Cardiovascular Factors Associated with COVID-19 from an International Registry of Primarily Japanese Patients
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. International Registry
2.3. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. COVID-19 Symptoms and Outcomes in Registry Patients with or without a History of CVD and by Sex
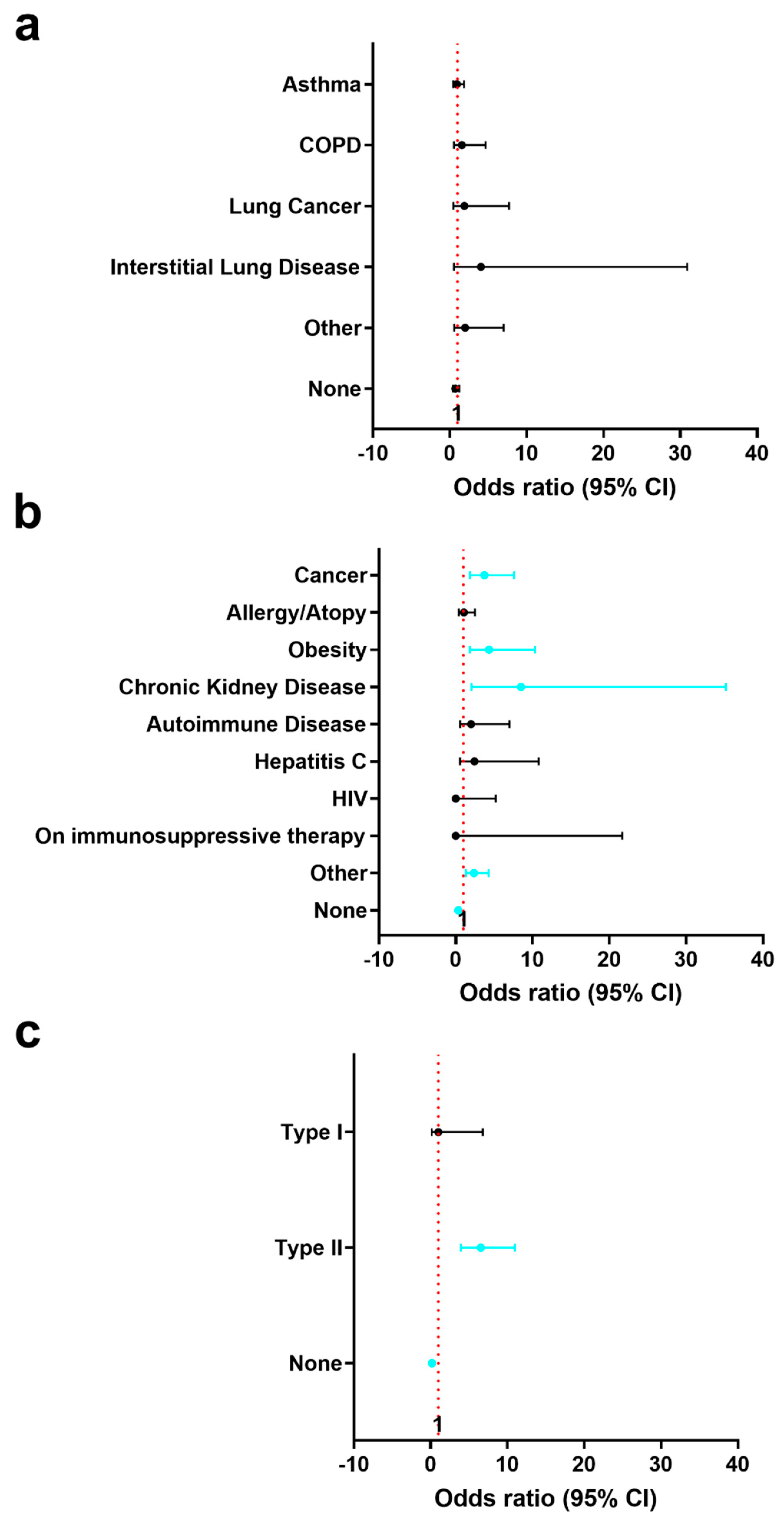
3.3. History of Pre-Existing Lung and Other Conditions in Registry Patients with or without a History of CVD and by Sex
3.4. Lung Imaging and Respiratory Support for Registry Patients with or without a History of CVD and by Sex
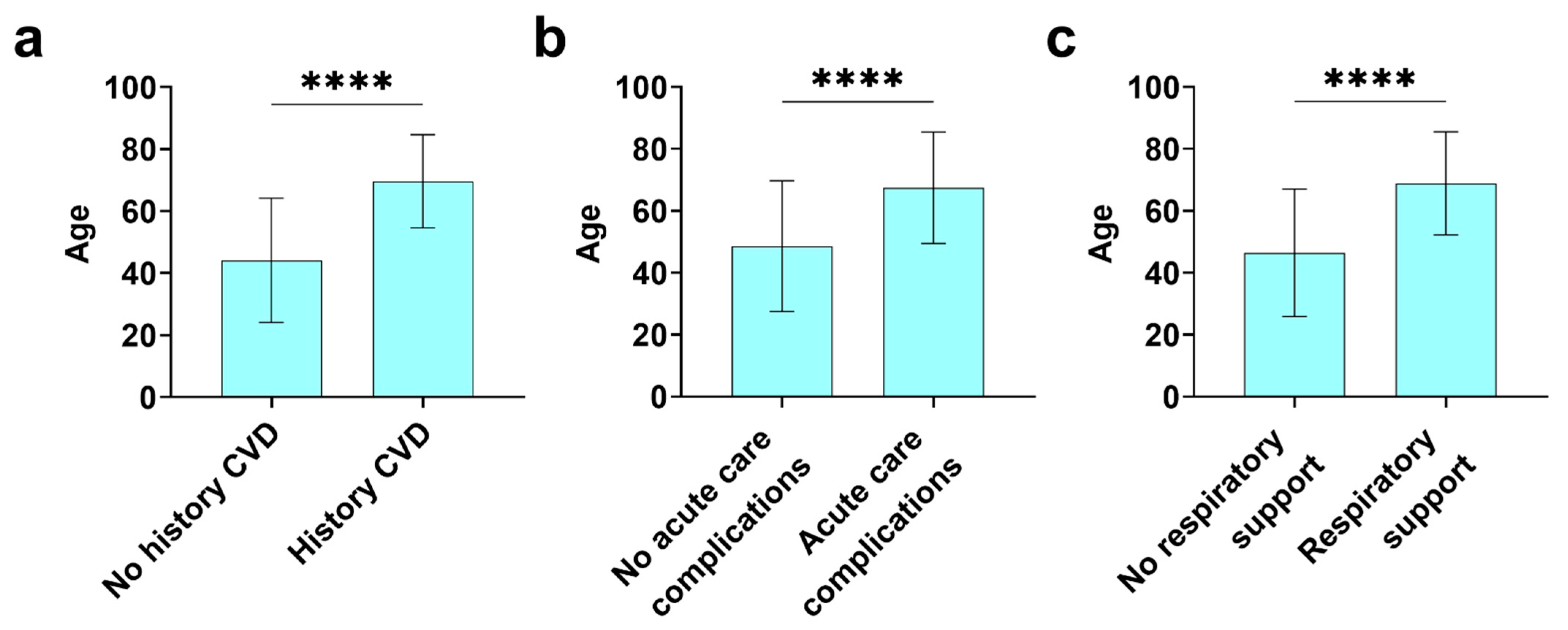
3.5. Mortality and Acute Care Complications in Registry Patients with or without a History of CVD
3.6. Cardiovascular Findings in Registry Patients with or without a History of CVD
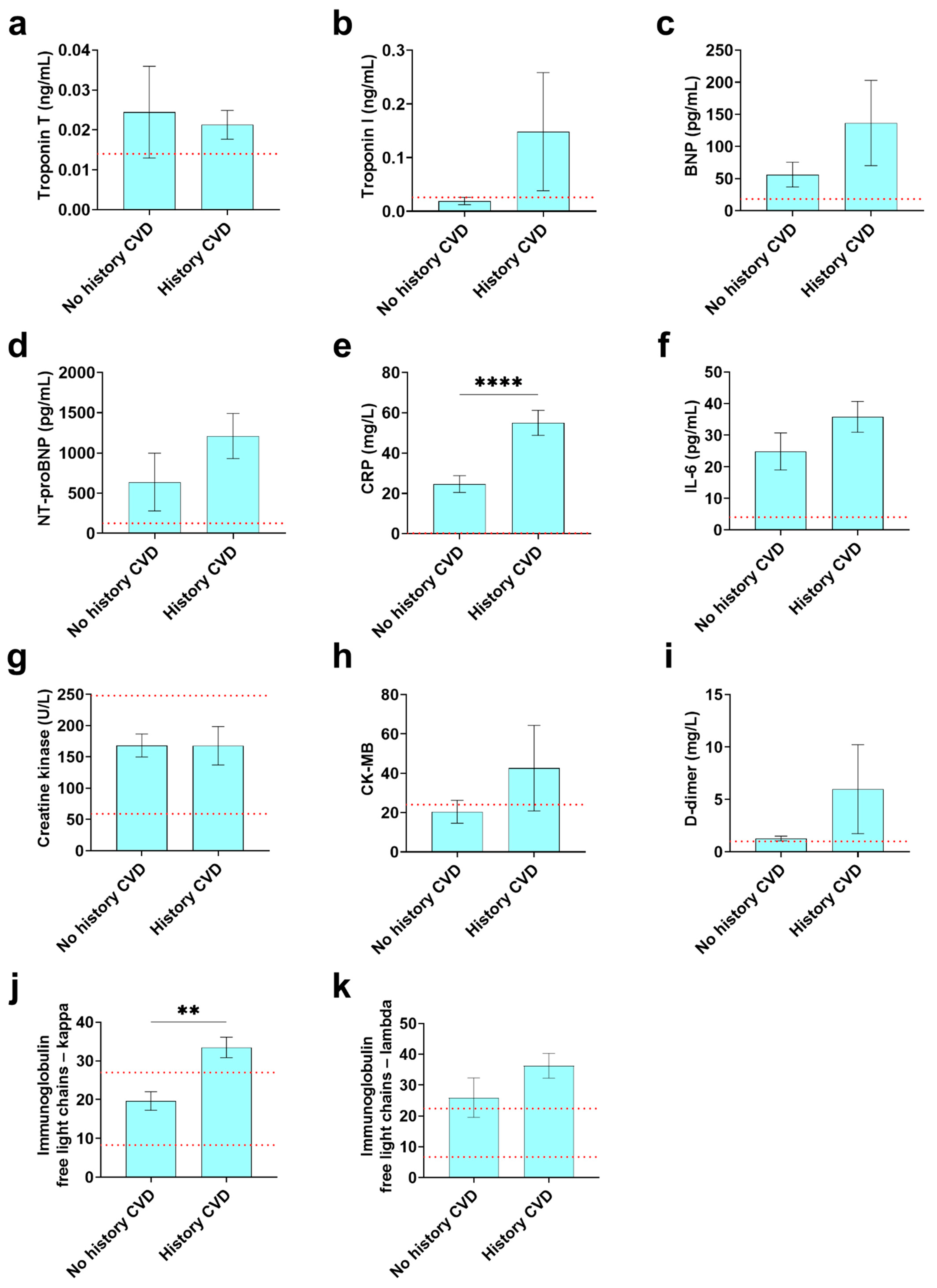
3.7. Cardiovascular and Inflammatory Biomarkers Found in COVID-19 Registry Patients with or without a History of CVD
4. Discussion
5. Limitations of the Study
6. Conclusions
7. Perspectives and Significance
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- The Lancet, COVID-19 in the USA: A question of time. Lancet 2020, 395, 1229. [CrossRef]
- Perez-Fernandez, X.L.; Sabater-Riera, J.; Fuset-Cabanes, M. COVID-19 ARDS: Getting ventilation right. Lancet 2022, 399, 22. [Google Scholar] [CrossRef]
- Ruaro, B.; Confalonieri, P.; Pozzan, R.; Tavano, S.; Mondini, L.; Baratella, E.; Pagnin, A.; Lerda, S.; Geri, P.; Biolo, M.; et al. Severe COVID-19 ARDS treated by bronchoalveolar lavage with diluted exogenous pulmonary surfactant as salvage therapy: In pursuit of the Holy Grail? J. Clin. Med. 2022, 11, 3577. [Google Scholar] [CrossRef]
- Cremer, S.; Jakob, C.; Berkowitsch, A.; Borgmann, S.; Pilgram, L.; Tometten, L.; Classen, A.; Wille, K.; Weidlich, S.; Gruener, B.; et al. Elevated markers of thrombo-inflammatory activation predict outcome in patients with cardiovascular comorbidities and COVID-19 disease: Insights from the LEOSS registry. Clin. Res. Cardiol. 2021, 110, 1029–1040. [Google Scholar] [CrossRef] [PubMed]
- Sokolski, M.; Trenson, S.; Sokolska, J.M.; D’Amario, D.; Meyer, P.; Poku, N.K.; Biering-Sorensen, T.; Hojbjerg Lassen, M.C.; Skaarup, K.G.; Barge-Caballero, E.; et al. Heart failure in COVID-19: The multicentre, multinational PCHF-COVICAV registry. ESC Heart Fail. 2021, 8, 4955–4967. [Google Scholar] [CrossRef] [PubMed]
- Singh, J.; Durr, M.R.; Deptuch, E.; Sultana, S.; Mehta, N.; Garcia, S.; Henry, T.D.; Dehghani, P. Cardiac registries during the COVID-19 pandemic: Lessons learned. Curr. Cardiol. Rep. 2022, 24, 659–665. [Google Scholar] [CrossRef]
- Coronado, M.J.; Bruno, K.A.; Blauwet, L.A.; Tschope, C.; Cunningham, M.W.; Pankuweit, S.; Van Linthout, S.; Jeon, E.S.; McNamara, D.M.; Krejci, J.; et al. Elevated sera sST2 is associated with heart failure in men ≤50 years old with myocarditis. J. Am. Heart Assoc. 2019, 8, e008968. [Google Scholar] [CrossRef] [PubMed]
- Fairweather, D.; Cooper, L.T., Jr.; Blauwet, L.A. Sex and gender differences in myocarditis and dilated cardiomyopathy. Curr. Probl. Cardiol. 2013, 38, 7–46. [Google Scholar] [CrossRef]
