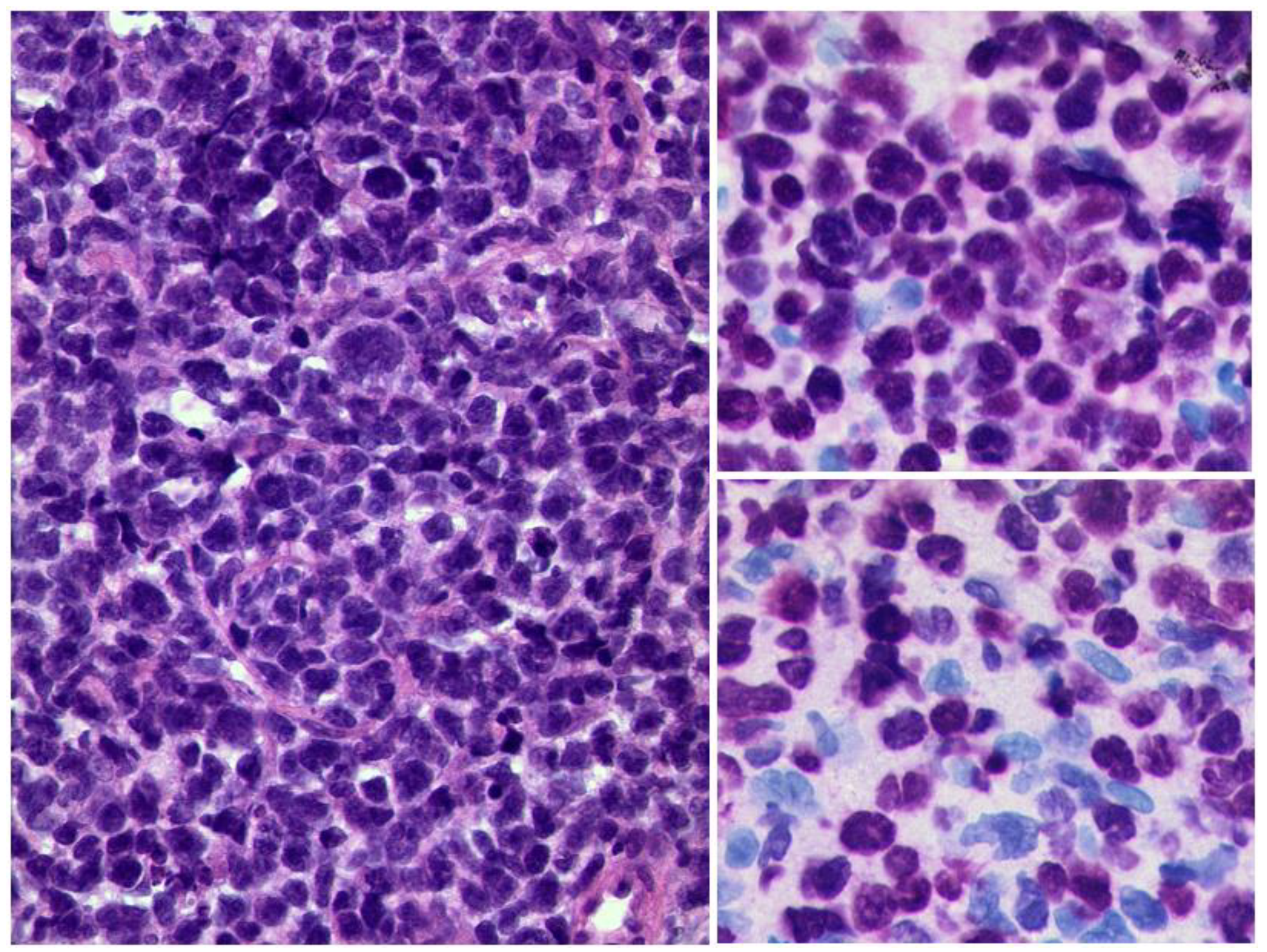
3.3.2. Lymphoma
3.3.3. Tumors of the Lacrimal Gland
3.4. N. opticus
3.4.1. Optic Nerve Glioma
3.4.2. Optic Nerve Sheath Meningioma
3.5. Peripheral Nerve Sheath
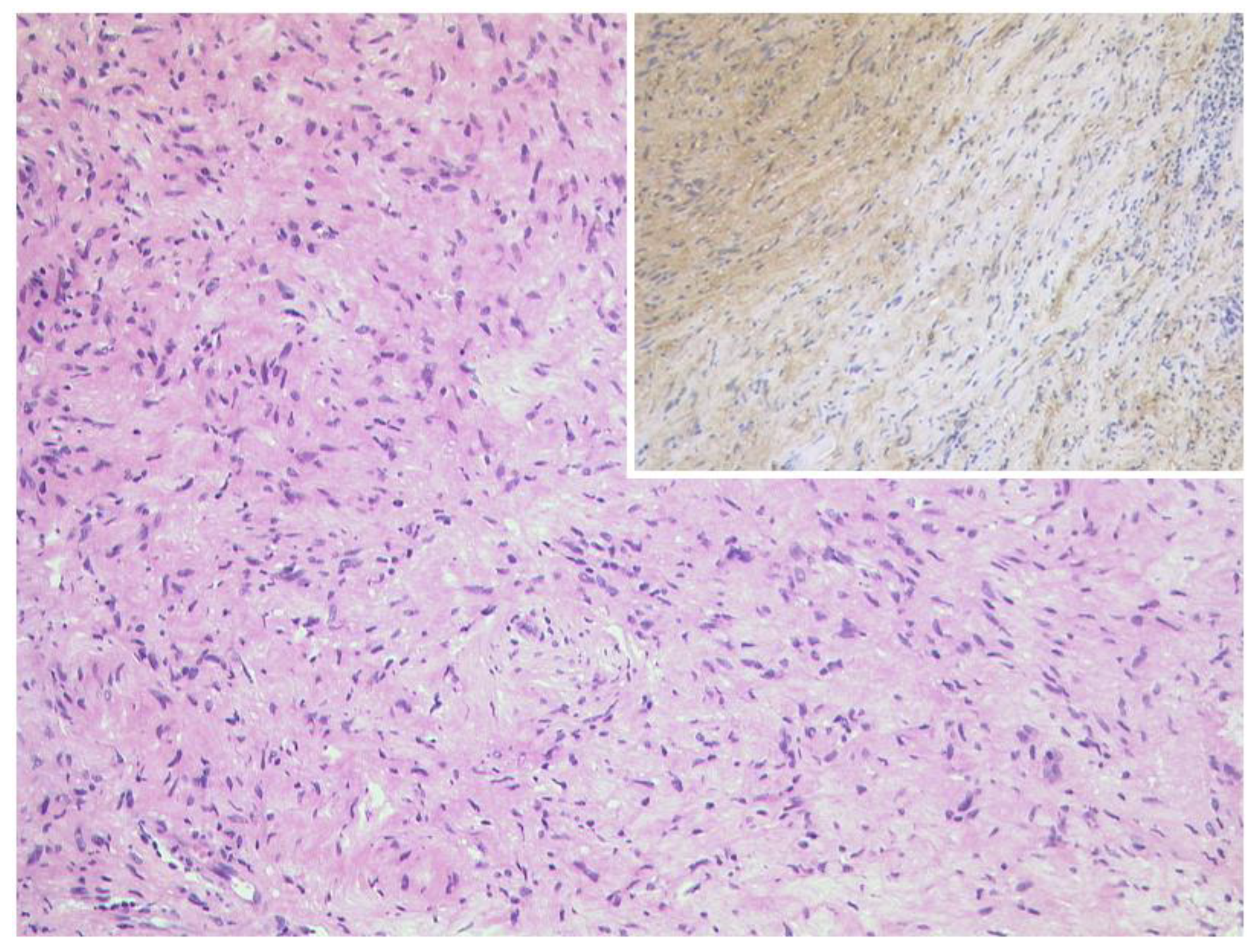
3.5.1. Schwannoma
3.5.2. Neurofibroma
3.6. Masses with Involvement of Different Parts of the Orbit
3.6.1. Metastasis
| Primary Site | Tumor Type | % |
|---|---|---|
| Breast | Carcinoma | 53 |
| Prostate gland | Carcinoma | 12 |
| Lung | Carcinoma | 8 |
| Skin (melanoma) | Melanoma | 6 |
| Kidney | Carcinoma/Sarcoma | 5 |
| Alimentary tract | Carcinoma/Carcinoid | 5 |
3.6.2. Rhabdomyosarcoma
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nguyen, V.D.; Singh, A.K.; Altmeyer, W.B.; Tantiwongkosi, B. Demystifying Orbital Emergencies: A Pictorial Review. Radiographics 2017, 37, 947–962. [Google Scholar] [CrossRef] [PubMed]
- Tailor, T.D.; Gupta, D.; Dalley, R.W.; Keene, C.D.; Anzai, Y. Orbital neoplasms in adults: Clinical, radiologic, and pathologic review. Radiographics 2013, 33, 1739–1758. [Google Scholar] [CrossRef]
- Shields, J.A.; Shields, C.L.; Scartozzi, R. Survey of 1264 patients with orbital tumors and simulating lesions: The 2002 Montgomery Lecture, part 1. Ophthalmology 2004, 111, 997–1008. [Google Scholar] [CrossRef]
- Mafee, M.F.; Inoue, Y.; Mafee, R.F. Ocular and orbital imaging. Neuroimaging Clin. N Am. 1996, 6, 291–318. [Google Scholar] [PubMed]
- Jackson, A.; Sheppard, S.; Johnson, A.C.; Annesley, D.; Laitt, R.D.; Kassner, A. Combined fat- and water-suppressed MR imaging of orbital tumors. AJNR Am. J. Neuroradiol. 1999, 20, 1963–1969. [Google Scholar]
- Bernardini, F.P.; Bazzan, M. Lymphoproliferative disease of the orbit. Curr. Opin. Ophthalmol. 2007, 18, 398–401. [Google Scholar] [CrossRef]
- Goh, P.S.; Gi, M.T.; Charlton, A.; Tan, C.; Gangadhara Sundar, J.K.; Amrith, S. Review of orbital imaging. Eur. J. Radiol. 2008, 66, 387–395. [Google Scholar] [CrossRef]
- Purohit, B.S.; Vargas, M.I.; Ailianou, A.; Merlini, L.; Poletti, P.A.; Platon, A.; Delattre, B.M.; Rager, O.; Burkhardt, K.; Becker, M. Orbital tumours and tumour-like lesions: Exploring the armamentarium of multiparametric imaging. Insights Imaging 2016, 7, 43–68. [Google Scholar] [CrossRef]
- Dimaras, H.; Kimani, K.; Dimba, E.A.; Gronsdahl, P.; White, A.; Chan, H.S.; Gallie, B.L. Retinoblastoma. Lancet 2012, 379, 1436–1446. [Google Scholar] [CrossRef]
- Rauschecker, A.M.; Patel, C.V.; Yeom, K.W.; Eisenhut, C.A.; Gawande, R.S.; O’Brien, J.M.; Ebrahimi, K.B.; Daldrup-Link, H.E. High-resolution MR imaging of the orbit in patients with retinoblastoma. Radiographics 2012, 32, 1307–1326. [Google Scholar] [CrossRef]
- Dunkel, I.J.; Chan, H.S.; Jubran, R.; Chantada, G.L.; Goldman, S.; Chintagumpala, M.; Khakoo, Y.; Abramson, D.H. High-dose chemotherapy with autologous hematopoietic stem cell rescue for stage 4B retinoblastoma. Pediatr. Blood Cancer 2010, 55, 149–152. [Google Scholar] [CrossRef]