- Kindermann, I.; Kindermann, M.; Kandolf, R.; Klingel, K.; Bultmann, B.; Muller, T.; Lindinger, A.; Bohm, M. Predictors of outcome in patients with suspected myocarditis. Circulation 2008, 118, 639–648. [Google Scholar] [CrossRef]
- McNamara, D.M.; Starling, R.C.; Cooper, L.T.; Boehmer, J.P.; Mather, P.J.; Janosko, K.M.; Gorcsan, J., 3rd; Kip, K.E.; Dec, G.W.; IMAC Investigators. Clinical and demographic predictors of outcomes in recent onset dilated cardiomyopathy: Results of the IMAC (Intervention in Myocarditis and Acute Cardiomyopathy)-2 study. J. Am. Coll. Cardiol. 2011, 58, 1112–1118. [Google Scholar] [CrossRef]
- Fairweather, D.; Kaya, Z.; Shellam, G.R.; Lawson, C.M.; Rose, N.R. From infection to autoimmunity. J. Autoimmun. 2001, 16, 175–186. [Google Scholar] [CrossRef] [PubMed]
- Fairweather, D.; Frisancho-Kiss, S.; Yusung, S.A.; Barrett, M.A.; Davis, S.E.; Gatewood, S.J.; Njoku, D.B.; Rose, N.R. Interferon-gamma protects against chronic viral myocarditis by reducing mast cell degranulation, fibrosis, and the profibrotic cytokines transforming growth factor-beta 1, interleukin-1 beta, and interleukin-4 in the heart. Am. J. Pathol. 2004, 165, 1883–1894. [Google Scholar] [CrossRef]
- Saleh, A.; Matsumori, A.; Abdelrazek, S.; Eltaweel, S.; Salous, A.; Neumann, F.J.; Antz, M. Myocardial involvement in coronavirus disease 19. Herz 2020, 45, 719–725. [Google Scholar] [CrossRef] [PubMed]
- Matsumori, A.; Shimada, M.; Jie, X.; Higuchi, H.; Groot Kormelink, T.; Redegeld, F.A. Effects of free immunoglobulin light chains on viral myocarditis. Circ. Res. 2010, 106, 1533–1540. [Google Scholar] [CrossRef]
- Matsumori, A.; Shimada, T.; Nakatani, E.; Shimada, M.; Tracy, S.; Chapman, N.M.; Drayson, M.T.; Hartz, V.L.; Mason, J.W. Immunoglobulin free light chains as an inflammatory biomarker of heart failure with myocarditis. Clin. Immunol. 2020, 217, 108455. [Google Scholar] [CrossRef]
- Krittanawong, C.; Kumar, A.; Hahn, J.; Wang, Z.; Zhang, H.J.; Sun, T.; Bozkurt, B.; Ballantyne, C.M.; Virani, S.S.; Halperin, J.L.; et al. Cardiovascular risk and complications associated with COVID-19. Am. J. Cardiovasc. Dis. 2020, 10, 479–489. [Google Scholar]
- Hendren, N.S.; Drazner, M.H.; Bozkurt, B.; Cooper, L.T., Jr. Description and proposed management of the acute COVID-19 cardiovascular syndrome. Circulation 2020, 141, 1903–1914. [Google Scholar] [CrossRef]
- Goyal, P.; Reshetnyak, E.; Khan, S.; Musse, M.; Navi, B.B.; Kim, J.; Allen, L.A.; Banerjee, S.; Elkind, M.S.V.; Shah, S.J.; et al. Clinical characteristics and outcomes of adults with a history of heart failure hospitalized for COVID-19. Circ. Heart Fail. 2021, 14, e008354. [Google Scholar] [CrossRef]
- Richardson, S.; Hirsch, J.S.; Narasimhan, M.; Crawford, J.M.; McGinn, T.; Davidson, K.W.; Northwell, C.-R.C.; Barnaby, D.P.; Becker, L.B.; Chelico, J.D.; et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA 2020, 323, 2052–2059. [Google Scholar] [CrossRef]
- Joyner, M.J.; Wright, R.S.; Fairweather, D.; Senefeld, J.W.; Bruno, K.A.; Klassen, S.A.; Carter, R.E.; Klompas, A.M.; Wiggins, C.C.; Shepherd, J.R.; et al. Early safety indicators of COVID-19 convalescent plasma in 5000 patients. J. Clin. Investig. 2020, 130, 4791–4797. [Google Scholar] [CrossRef]