- Kivelä, T. Trilateral retinoblastoma: A meta-analysis of hereditary retinoblastoma associated with primary ectopic intracranial retinoblastoma. J. Clin. Oncol. 1999, 17, 1829–1837. [Google Scholar] [CrossRef] [PubMed]
- Lemke, A.J.; Kazi, I.; Mergner, U.; Foerster, P.I.; Heimann, H.; Bechrakis, N.; Schuler, A.; von Pilsach, M.I.; Foerster, M.; Felix, R.; et al. Retinoblastoma—MR appearance using a surface coil in comparison with histopathological results. Eur. Radiol. 2007, 17, 49–60. [Google Scholar] [CrossRef]
- Razek, A.A.; Elkhamary, S. MRI of retinoblastoma. Br. J. Radiol. 2011, 84, 775–784. [Google Scholar] [CrossRef]
- de Graaf, P.; Göricke, S.; Rodjan, F.; Galluzzi, P.; Maeder, P.; Castelijns, J.A.; Brisse, H.J. Guidelines for imaging retinoblastoma: Imaging principles and MRI standardization. Pediatr. Radiol. 2012, 42, 2–14. [Google Scholar] [CrossRef]
- Khurana, A.; Eisenhut, C.A.; Wan, W.; Ebrahimi, K.B.; Patel, C.; O’Brien, J.M.; Yeom, K.; Daldrup-Link, H.E. Comparison of the diagnostic value of MR imaging and ophthalmoscopy for the staging of retinoblastoma. Eur. Radiol. 2013, 23, 1271–1280. [Google Scholar] [CrossRef]
- Song, K.D.; Eo, H.; Kim, J.H.; Yoo, S.Y.; Jeon, T.Y. Can preoperative MR imaging predict optic nerve invasion of retinoblastoma? Eur. J. Radiol. 2012, 81, 4041–4045. [Google Scholar] [CrossRef]
- Deike-Hofmann, K.; von Lampe, P.; Eerikaeinen, M.; Ting, S.; Schlüter, S.; Schlemmer, H.P.; Bechrakis, N.E.; Forsting, M.; Radbruch, A. Anterior chamber enhancement predicts optic nerve infiltration in retinoblastoma. Eur. Radiol. 2022. [Google Scholar] [CrossRef] [PubMed]
- Linn Murphree, A. Intraocular retinoblastoma: The case for a new group classification. Ophthalmol. Clin. N. Am. 2005, 18, 41–53. [Google Scholar] [CrossRef]
- Novetsky, D.E.; Abramson, D.H.; Kim, J.W.; Dunkel, I.J. Published international classification of retinoblastoma (ICRB) definitions contain inconsistencies—An analysis of impact. Ophthalmic Genet. 2009, 30, 40–44. [Google Scholar] [CrossRef]
- Blach, L.E.; McCormick, B.; Abramson, D.H.; Ellsworth, R.M. Trilateral retinoblastoma—Incidence and outcome: A decade of experience. Int. J. Radiat. Oncol. Biol. Phys. 1994, 29, 729–733. [Google Scholar] [CrossRef]
- Carvajal, R.D.; Schwartz, G.K.; Tezel, T.; Marr, B.; Francis, J.H.; Nathan, P.D. Metastatic disease from uveal melanoma: Treatment options and future prospects. Br. J. Ophthalmol. 2017, 101, 38–44. [Google Scholar] [CrossRef]
- Chang, A.E.; Karnell, L.H.; Menck, H.R. The National Cancer Data Base report on cutaneous and noncutaneous melanoma: A summary of 84,836 cases from the past decade. The American College of Surgeons Commission on Cancer and the American Cancer Society. Cancer 1998, 83, 1664–1678. [Google Scholar] [CrossRef]
- Singh, A.D.; Turell, M.E.; Topham, A.K. Uveal melanoma: Trends in incidence, treatment, and survival. Ophthalmology 2011, 118, 1881–1885. [Google Scholar] [CrossRef] [PubMed]
- Spagnolo, F.; Caltabiano, G.; Queirolo, P. Uveal melanoma. Cancer Treat. Rev. 2012, 38, 549–553. [Google Scholar] [CrossRef]
- Ferreira, T.A.; Jaarsma-Coes, M.G.; Marinkovic, M.; Verbist, B.; Verdijk, R.M.; Jager, M.J.; Luyten, G.P.M.; Beenakker, J.M. MR imaging characteristics of uveal melanoma with histopathological validation. Neuroradiology 2022, 64, 171–184. [Google Scholar] [CrossRef] [PubMed]
- Foti, P.V.; Travali, M.; Farina, R.; Palmucci, S.; Spatola, C.; Raffaele, L.; Salamone, V.; Caltabiano, R.; Broggi, G.; Puzzo, L.; et al. Diagnostic methods and therapeutic options of uveal melanoma with emphasis on MR imaging-Part I: MR imaging with pathologic correlation and technical considerations. Insights Imaging 2021, 12, 66. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, T.A.; Grech Fonk, L.; Jaarsma-Coes, M.G.; van Haren, G.G.R.; Marinkovic, M.; Beenakker, J.M. MRI of Uveal Melanoma. Cancers 2019, 11, 377. [Google Scholar] [CrossRef]
- Karcioğlu, Z.A. Orbital Tumors, Diagnosis and Treatment; Springer: Berlin/Heidelberg, Germany, 2005; ISBN 038721321X. [Google Scholar]
- Pappas, A.; Araque, J.M.; Sarup, V. Orbital Venous Varices: A Rare Bilateral Asymptomatic Presentation. Cureus 2018, 10, e3302. [Google Scholar] [CrossRef]
- Weill, A.; Cognard, C.; Castaings, L.; Robert, G.; Moret, J. Embolization of an orbital varix after surgical exposure. AJNR Am. J. Neuroradiol. 1998, 19, 921–923. [Google Scholar]
- Bullock, J.D.; Goldbert, S.H.; Connelly, P.J. Orbital varix thrombosis. Trans. Am. Ophthalmol. Soc. 1989, 87, 463–484. [Google Scholar] [CrossRef]
- Shields, J.A.; Dolinskas, C.; Augsburger, J.J.; Shah, H.G.; Shapiro, M.L. Demonstration of orbital varix with computed tomography and valsalva maneuver. Am. J. Ophthalmol. 1984, 97, 108–110. [Google Scholar] [CrossRef]
- Shnier, R.; Parker, G.D.; Hallinan, J.M.; Pollei, S.R.; Smoker, W.R. Orbital varices: A new technique for noninvasive diagnosis. AJNR Am. J. Neuroradiol. 1991, 12, 717–718. [Google Scholar]
- Wu, E.H.; Bai, R.J.; Zhang, Y.T.; Song, G.X. CT with pressure exerted on neck veins in the diagnosis of primary orbital varices. Chin. Med. J. 1985, 98, 287–288. [Google Scholar] [PubMed]
- Calandriello, L.; Grimaldi, G.; Petrone, G.; Rigante, M.; Petroni, S.; Riso, M.; Savino, G. Cavernous venous malformation (cavernous hemangioma) of the orbit: Current concepts and a review of the literature. Surv. Ophthalmol. 2017, 62, 393–403. [Google Scholar] [CrossRef]
- Hegde, A.; Prasad, G.L.; Menon, G.; Jaiprakash, P. Spontaneous orbital haemorrhage secondary to cavernous haemangioma—A case summary and review of literature. J. Clin. Neurosci. 2019, 67, 272–275. [Google Scholar] [CrossRef] [PubMed]
- Wilms, G. Orbital cavernous hemangiomas. AJNR Am. J. Neuroradiol. 2009, 30, E7. [Google Scholar] [CrossRef] [PubMed]
- Scheuerle, A.F.; Steiner, H.H.; Kolling, G.; Kunze, S.; Aschoff, A. Treatment and long-term outcome of patients with orbital cavernomas. Am. J. Ophthalmol. 2004, 138, 237–244. [Google Scholar] [CrossRef] [PubMed]
- Harris, G.J. Cavernous hemangioma of the orbital apex: Pathogenetic considerations in surgical management. Am. J. Ophthalmol. 2010, 150, 764–773. [Google Scholar] [CrossRef]