- Joyner, M.J.; Bruno, K.A.; Klassen, S.A.; Kunze, K.L.; Johnson, P.W.; Lesser, E.R.; Wiggins, C.C.; Senefeld, J.W.; Klompas, A.M.; Hodge, D.O.; et al. Safety update: COVID-19 convalescent plasma in 20,000 hospitalized patients. Mayo Clin. Proc. 2020, 95, 1888–1897. [Google Scholar] [CrossRef] [PubMed]
- Coe, C.L.; Love, G.D.; Karasawa, M.; Kawakami, N.; Kitayama, S.; Markus, H.R.; Tracy, R.P.; Ryff, C.D. Population differences in proinflammatory biology: Japanese have healthier profiles than Americans. Brain Behav. Immun. 2011, 25, 494–502. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, F.; Solomon, N.; De Lemos, J.A.; Das, S.R.; Morrow, D.A.; Bradley, S.M.; Elkind, M.S.V.; Williams, J.H.; Holmes, D.; Matsouaka, R.A.; et al. Racial and ethnic differences in presentation and outcomes for patients hospitalized with COVID-19: Findings from the American Heart Association’s COVID-19 Cardiovascular Disease Registry. Circulation 2021, 143, 2332–2342. [Google Scholar] [CrossRef] [PubMed]
- Mehraeen, E.; Karimi, A.; Barzegary, A.; Vahedi, F.; Afsahi, A.M.; Dadras, O.; Moradmand-Badie, B.; Seyed Alinaghi, S.A.; Jahanfar, S. Predictors of mortality in patients with COVID-19- A systematic review. Eur. J. Integr. Med. 2020, 40, 101226. [Google Scholar] [CrossRef]
- Roth, G.A.; Emmons-Bell, S.; Alger, H.M.; Bradley, S.M.; Das, S.R.; De Lemos, J.A.; Gakidou, E.; Elkind, M.S.V.; Hay, S.; Hall, J.L.; et al. Trends in patient characteristics and COVID-19 in-hospital mortality in the United States during the COVID-19 pandemic. JAMA Netw. Open 2021, 4, e218828. [Google Scholar] [CrossRef] [PubMed]
- Peckham, H.; De Gruijter, N.M.; Raine, C.; Radziszewska, A.; Ciurtin, C.; Wedderburn, L.R.; Rosser, E.C.; Webb, K.; Deakin, C.T. Male sex identified by global COVID-19 meta-analysis as a risk factor for death and ITU admission. Nat. Commun. 2020, 11, 6317. [Google Scholar] [CrossRef]
- Lau, E.S.; McNeill, J.N.; Paniagua, S.M.; Liu, E.E.; Wang, J.K.; Bassett, I.V.; Selvaggi, C.A.; Lubitz, S.A.; Foulkes, A.S.; Ho, J.E. Sex differences in inflammatory markers in patients hospitalized with COVID-19 infection: Insights from the MGH COVID-19 patient registry. PLoS ONE 2021, 16, e0250774. [Google Scholar] [CrossRef]
- Scully, E.P.; Schumock, G.; Fu, M.; Massaccesi, G.; Muschelli, J.; Betz, J.; Klein, E.Y.; West, N.E.; Robinson, M.; Garibaldi, B.T.; et al. Sex and gender differences in testing, hospital admission, clinical presentation, and drivers of severe outcomes from COVID-19. Open Forum Infect. Dis. 2021, 8, ofab448. [Google Scholar] [CrossRef]
- Vahidy, F.S.; Pan, A.P.; Ahnstedt, H.; Munshi, Y.; Choi, H.A.; Tiruneh, Y.; Nasir, K.; Kash, B.A.; Andrieni, J.D.; McCullough, L.D. Sex differences in susceptibility, severity, and outcomes of coronavirus disease 2019: Cross-sectional analysis from a diverse US metropolitan area. PLoS ONE 2021, 16, e0245556. [Google Scholar] [CrossRef]
- Sagar, S.; Liu, P.P.; Cooper, L.T., Jr. Myocarditis. Lancet 2012, 379, 738–747. [Google Scholar] [CrossRef]
- Manka, R.; Karolyi, M.; Polacin, M.; Holy, E.W.; Nemeth, J.; Steiger, P.; Schuepbach, R.A.; Zinkernagel, A.S.; Alkadhi, H.; Mehra, M.R.; et al. Myocardial edema in COVID-19 on cardiac MRI. J. Heart Lung Transplant. 2020, 39, 730–732. [Google Scholar] [CrossRef] [PubMed]
- Puntmann, V.O.; Carerj, M.L.; Wieters, I.; Fahim, M.; Arendt, C.; Hoffmann, J.; Shchendrygina, A.; Escher, F.; Vasa-Nicotera, M.; Zeiher, A.M.; et al. Outcomes of cardiovascular magnetic resonance imaging in patients recently recovered from Coronavirus Disease 2019 (COVID-19). JAMA Cardiol. 2020, 5, 1265–1273. [Google Scholar] [CrossRef] [PubMed]
- Siripanthong, B.; Nazarian, S.; Muser, D.; Deo, R.; Santangeli, P.; Khanji, M.Y.; Cooper, L.T., Jr.; Chahal, C.A.A. Recognizing COVID-19-related myocarditis: The possible pathophysiology and proposed guideline for diagnosis and management. Heart Rhythm 2020, 17, 1463–1471. [Google Scholar] [CrossRef]
- Bertini, M.; Ferrari, R.; Guardigli, G.; Malagu, M.; Vitali, F.; Zucchetti, O.; D’Aniello, E.; Volta, C.A.; Cimaglia, P.; Piovaccari, G.; et al. Electrocardiographic features of 431 consecutive, critically ill COVID-19 patients: An insight into the mechanisms of cardiac involvement. Europace 2020, 22, 1848–1854. [Google Scholar] [CrossRef] [PubMed]
- Lanza, G.A.; De Vita, A.; Ravenna, S.E.; D’Aiello, A.; Covino, M.; Franceschi, F.; Crea, F. Electrocardiographic findings at presentation and clinical outcome in patients with SARS-CoV-2 infection. Europace 2021, 23, 123–129. [Google Scholar] [CrossRef]
- Bergamaschi, L.; D’Angelo, E.C.; Paolisso, P.; Toniolo, S.; Fabrizio, M.; Angeli, F.; Donati, F.; Magnani, I.; Rinaldi, A.; Bartoli, L.; et al. The value of ECG changes in risk stratification of COVID-19 patients. Ann. Noninvasive Electrocardiol. 2021, 26, e12815. [Google Scholar] [CrossRef]
- Acherjee, T.; Behara, A.; Saad, M.; Vittorio, T.J. Mechanisms and management of prothrombotic state in COVID-19 disease. Ther. Adv. Cardiovasc. Dis. 2021, 15, 17539447211053470. [Google Scholar] [CrossRef]
- Allotey, J.; Stallings, E.; Bonet, M.; Yap, M.; Chatterjee, S.; Kew, T.; Debenham, L.; Llavall, A.C.; Dixit, A.; Zhou, D.; et al. Clinical manifestations, risk factors, and maternal and perinatal outcomes of coronavirus disease 2019 in pregnancy: Living systematic review and meta-analysis. BMJ 2020, 370, m3320. [Google Scholar] [CrossRef]
- Mullins, E.; Hudak, M.L.; Banerjee, J.; Getzlaff, T.; Townson, J.; Barnette, K.; Playle, R.; Perry, A.; Bourne, T.; Lees, C.C.; et al. Pregnancy and neonatal outcomes of COVID-19: Coreporting of common outcomes from PAN-COVID and AAP-SONPM registries. Ultrasound Obstet. Gynecol. 2021, 57, 573–581. [Google Scholar] [CrossRef]
- Di Mascio, D.; Khalil, A.; Saccone, G.; Rizzo, G.; Buca, D.; Liberati, M.; Vecchiet, J.; Nappi, L.; Scambia, G.; Berghella, V.; et al. Outcome of coronavirus spectrum infections (SARS, MERS, COVID-19) during pregnancy: A systematic review and meta-analysis. Am. J. Obstet. Gynecol. MFM 2020, 2, 100107. [Google Scholar] [CrossRef]
- Sisman, J.; Jaleel, M.A.; Moreno, W.; Rajaram, V.; Collins, R.R.J.; Savani, R.C.; Rakheja, D.; Evans, A.S. Intrauterine transmission of SARS-CoV-2 infection in a preterm infant. Pediatr. Infect. Dis. J. 2020, 39, e265–e267. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, D.A. An analysis of 38 pregnant women with COVID-19, their newborn infants, and maternal-fetal transmission of SARS-CoV-2: Maternal coronavirus infections and pregnancy outcomes. Arch. Pathol. Lab. Med. 2020, 144, 799–805. [Google Scholar] [CrossRef] [PubMed]