- Enjolras, O.; Mulliken, J.B. Vascular tumors and vascular malformations (new issues). Adv. Dermatol. 1997, 13, 375–423. [Google Scholar]
- Enjolras, O. Classification and management of the various superficial vascular anomalies: Hemangiomas and vascular malformations. J. Dermatol. 1997, 24, 701–710. [Google Scholar] [CrossRef] [PubMed]
- Belov, S. Anatomopathological classification of congenital vascular defects. Semin. Vasc. Surg. 1993, 6, 219–224. [Google Scholar]
- Spiteri Cornish, K.; Reddy, A.R. The use of propranolol in the management of periocular capillary haemangioma—A systematic review. Eye 2011, 25, 1277–1283. [Google Scholar] [CrossRef]
- Drolet, B.A.; Esterly, N.B.; Frieden, I.J. Hemangiomas in children. N. Engl. J. Med. 1999, 341, 173–181. [Google Scholar] [CrossRef]
- Thoumazet, F.; Léauté-Labrèze, C.; Colin, J.; Mortemousque, B. Efficacy of systemic propranolol for severe infantile haemangioma of the orbit and eyelid: A case study of eight patients. Br. J. Ophthalmol. 2012, 96, 370–374. [Google Scholar] [CrossRef]
- Haik, B.G.; Jakobiec, F.A.; Ellsworth, R.M.; Jones, I.S. Capillary hemangioma of the lids and orbit: An analysis of the clinical features and therapeutic results in 101 cases. Ophthalmology 1979, 86, 760–792. [Google Scholar] [CrossRef]
- Dubois, J.; Milot, J.; Jaeger, B.I.; McCuaig, C.; Rousseau, E.; Powell, J. Orbit and eyelid hemangiomas: Is there a relationship between location and ocular problems? J. Am. Acad. Dermatol. 2006, 55, 614–619. [Google Scholar] [CrossRef]
- Zvizdic, D.; Bulja, D.; Sidran, A.; Skenderi, F.; Zvizdic, Z.; Vranic, S. Isolated deep orbital hemangioma treated successfully with oral propranolol in a 2-month-old infant: Case report with literature review. Am. J. Ophthalmol. Case Rep. 2021, 22, 101095. [Google Scholar] [CrossRef] [PubMed]
- Frieden, I.J.; Reese, V.; Cohen, D. PHACE syndrome. The association of posterior fossa brain malformations, hemangiomas, arterial anomalies, coarctation of the aorta and cardiac defects, and eye abnormalities. Arch. Dermatol. 1996, 132, 307–311. [Google Scholar] [CrossRef]
- Kralik, S.F.; Haider, K.M.; Lobo, R.R.; Supakul, N.; Calloni, S.F.; Soares, B.P. Orbital infantile hemangioma and rhabdomyosarcoma in children: Differentiation using diffusion-weighted magnetic resonance imaging. J. Am. Assoc. Pediatr. Ophthalmol. Strabismus 2018, 22, 27–31. [Google Scholar] [CrossRef] [PubMed]
- Margo, C.E.; Mulla, Z.D. Malignant tumors of the orbit. Analysis of the Florida Cancer Registry. Ophthalmology 1998, 105, 185–190. [Google Scholar] [CrossRef]
- Olsen, T.G.; Heegaard, S. Orbital lymphoma. Surv. Ophthalmol. 2019, 64, 45–66. [Google Scholar] [CrossRef] [PubMed]
- Valvassori, G.E.; Sabnis, S.S.; Mafee, R.F.; Brown, M.S.; Putterman, A. Imaging of orbital lymphoproliferative disorders. Radiol. Clin. N. Am. 1999, 37, 135–150. [Google Scholar] [CrossRef]
- Hosten, N.; Schörner, W.; Zwicker, C.; Lietz, A.; Serke, S.; Huhn, D.; Felix, R. Lymphocytic infiltrations of the orbit in MRT and CT. Lymphoma, pseudolymphoma and inflammatory pseudotumor. Rofo 1991, 155, 445–451. [Google Scholar] [CrossRef]