- Resta, L.; Vimercati, A.; Cazzato, G.; Mazzia, G.; Cicinelli, E.; Colagrande, A.; Fanelli, M.; Scarcella, S.V.; Ceci, O.; Rossi, R. SARS-CoV-2 and placenta: New insights and perspectives. Viruses 2021, 13, 723. [Google Scholar] [CrossRef] [PubMed]
- Del Valle, D.M.; Kim-Schulze, S.; Huang, H.H.; Beckmann, N.D.; Nirenberg, S.; Wang, B.; Lavin, Y.; Swartz, T.H.; Madduri, D.; Stock, A.; et al. An inflammatory cytokine signature predicts COVID-19 severity and survival. Nat. Med. 2020, 26, 1636–1643. [Google Scholar] [CrossRef]
- Schultheiss, H.P.; Fairweather, D.; Caforio, A.L.P.; Escher, F.; Hershberger, R.E.; Lipshultz, S.E.; Liu, P.P.; Matsumori, A.; Mazzanti, A.; McMurray, J.; et al. Dilated cardiomyopathy. Nat. Rev. Dis. Primers 2019, 5, 32. [Google Scholar] [CrossRef]
- Chadda, K.R.; Blakey, E.E.; Huang, C.L.; Jeevaratnam, K. Long COVID-19 and postural orthostatic tachycardia syndrome-is dysautonomia to be blamed? Front. Cardiovasc. Med. 2022, 9, 860198. [Google Scholar] [CrossRef]
- Del Prete, A.; Conway, F.; Della Rocca, D.G.; Biondi-Zoccai, G.; De Felice, F.; Musto, C.; Piciche, M.; Martuscelli, E.; Natale, A.; Versaci, F. COVID-19, acute myocardial injury, and infarction. Card. Electrophysiol. Clin. 2022, 14, 29–39. [Google Scholar] [CrossRef]

| Answered n | n (%) | Mean | SD | |
|---|---|---|---|---|
| Sex | 694 | Male: 411 (59.2) Female: 283 (40.8) | ||
| Country | 695 | Japan: 664 (95.5) Germany: 26 (3.7) Other: 5 (0.7) | ||
| Ethnicity | 688 | Asian: 645 (93.8) Caucasian: 21 (3.1) Hispanic: 13 (1.9) Middle Eastern: 4 (0.6) Black: 2 (0.3) Pacific Islander: 0 (0.0) American Indian/Alaska Native/Indigenous: 0 (0.0) Other: 0 (0.0) Unknown: 3 (0.4) | ||
| Vulnerable Populations | 696 | Elder (>65 years): 219 (31.5) Child (<18 years): 32 (4.6) Pregnant: 17 (2.4) | ||
| Pregnancy Trimester | 17 | 1st: 6 (35.3) 2nd: 2 (11.8) 3rd: 9 (52.9) | ||
| Age | 692 | ≤50 years: 331 (47.8) >50 years: 361 (52.2) | 52.0 | 21.9 |
| Answered n = 672 (467 No history CVD/205 History CVD) a | n (%) | No History CVD n (%) | History CVD n (%) | p-Value b |
|---|---|---|---|---|
| Fever | 498 (74.1) | 352 (75.4) | 146 (71.2) | 0.29 |
| Dry cough | 288 (42.9) | 214 (45.8) | 74 (36.1) | 0.022c |
| Fatigue | 252 (37.5) | 173 (37.0) | 79 (38.5) | 0.73 |
| Loss of smell/taste | 177 (26.3) | 145 (31.0) | 32 (15.6) | <0.0001 |
| Shortness of Breath | 177 (26.3) | 110 (23.6) | 67 (32.7) | 0.017 |
| Sore throat | 161 (24.0) | 114 (24.4) | 47 (22.9) | 0.70 |
| Headache | 152 (22.6) | 113 (24.2) | 39 (19.0) | 0.16 |
| Myalgia | 102 (15.2) | 76 (16.3) | 26 (12.7) | 0.25 |
| Diarrhea | 98 (14.6) | 75 (16.1) | 23 (11.2) | 0.12 |
| Decreased appetite | 92 (13.7) | 53 (11.3) | 39 (19.0) | 0.010 |
| Chills | 57 (8.5) | 34 (7.3) | 23 (11.2) | 0.10 |
| Chest discomfort | 33 (4.9) | 25 (5.4) | 8 (3.9) | 0.56 |
| Nausea | 28 (4.2) | 21 (4.5) | 7 (3.4) | 0.68 |
| Stomach/abdominal pain | 19 (2.8) | 9 (1.9) | 10 (4.9) | 0.043 |
| Vomiting | 12 (1.8) | 10 (2.1) | 2 (1.0) | 0.36 |