- Andreasen, S.; Esmaeli, B.; Holstein, S.L.; Mikkelsen, L.H.; Rasmussen, P.K.; Heegaard, S. An Update on Tumors of the Lacrimal Gland. Asia Pac. J. Ophthalmol. 2017, 6, 159–172. [Google Scholar] [CrossRef]
- Young, S.M.; Kim, Y.D.; Shin, H.J.; Imagawa, Y.; Lang, S.S.; Woo, K.I. Lacrimal gland pleomorphic adenoma and malignant epithelial tumours: Clinical and imaging differences. Br. J. Ophthalmol. 2019, 103, 264–268. [Google Scholar] [CrossRef] [PubMed]
- Clauser, L.; Sarti, E.; Dallera, V.; Galiè, M. Integrated reconstructive strategies for treating the anophthalmic orbit. J. Craniomaxillofac. Surg. 2004, 32, 279–290. [Google Scholar] [CrossRef] [PubMed]
- Qin, W.; Chong, R.; Huang, X.; Liu, M.; Yin, Z.Q. Adenoid cystic carcinoma of the lacrimal gland: CT and MRI findings. Eur. J. Ophthalmol. 2012, 22, 316–319. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Moonis, G.; Cunnane, M.E.; Eisenberg, R.L. Lacrimal gland masses. AJR Am. J. Roentgenol. 2013, 201, W371–W381. [Google Scholar] [CrossRef]
- Lin, Y.H.; Huang, S.M.; Yap, W.K.; Yang, J.W.; Yeung, L.; Tsan, D.L.; Chang, J.T.; Chen, L.C. Outcomes in patients with lacrimal gland carcinoma treated with definitive radiotherapy or eye-sparing surgery followed by adjuvant radiotherapy. Radiat. Oncol. 2020, 15, 156. [Google Scholar] [CrossRef] [PubMed]
- Touil, A.; El Abbassi, S.; Echchikhi, Y.; Maher, M.; Kebdani, T.; Benjaafar, N. Adenocarcinoma of the lacrimal gland: A case report. J. Med. Case Rep. 2017, 11, 257. [Google Scholar] [CrossRef] [PubMed]
- Shapey, J.; Danesh-Meyer, H.V.; Kaye, A.H. Diagnosis and management of optic nerve glioma. J. Clin. Neurosci. 2011, 18, 1585–1591. [Google Scholar] [CrossRef] [PubMed]
- Thompson, C.R.; Lessell, S. Anterior visual pathway gliomas. Int. Ophthalmol. Clin. 1997, 37, 261–279. [Google Scholar] [CrossRef]
- Chung, E.M.; Specht, C.S.; Schroeder, J.W. From the archives of the AFIP: Pediatric orbit tumors and tumorlike lesions: Neuroepithelial lesions of the ocular globe and optic nerve. Radiographics 2007, 27, 1159–1186. [Google Scholar] [CrossRef]
- Wladis, E.J.; Adamo, M.A.; Weintraub, L. Optic Nerve Gliomas. J. Neurol. Surg. B Skull Base 2021, 82, 91–95. [Google Scholar] [CrossRef] [PubMed]
- Thiagalingam, S.; Flaherty, M.; Billson, F.; North, K. Neurofibromatosis type 1 and optic pathway gliomas: Follow-up of 54 patients. Ophthalmology 2004, 111, 568–577. [Google Scholar] [CrossRef]
- DeAngelis, L.M. Brain tumors. N. Engl. J. Med. 2001, 344, 114–123. [Google Scholar] [CrossRef]
- Lin, C.Y.; Huang, H.M. Unilateral malignant optic glioma following glioblastoma multiforme in the young: A case report and literature review. BMC Ophthalmol. 2017, 17, 21. [Google Scholar] [CrossRef]
- Dutton, J.J. Optic nerve sheath meningiomas. Surv. Ophthalmol. 1992, 37, 167–183. [Google Scholar] [CrossRef]
- Shapey, J.; Sabin, H.I.; Danesh-Meyer, H.V.; Kaye, A.H. Diagnosis and management of optic nerve sheath meningiomas. J. Clin. Neurosci. 2013, 20, 1045–1056. [Google Scholar] [CrossRef]