| Dizziness/Light-Headedness | 11 (1.6) | 6 (1.3) | 5 (2.4) | 0.32 |
| Palpitations | 3 (0.4) | 1 (0.2) | 2 (1.0) | 0.22 |
| Angina | 3 (0.4) | 1 (0.2) | 2 (1.0) | 0.22 |
| Confusion/Altered mental status | 1 (0.1) | 1 (0.2) | 0 (0.0) | 0.99 |
| Myocarditis | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0.99 |
| Other | 35 (5.2) | 25 (5.4) | 10 (4.9) | 0.85 |
| None | 38 (5.7) | 26 (5.6) | 12 (5.9) | 0.86 |
| Answered n (no History CVD, History CVD) | Condition n (%) | No History CVD n (%) | History CVD n (%) | p-Value a | |
|---|---|---|---|---|---|
| History of Lung Conditions | 659 (466,193) | Asthma: 40 (6.1) | 29 (6.2) | 11 (5.7) | 0.86 |
| COPD: 13 (2.0) | 8 (1.7) | 5 (2.6) | 0.54 | ||
| Lung Cancer: 7 (1.1) | 4 (0.9) | 3 (1.6) | 0.42 | ||
| Interstitial Lung Disease: 3 (0.5) | 1 (0.2) | 2 (1.0) | 0.21 | ||
| Other: 9 (1.4) | 5 (1.1) | 4 (2.1) | 0.46 | ||
| None: 589 (89.4) | 421 (90.3) | 168 (87.0) | 0.21 | ||
| Other Pre-Existing Conditions | 658 (465,193) | Cancer (excl. lung): 34 (5.2) | 14 (3.0) | 20 (10.4) | 0.0003b |
| Allergy/Atopy: 23 (3.5) | 16 (3.4) | 7 (3.6) | >0.99 | ||
| Obesity: 22 (3.3) | 8 (1.7) | 14 (7.3) | 0.0011 | ||
| Chronic Kidney Disease: 10 (1.5) | 2 (0.4) | 8 (4.1) | 0.0012 | ||
| Autoimmune Disease: 9 (1.4) | 5 (1.1) | 4 (2.1) | 0.46 | ||
| Hepatitis C: 6 (0.9) | 3 (0.6) | 3 (1.6) | 0.37 | ||
| HIV: 2 (0.3) | 2 (0.4) | 0 (0.0) | 0.99 | ||
| On immunosuppressive therapy: 1 (0.2) | 1 (0.2) | 0 (0.0) | 0.99 | ||
| Other: 48 (7.3) | 25 (5.4) | 23 (11.9) | 0.0049 | ||
| None: 533 (81.0) | 401 (86.2) | 132 (68.4) | <0.0001 | ||
| History of Diabetes | 662 (463,199) | Type I: 4 (0.6) | 3 (0.6) | 1 (0.5) | 0.99 |
| Type II: 79 (11.9) | 25 (5.4) | 54 (27.1) | <0.0001 | ||
| None: 579 (87.5) | 435 (94.0) | 144 (72.4) | <0.0001 |
| Answered n (no History CVD, History CVD) | All Patients n (%) | No History CVD n (%) | History CVD n (%) | p-Value a | |
|---|---|---|---|---|---|
| Lung Imaging | 677 (471,206) | Chest X-ray: 277 (40.9) | 203 (43.1) | 74 (35.9) | 0.089 |
| CT: 269 (39.7) | 164 (34.8) | 105 (51.0) | 0.0001b | ||
| Both Chest X-ray and CT: 171 (25.3) | 117 (24.8) | 54 (26.2) | 0.70 | ||
| No Imaging: 302 (44.6) | 221 (46.9) | 81 (39.3) | 0.078 | ||
| Lung Imaging Results | 369 (248,121) | Ground glass shadowing: 168 (45.5) | 109 (44.0) | 59 (48.8) | 0.44 |
| Pneumonia: 90 (24.4) | 48 (19.4) | 42 (34.7) | 0.0018 | ||
| Bilateral patchy shadowing: 60 (16.3) | 36 (14.5) | 24 (19.8) | 0.23 | ||
| Local patchy shadowing: 14 (3.8) | 10 (4.0) | 4 (3.3) | 0.99 | ||
| Interstitial infiltrates: 10 (2.7) | 7 (2.8) | 3 (2.5) | 0.99 | ||
| Pleural effusion: 10 (2.7) | 3 (1.2) | 7 (5.8) | 0.017 | ||
| Interstitial abnormalities: 6 (1.6) | 4 (1.6) | 2 (1.7) | 0.99 | ||
| CTR: 1 (0.3) | 1 (0.4) | 0 (0.0) | 0.99 | ||
| Other: 1 (0.3) | 1 (0.4) | 0 (0.0) | 0.99 | ||
| Normal: 98 (26.6) | 86 (34.7) | 12 (9.9) | <0.0001 | ||
| Highest Level of Respiratory Support | 663 (465,198) | Supplemental Oxygen: 115 (17.3) | 57 (12.3) | 58 (29.3) | <0.0001 |
| Mechanical Ventilation/Intubation: 16 (2.4) | 6 (1.3) | 10 (5.1) | 0.0094 | ||
| NIPPV: 13 (2.0) | 6 (1.3) | 7 (3.5) | 0.069 | ||
| VV Extra Corporeal Machine Oxygenation (ECMO): 6 (0.9) | 1 (0.2) | 5 (2.5) | 0.010 | ||