- Bradbury, P.G.; Levy, I.S.; McDonald, W.I. Transient uniocular visual loss on deviation of the eye in association with intraorbital tumours. J. Neurol. Neurosurg. Psychiatry 1987, 50, 615–619. [Google Scholar] [CrossRef]
- Kanamalla, U.S. The optic nerve tram-track sign. Radiology 2003, 227, 718–719. [Google Scholar] [CrossRef] [PubMed]
- Ansari, I.; Ansari, A.; Graison, A.A.; Patil, A.J.; Joshi, H. Head and Neck Schwannomas: A Surgical Challenge—A Series of 5 Cases. Case Rep. Otolaryngol. 2018, 2018, 4074905. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.H.; Yan, J.H. Imaging characteristics and surgical management of orbital neurilemmomas. Int. J. Ophthalmol. 2019, 12, 1108–1115. [Google Scholar] [CrossRef] [PubMed]
- Chaskes, M.B.; Rabinowitz, M.R. Orbital Schwannoma. J. Neurol. Surg. B Skull Base 2020, 81, 376–380. [Google Scholar] [CrossRef]
- Pointdujour-Lim, R.; Lally, S.E.; Shields, J.A.; Eagle, R.C., Jr.; Shields, C.L. Orbital Schwannoma: Radiographic and Histopathologic Correlation in 15 Cases. Ophthalmic Plast. Reconstr. Surg. 2018, 34, 162–167. [Google Scholar] [CrossRef]
- Rootman, J.; Goldberg, C.; Robertson, W. Primary orbital schwannomas. Br. J. Ophthalmol. 1982, 66, 194–204. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; Singh, U.; Zadeng, Z.; Pathak, A.; Sukhija, J. Clinico-Radiological Spectrum and Management of Orbital Schwannomas: A Tertiary Care Institute Study. Orbit 2013, 32, 171–177. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.S.; Jung, J.W.; Yoon, K.C.; Kwon, Y.J.; Hwang, J.H.; Lee, S.Y. Schwannoma of the Orbit. Arch. Craniofac. Surg. 2015, 16, 67–72. [Google Scholar] [CrossRef]
- Tanaka, A.; Mihara, F.; Yoshiura, T.; Togao, O.; Kuwabara, Y.; Natori, Y.; Sasaki, T.; Honda, H. Differentiation of cavernous hemangioma from schwannoma of the orbit: A dynamic MRI study. AJR Am. J. Roentgenol. 2004, 183, 1799–1804. [Google Scholar] [CrossRef] [PubMed]
- Milburn, J.M.; Gimenez, C.R., Jr.; Dutweiler, E. Clinical Images: Imaging Manifestations of Orbital Neurofibromatosis Type 1. Ochsner J. 2016, 16, 431–434. [Google Scholar] [PubMed]
- Lee, L.R.; Gigantelli, J.W.; Kincaid, M.C. Localized neurofibroma of the orbit: A radiographic and histopathologic study. Ophthalmic Plast. Reconstr. Surg. 2000, 16, 241–246. [Google Scholar] [CrossRef] [PubMed]
- Staser, K.; Yang, F.C.; Clapp, D.W. Pathogenesis of plexiform neurofibroma: Tumor-stromal/hematopoietic interactions in tumor progression. Annu. Rev. Pathol. 2012, 7, 469–495. [Google Scholar] [CrossRef] [PubMed]
- Bechtold, D.; Hove, H.D.; Prause, J.U.; Heegaard, S.; Toft, P.B. Plexiform neurofibroma of the eye region occurring in patients without neurofibromatosis type 1. Ophthalmic Plast. Reconstr. Surg. 2012, 28, 413–415. [Google Scholar] [CrossRef] [PubMed]
- DeBella, K.; Szudek, J.; Friedman, J.M. Use of the national institutes of health criteria for diagnosis of neurofibromatosis 1 in children. Pediatrics 2000, 105, 608–614. [Google Scholar] [CrossRef] [PubMed]