| VA Extra Corporeal Machine Oxygenation (ECMO): 0 (0.0) | 0 (0.0) | 0 (0.0) | 0.99 | ||
| None: 513 (77.4) | 395 (84.9) | 118 (59.6) | <0.0001 |
| Answered n (no History CVD, History CVD) | All Patients n (%) | No History CVD n (%) | History CVD n (%) | p-Value a | |
|---|---|---|---|---|---|
| Mortalityb | 677 (470,207) | 29 (4.3) | 8 (1.7) | 21 (10.1) | <0.0001c |
| Time in the ICU (Days) | 12 (5,7) | 5.7 ± 3.5 | 5.8 ± 4.1 | 5.6 ± 3.3 | 0.92 |
| Time to death after admission (Days) | 26 (5,21) | 18.0 ± 11.2 | 16.0 ± 17.3 | 18.4 ± 9.9 | 0.77 |
| Acute Care Complications | 663 (461,202) | Pneumonia: 91 (13.7) | 38 (8.2) | 53 (26.2) | <0.0001 |
| Acute Respiratory Distress Syndrome (ARDS): 16 (2.4) | 5 (1.1) | 11 (5.4) | 0.0016 | ||
| ICU Admission: 13 (2.0) | 5 (1.1) | 8 (4.0) | 0.028 | ||
| Shock: 6 (0.9) | 1 (0.2) | 5 (2.5) | 0.011 | ||
| Acute Kidney Injury (AKI): 4 (0.6) | 1 (0.2) | 3 (1.5) | 0.087 | ||
| VT Cardiac Arrest: 2 (0.3) | 1 (0.2) | 1 (0.5) | 0.52 | ||
| VF Cardiac Arrest: 2 (0.3) | 1 (0.2) | 1 (0.5) | 0.52 | ||
| PEA Cardiac Arrest: 2 (0.3) | 0 (0.0) | 2 (1.0) | 0.093 | ||
| Other: 5 (0.8) | 2 (0.4) | 3 (1.5) | 0.17 | ||
| None: 554 (83.6) | 415 (90.0) | 139 (68.8) | <0.0001 |
| Answered n (no History CVD, History CVD) | All Patients n (%) | No History CVD n (%) | History CVD n (%) | p-Value a | |
|---|---|---|---|---|---|
| CVD History | 681 (209,472) | Hypertension 159 (23.3) | 0 (0) | 159 (33.7) | <0.0001b |
| Stroke 27 (4.0) | 0 (0) | 27 (5.7) | <0.0001 | ||
| Arrhythmias 24 (3.5) | 0 (0) | 24 (5.1) | 0.0002 | ||
| Coronary Artery Disease/MI 15 (2.2) | 0 (0) | 15 (3.2) | 0.008 | ||
| Heart Failure 9 (1.3) | 0 (0) | 9 (1.9) | 0.06 | ||
| Valvular Heart Disease 5 (0.7) | 0 (0) | 5 (1.1) | 0.33 | ||
| Prior Coronary Artery Bypass Graft 2 (0.3) | 0 (0) | 2 (0.4) | >0.99 | ||
| Myocarditis 1 (0.1) | 0 (0) | 1 (0.2) | >0.99 | ||
| Cardiomyopathy 1 (0.1) | 0 (0) | 1 (0.2) | >0.99 | ||
| Other 53 (7.8) | 0 (0) | 53 (11.2) | <0.0001 | ||
| None 209 (30.7) | 209 (100.0) | 0 (0) | <0.0001 | ||
| Cardiac Decompensation | 643 (459,184) | 3 (0.5) | 0 (0.0) | 3 (1.6) | 0.023 |
| NYHA Class | 614 (441,173) | Class I 32 (5.2) | 17 (3.9) | 15 (8.7) | 0.025 |
| Class II 4 (0.7) | 0 (0.0) | 4 (2.3) | 0.0061 | ||
| Class I/II 36 (5.9) | 17 (3.9) | 19 (11.0) | 0.0017 | ||
| Class III 4 (0.7) | 2 (0.5) | 2 (1.2) | 0.32 | ||
| Class IV 1 (0.2) | 0 (0.0) | 1 (0.6) | 0.28 | ||
| Class III/IV 5 (0.8) | 2 (0.5) | 3 (1.7) | 0.14 | ||
| Unknown 36 (5.9) | 12 (2.7) | 24 (13.9) | <0.0001 | ||
| None 537 (87.5) | 410 (93.0) | 127 (73.4) | <0.0001 | ||
| ECG Performed | 657 (462,195) | 48 (7.3) | 16 (3.5) | 32 (16.4) | <0.0001 |
| ECG Abnormalities | 48 (16,32) | Arrhythmia 7 (14.6) | 1 (6.3) | 6 (18.8) | 0.40 |
| Atrial Fibrillation 5 (10.4) | 0 (0.0) | 5 (15.6) | 0.15 | ||
| ST Elevation 4 (8.3) | 1 (6.3) | 3 (9.4) | 0.99 | ||
| AV Block 3 (6.3) | 0 (0.0) | 3 (9.4) | 0.54 | ||
| Non-sustained Ventricular Tachycardia 2 (4.2) | 1 (6.3) | 1 (3.1) | 0.99 | ||
| QT Prolongation 1 (2.1) | 0 (0.0) | 1 (3.1) | 0.99 | ||
| Abnormal Q 0 (0.0) | 0 (0.0) | 0 (0.0) | 0.99 | ||
| ST Depression 0 (0.0) | 0 (0.0) | 0 (0.0) | 0.99 | ||
| Atrial Flutter 0 (0.0) | 0 (0.0) | 0 (0.0) | 0.99 | ||
| Other 3 (6.3) | 1 (6.3) | 2 (6.3) | 0.99 | ||
| None 26 (54.2) | 13 (81.3) | 13 (40.6) | 0.013 | ||
| LVEF at admission | 8 (6,2) | 60.4 ± 7.5 | 62.0 ± 8.1 | 55.5 ± 0.7 | 0.11 |
| Heart Imaging | 674 (469,205) | Cardiac Echo 12 (1.8) | 6 (1.3) | 6 (2.9) | 0.20 |
| CMR 0 (0.0) | 0 (0.0) | 0 (0.0) | 0.99 | ||
| Cardiac Echo and CMR 0 (0.0) | 0 (0.0) | 0 (0.0) | 0.99 | ||
| No Imaging 662 (98.2) | 463 (98.7) | 199 (97.1) | 0.20 |
| Biomarker | n (no History CVD, History CVD a) | No History CVD Mean ± SD n (%) | History CVD Mean ± SD n (%) | Reference Range | p-Value b |
|---|---|---|---|---|---|
| Troponin T (ng/mL) | 18 (7,11) | 0.02 ± 0.03 7 (38.9) | 0.02 ± 0.01 11 (61.1) | <0.014 | 0.80 |
| Troponin I (ng/mL) | 26 (9,17) | 0.02 ± 0.02 9 (34.6) | 0.1 ± 0.5 17 (65.4) | <0.026 | 0.26 |
| BNP (pg/mL) | 21 (13,8) | 56.2 ± 69.5 13 (61.9) | 136.7 ± 187.7 8 (38.1) | <18.4 | 0.28 |
| NT-proBNP (pg/mL) | 76 (29,47) | 637.7 ± 1940.5 29 (38.2) | 1210.1 ± 1919.1 47 (61.8) | <125 | 0.21 |
| Peak CRP (mg/L) | 287 (184,103) | 24.6 ± 56.8 184 (64.1) | 55.0 ± 63.5 103 (35.9) | <0.14 | 7.98 × 10−5c |
| IL-6 (pg/mL) | 121 (84,37) | 24.8 ± 53.5 84 (69.4) | 45.1 ± 73.8 37 (30.6) | <4 | 0.14 |
| Creatine kinase (U/L) | 238 (178,60) | 169.4 ± 311.5 178 (74.8) | 167.9 ± 237.5 60 (25.2) | 59–248 | 0.97 |
| CK-MB | 30 (14,16) | 20.4 ± 21.7 14 (46.7) | 42.6 ± 87.0 16 (53.3) | <24 | 0.34 |
| D-dimer (mg/L) | 211 (154,57) | 1.3 ± 2.8 154 (72.9) | 6.0 ± 32.0 57 (27.0) | <1 | 0.27 |
| Immunoglobulin free light chains—kappa | 14 (4,10) | 19.6 ± 4.8 4 (28.6) | 33.5 ± 8.4 10 (71.4) | 8.3–27.0 | 0.0032 |
| Immunoglobulin free light chains—lambda | 16 (6,10) | 26.0 ± 15.7 6 (37.5) | 36.3 ± 12.8 10 (62.5) | 6.7–22.4 | 0.21 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matsumori, A.; Auda, M.E.; Bruno, K.A.; Shapiro, K.A.; Kato, T.; Nakamura, T.; Hasegawa, K.; Saleh, A.; Abdelrazek, S.; Negm, H.; et al. Cardiovascular Factors Associated with COVID-19 from an International Registry of Primarily Japanese Patients. Diagnostics 2022, 12, 2350. https://doi.org/10.3390/diagnostics12102350
Matsumori A, Auda ME, Bruno KA, Shapiro KA, Kato T, Nakamura T, Hasegawa K, Saleh A, Abdelrazek S, Negm H, et al. Cardiovascular Factors Associated with COVID-19 from an International Registry of Primarily Japanese Patients. Diagnostics. 2022; 12(10):2350. https://doi.org/10.3390/diagnostics12102350
Chicago/Turabian StyleMatsumori, Akira, Matthew E. Auda, Katelyn A. Bruno, Katie A. Shapiro, Toru Kato, Toshihiro Nakamura, Koji Hasegawa, Ahmed Saleh, Sherif Abdelrazek, Hany Negm, and et al. 2022. "Cardiovascular Factors Associated with COVID-19 from an International Registry of Primarily Japanese Patients" Diagnostics 12, no. 10: 2350. https://doi.org/10.3390/diagnostics12102350
APA StyleMatsumori, A., Auda, M. E., Bruno, K. A., Shapiro, K. A., Kato, T., Nakamura, T., Hasegawa, K., Saleh, A., Abdelrazek, S., Negm, H., Karunawan, N. H., Cooper, L. T., Jr., & Fairweather, D. (2022). Cardiovascular Factors Associated with COVID-19 from an International Registry of Primarily Japanese Patients. Diagnostics, 12(10), 2350. https://doi.org/10.3390/diagnostics12102350