- Kottler, U.B.; Conway, R.M.; Schlötzer-Schrehardt, U.; Holbach, L.M. Isolated neurofibroma of the orbit with extensive myxoid changes: A clinicopathologic study including MRI and electron microscopic findings. Orbit 2004, 23, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Allen, R.C. Orbital Metastases: When to Suspect? When to biopsy? Middle East Afr. J. Ophthalmol. 2018, 25, 60–64. [Google Scholar] [CrossRef] [PubMed]
- Madabhavi, I.; Ks, S.; Dharmarajan Lethika, R.; Tumbal, S.; Miskin, A.T.; Sarkar, M.; Modi, M. Intraconal Metastasis Leading to Diagnosis of Hepatocellular Carcinoma. Middle East J. Dig. Dis. 2020, 12, 48–51. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yan, J.; Gao, S. Metastatic orbital tumors in southern China during an 18-year period. Graefe’s Arch. Clin. Exp. Ophthalmol. 2011, 249, 1387–1393. [Google Scholar] [CrossRef]
- Valenzuela, A.A.; Archibald, C.W.; Fleming, B.; Ong, L.; O’Donnell, B.; Crompton, J.J.; Selva, D.; McNab, A.A.; Sullivan, T.J. Orbital metastasis: Clinical features, management and outcome. Orbit 2009, 28, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Shields, J.A.; Shields, C.L.; Brotman, H.K.; Carvalho, C.; Perez, N.; Eagle, R.C., Jr. Cancer metastatic to the orbit: The 2000 Robert M. Curts Lecture. Ophthalmic Plast. Reconstr. Surg. 2001, 17, 346–354. [Google Scholar] [CrossRef] [PubMed]
- Dagher, R.; Helman, L. Rhabdomyosarcoma: An overview. Oncologist 1999, 4, 34–44. [Google Scholar] [CrossRef] [PubMed]
- Elsas, F.J.; Mroczek, E.C.; Kelly, D.R.; Specht, C.S. Primary rhabdomyosarcoma of the iris. Arch. Ophthalmol. 1991, 109, 982–984. [Google Scholar] [CrossRef] [PubMed]
- Karcioglu, Z.A.; Hadjistilianou, D.; Rozans, M.; DeFrancesco, S. Orbital rhabdomyosarcoma. Cancer Control 2004, 11, 328–333. [Google Scholar] [CrossRef] [PubMed]
- Inarejos Clemente, E.J.; Navallas, M.; Barber Martínez de la Torre, I.; Suñol, M.; Munuera Del Cerro, J.; Torner, F.; Garraus, M.; Navarro, O.M. MRI of Rhabdomyosarcoma and Other Soft-Tissue Sarcomas in Children. Radiographics 2020, 40, 791–814. [Google Scholar] [CrossRef]
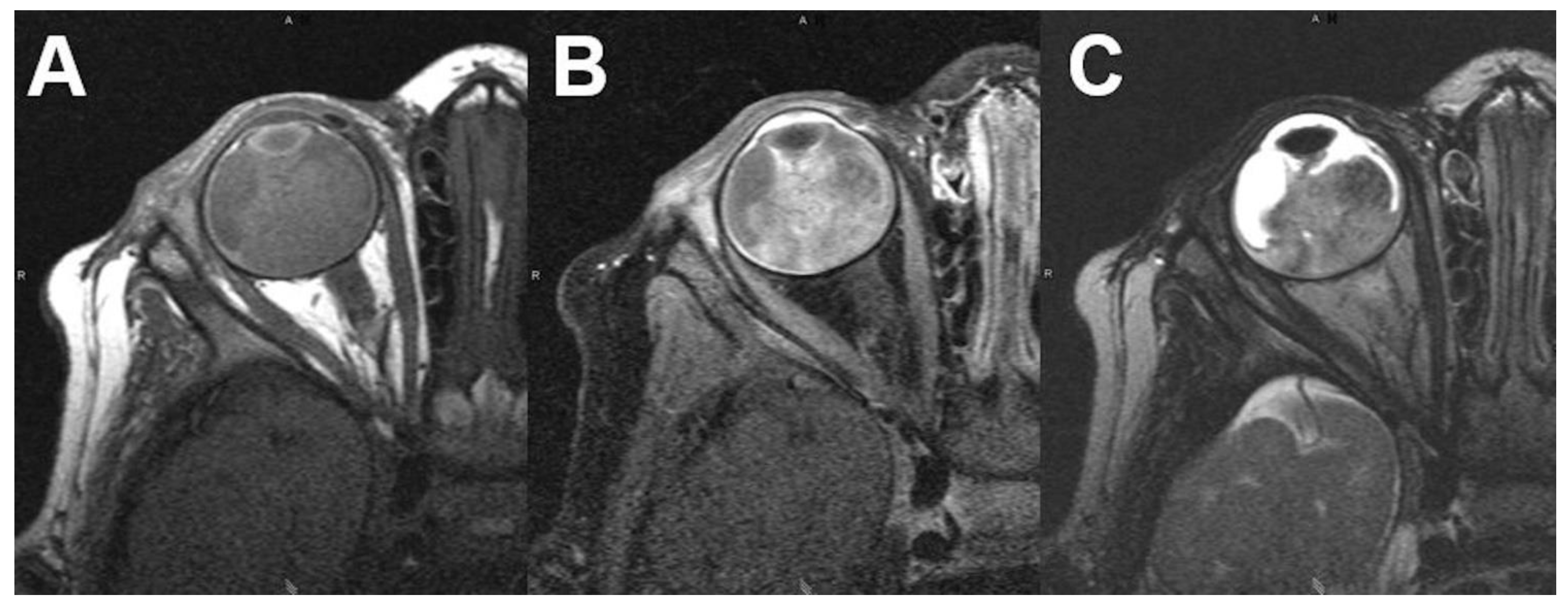
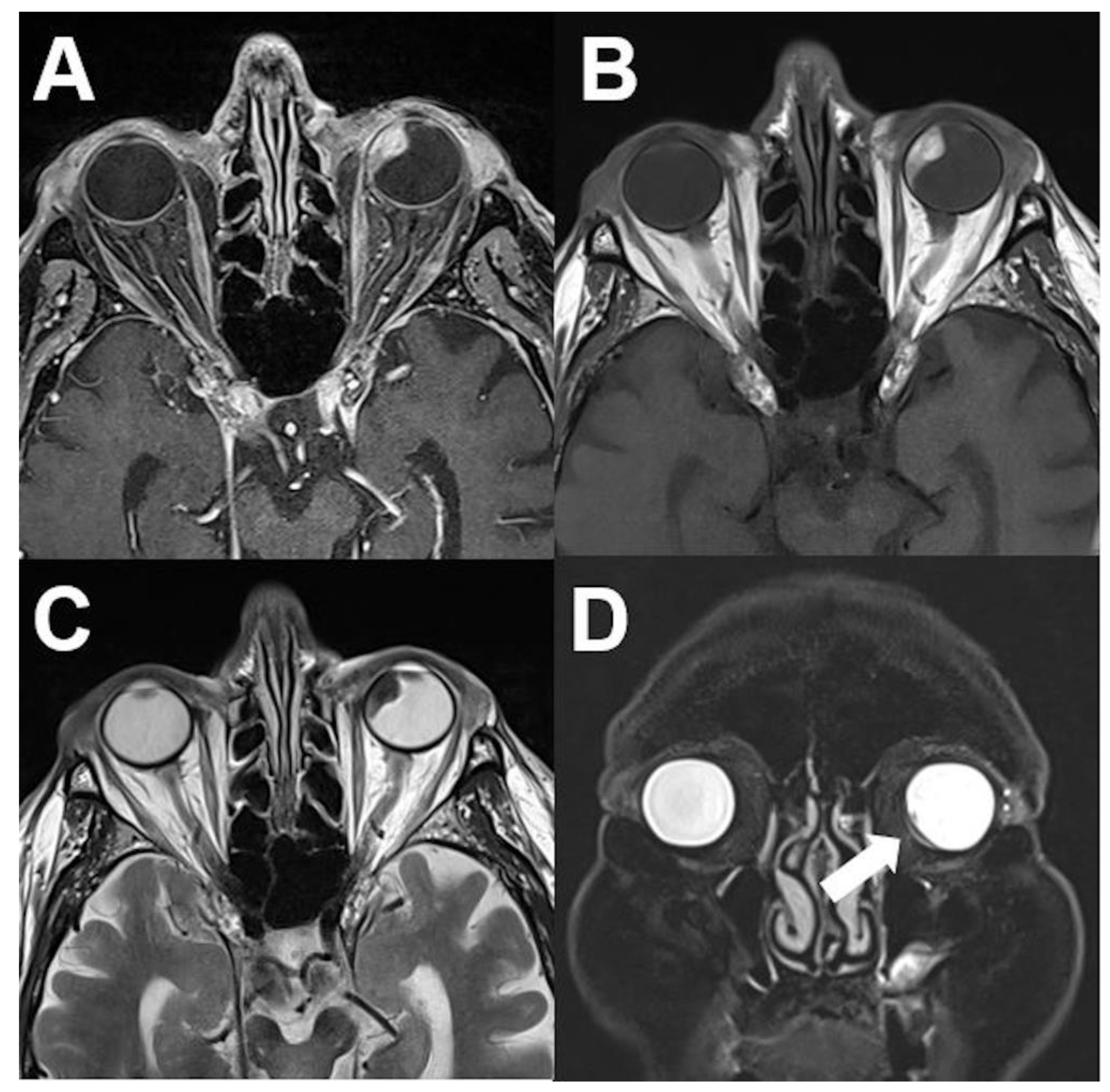

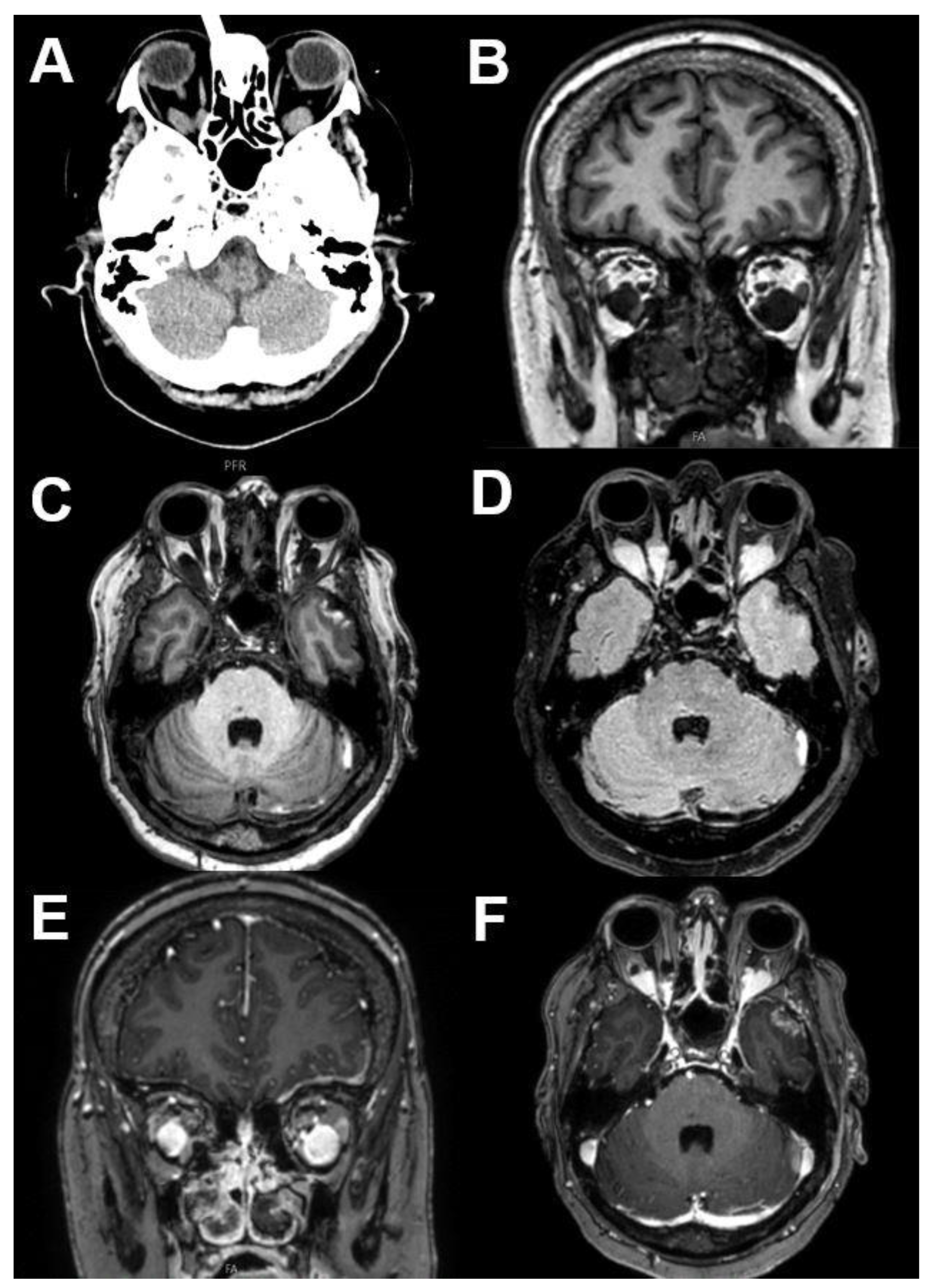
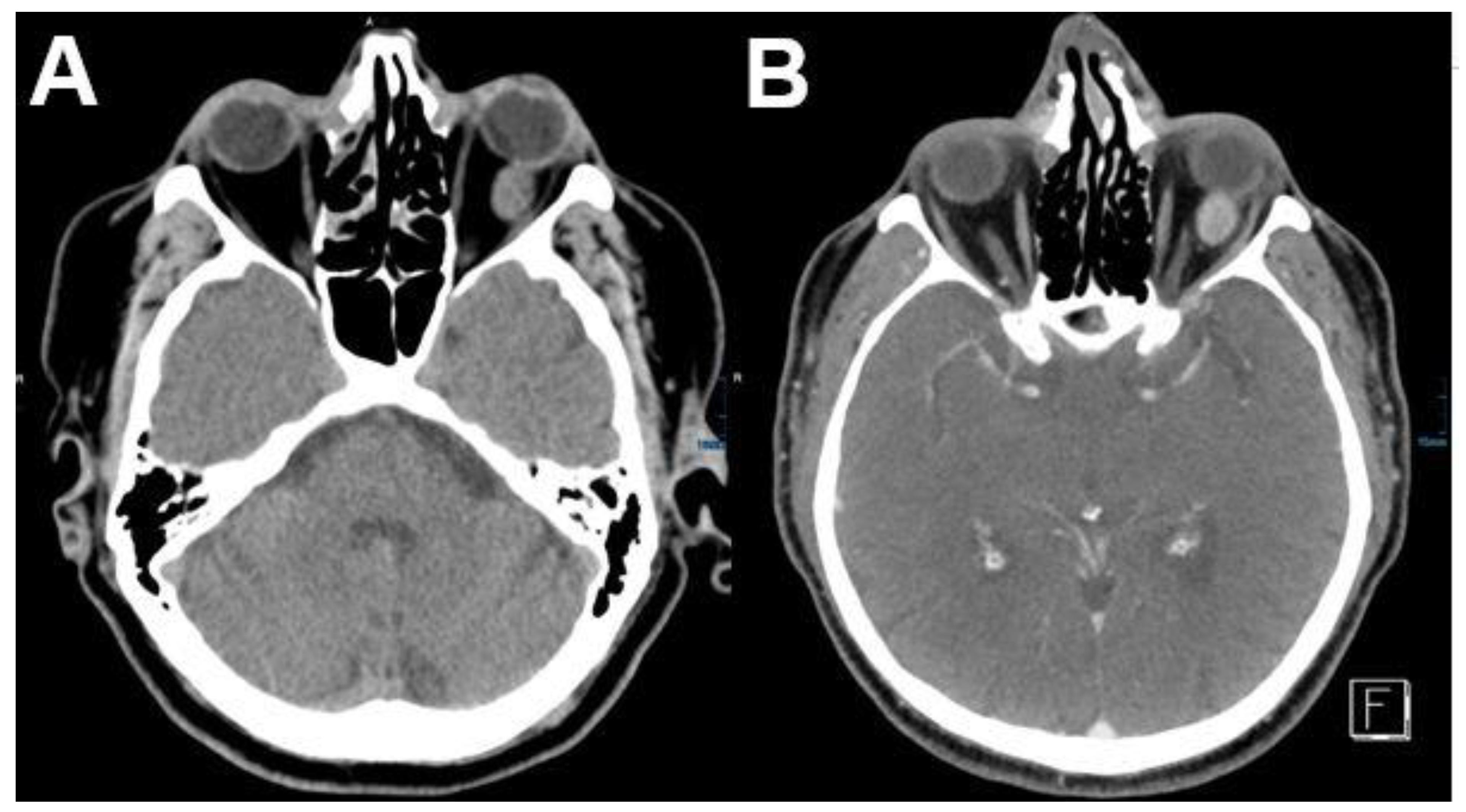
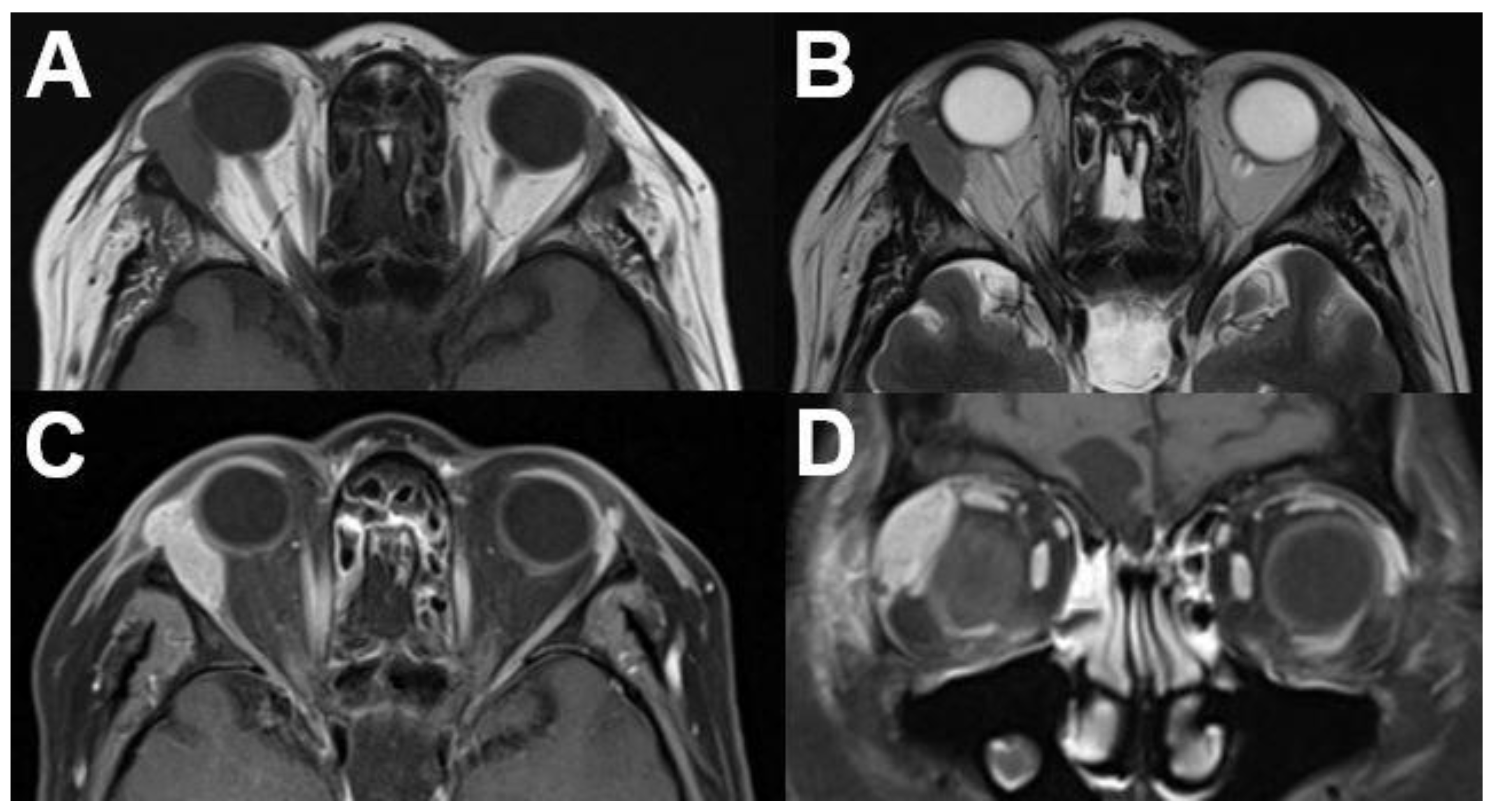

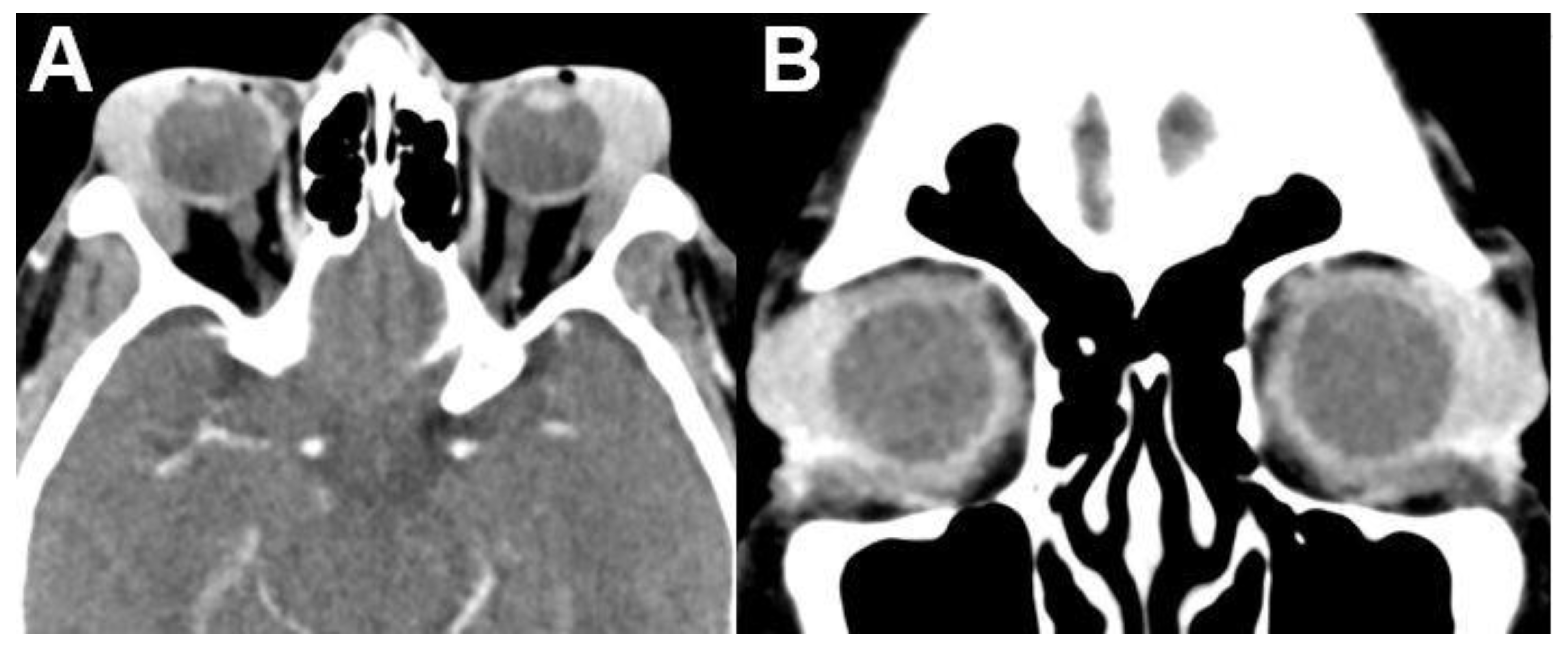

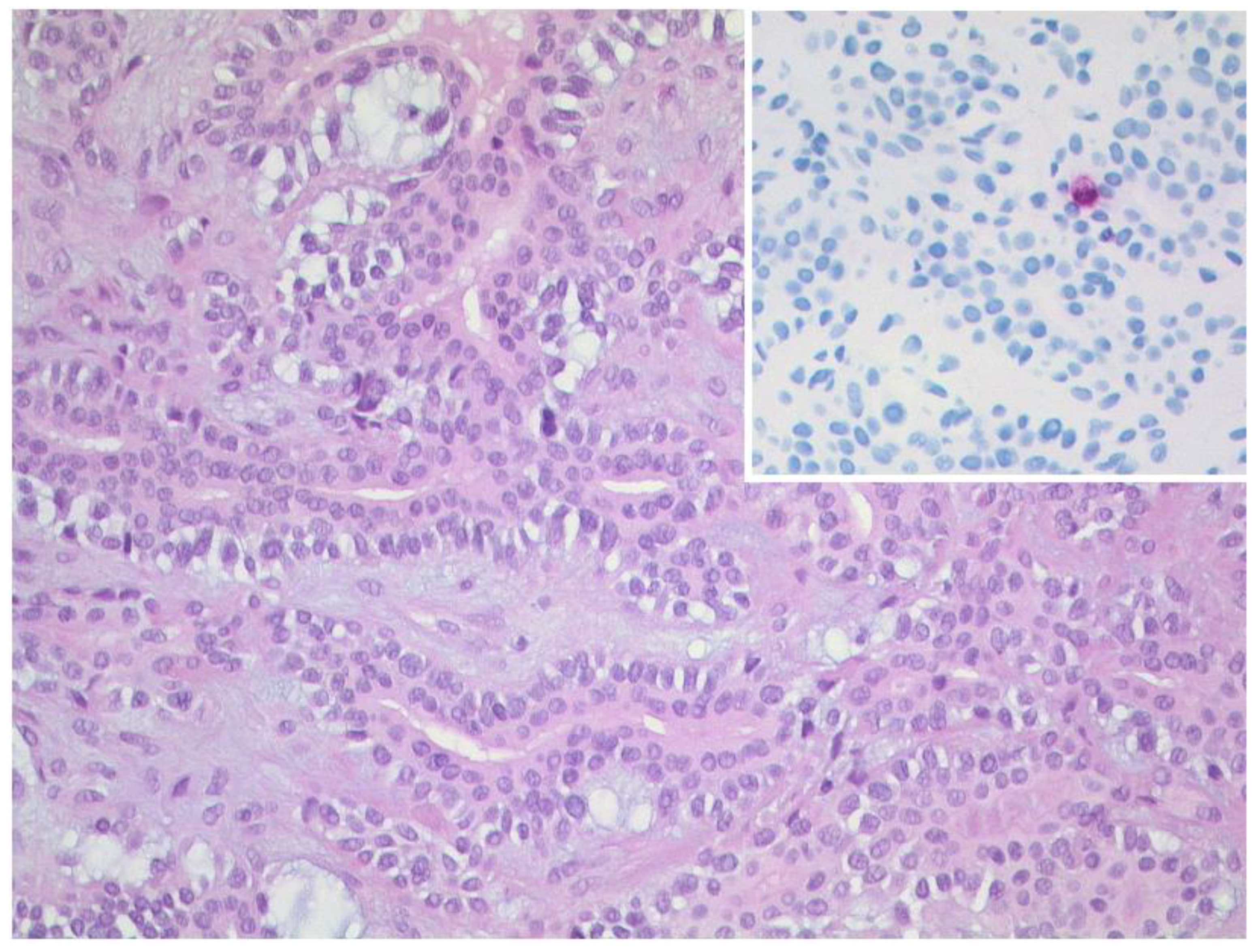
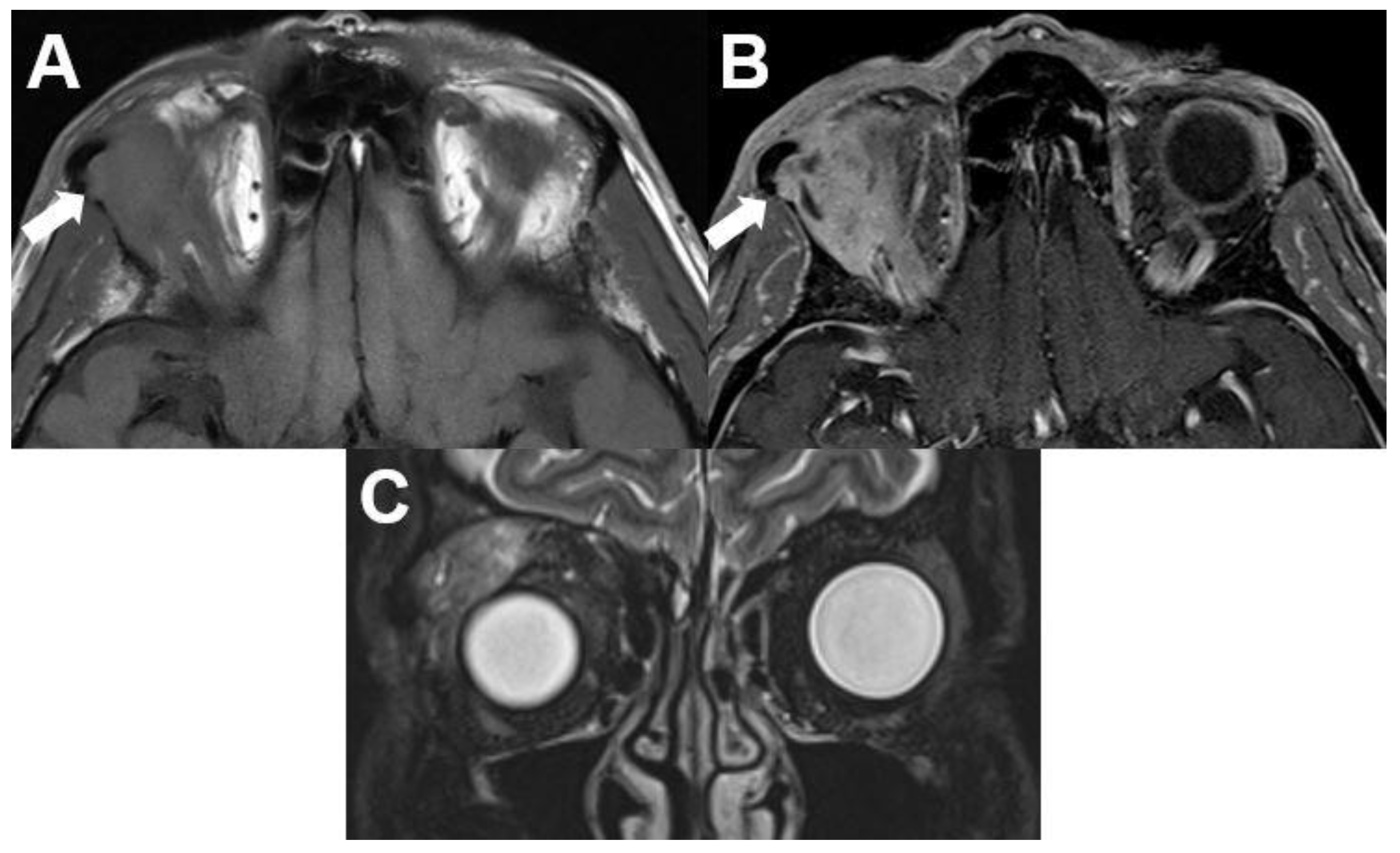

| Signal | Sequence | Plane | Slice Thickness | Description |
|---|---|---|---|---|
| T1 weighted | T1-TSE | axial | 3 mm | |
| T2-weighted | T2-TSE | axial | 3 mm | |
| TIRM | coronal | 4 mm | from the orbit to the chiasm | |
| Post-contrast | T1-VIBE fat-saturated | axial and coronal, sagittal (optional) | axial 1 mm coronal 2 mm sagittal 3 mm | axial for the orbits, coronal from the orbit to the chiasm |
| DWI | b = 0; b = 1000; ADC-map | axial | 3 mm |
| A | Small |
|
| B | Large | macular or juxtapapillary location |
| C | Local Dissemination | discrete vitreous or subretinal seeding |
| D | Diffuse |
|
| E | Unsalvageable or extensive |
|
| F | Extrascleral | extrascleral spread (optic nerve, orbit or distant metastases) |
| Vascular Anomalies | ||||
|---|---|---|---|---|
| Vascular Tumors | Vascular Malformation | |||
| Simple | Combined | of Major Named Vessels | Associated with Other Anomalies | |
| Benign | Capillary malformations | CVM, CLM | ||
| Local aggressive or borderline | Lymphatic malformations | LVM, CLVM | ||
| Venous malformations | CAVM | |||
| Malignant | Arteriovenous malformations | CLAVM | ||
| Arteriovenous fistula | others | |||
| Pattern | Different Types |
|---|---|
| focal | Superficial |
| multifocal | Deep |
| segmental | Mixed (superficial + deep) |
| indeterminate | Reticular/abortive/minimal growth |
| others |
| Inflammatory | Neoplastic | ||
|---|---|---|---|
| Sarcoidosis | Benign epithelial tumors | Malignant epithelial tumors | Non-epithelial neoplasmas |
| Orbital inflammatory pseudotumor | Pleomorphic adenoma | Adenoid cystic carcinoma | Lymphoma |
| Sjögren syndrome | Oncocytoma | Adenocarcinoma | Chloroma |
| Infectous dacryoadenitis | Dermoid cyst | Acinic cell carcinoma | Haemangioma |
| Squamous cell carcinoma | Metastatic or secondary tumors | ||
| Optic Nerve Meningioma | Optic Nerve Glioma |
|---|---|
| Calcification of the optic nerve sheath and adjacent bony hyperostosis on CT | No calcification or hyperostosis on CT |
| Apical expansion of the tumor | Kinking or buckling of the optic nerve |
| Optical nerve sheath thickening and enhancement with relative sparing of the optic nerve substance (tram tack sign) | Thickening of optic nerve and sheath by the tumor |
| Prominent contrast enhancement on MRI and CT | Variable contrast enhancement on MRI and CT |
| Extradural tumor extension | Cystic spaces within the optic nerve |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vogele, D.; Sollmann, N.; Beck, A.; Haggenmüller, B.; Schmidt, S.A.; Schmitz, B.; Kapapa, T.; Ozpeynirci, Y.; Beer, M.; Kloth, C. Orbital Tumors—Clinical, Radiologic and Histopathologic Correlation. Diagnostics 2022, 12, 2376. https://doi.org/10.3390/diagnostics12102376
Vogele D, Sollmann N, Beck A, Haggenmüller B, Schmidt SA, Schmitz B, Kapapa T, Ozpeynirci Y, Beer M, Kloth C. Orbital Tumors—Clinical, Radiologic and Histopathologic Correlation. Diagnostics. 2022; 12(10):2376. https://doi.org/10.3390/diagnostics12102376
Chicago/Turabian StyleVogele, Daniel, Nico Sollmann, Annika Beck, Benedikt Haggenmüller, Stefan Andreas Schmidt, Bernd Schmitz, Thomas Kapapa, Yigit Ozpeynirci, Meinrad Beer, and Christopher Kloth. 2022. "Orbital Tumors—Clinical, Radiologic and Histopathologic Correlation" Diagnostics 12, no. 10: 2376. https://doi.org/10.3390/diagnostics12102376
APA StyleVogele, D., Sollmann, N., Beck, A., Haggenmüller, B., Schmidt, S. A., Schmitz, B., Kapapa, T., Ozpeynirci, Y., Beer, M., & Kloth, C. (2022). Orbital Tumors—Clinical, Radiologic and Histopathologic Correlation. Diagnostics, 12(10), 2376. https://doi.org/10.3390/diagnostics12102376









