Ultrasonographical Evaluation of the Median Nerve Mobility in Carpal Tunnel Syndrome: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Information Sources and Search Strategy
2.2. Study Selection
2.3. Data Collection
2.4. Study Risk of Bias Assessment
2.5. Statistical Analysis
3. Results
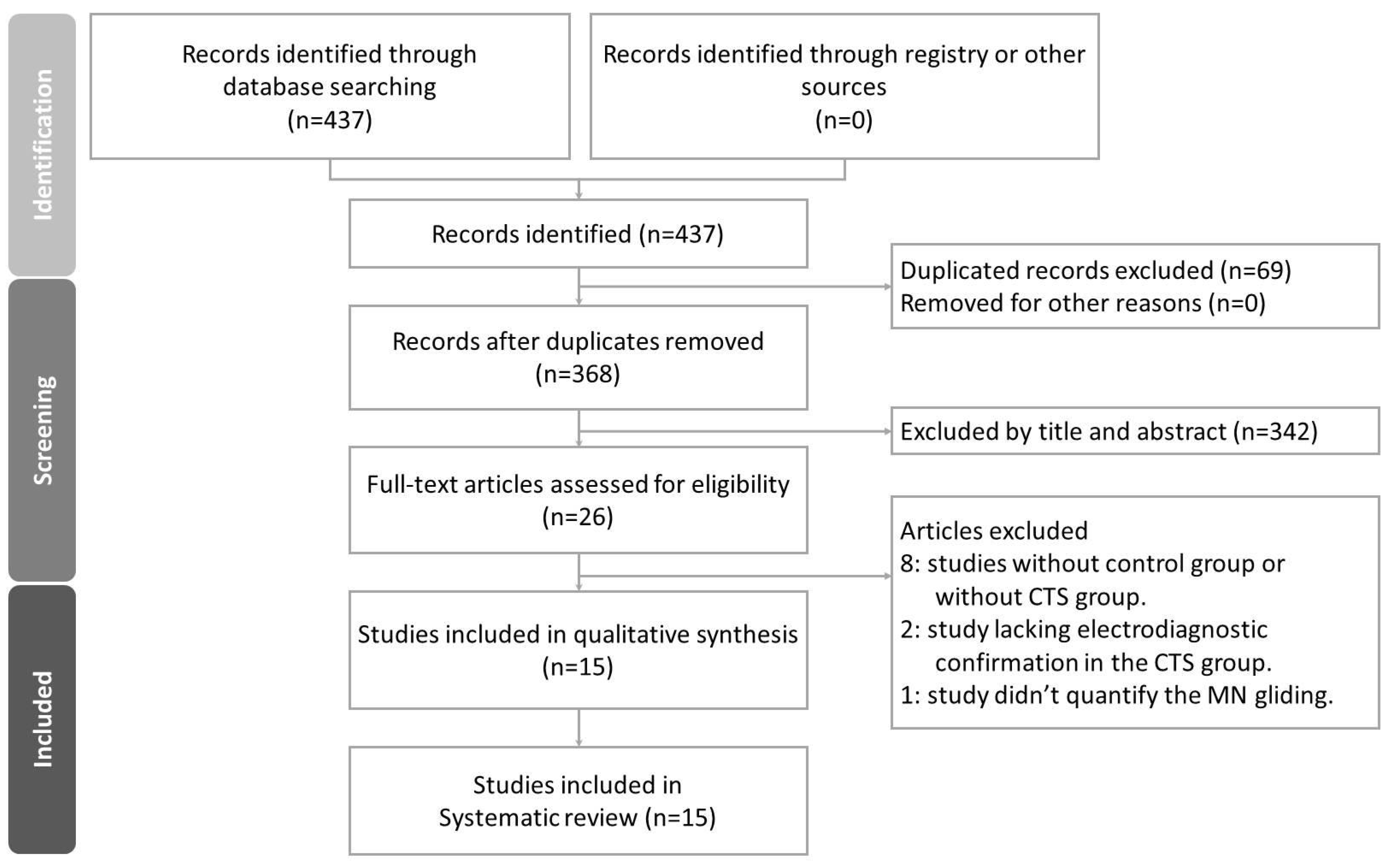
3.1. Study Selection
3.2. Study Characteristics
3.3. Risk of Bias in Studies
3.4. Results of Syntheses
3.4.1. Transverse MN Gliding
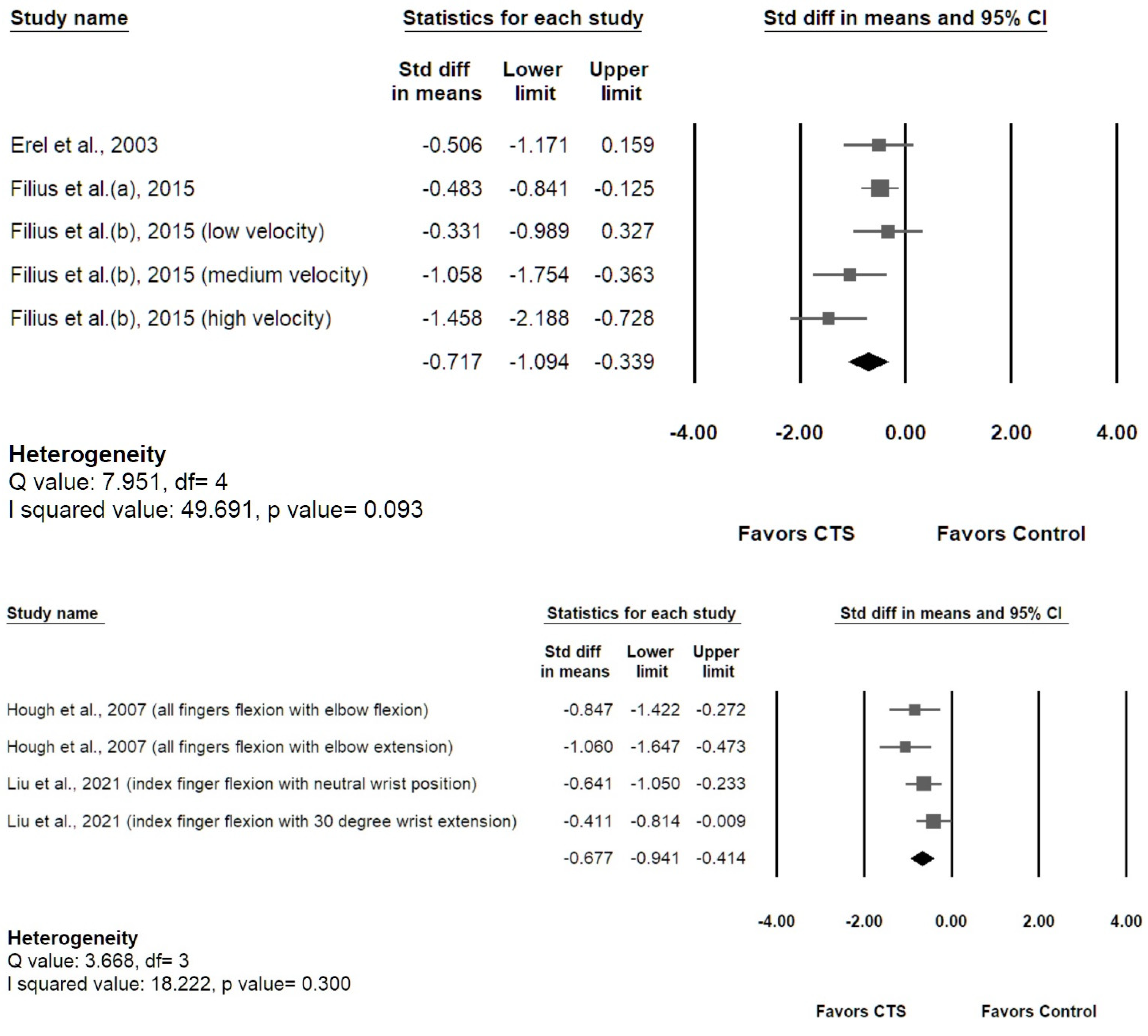
3.4.2. Longitudinal MN Gliding
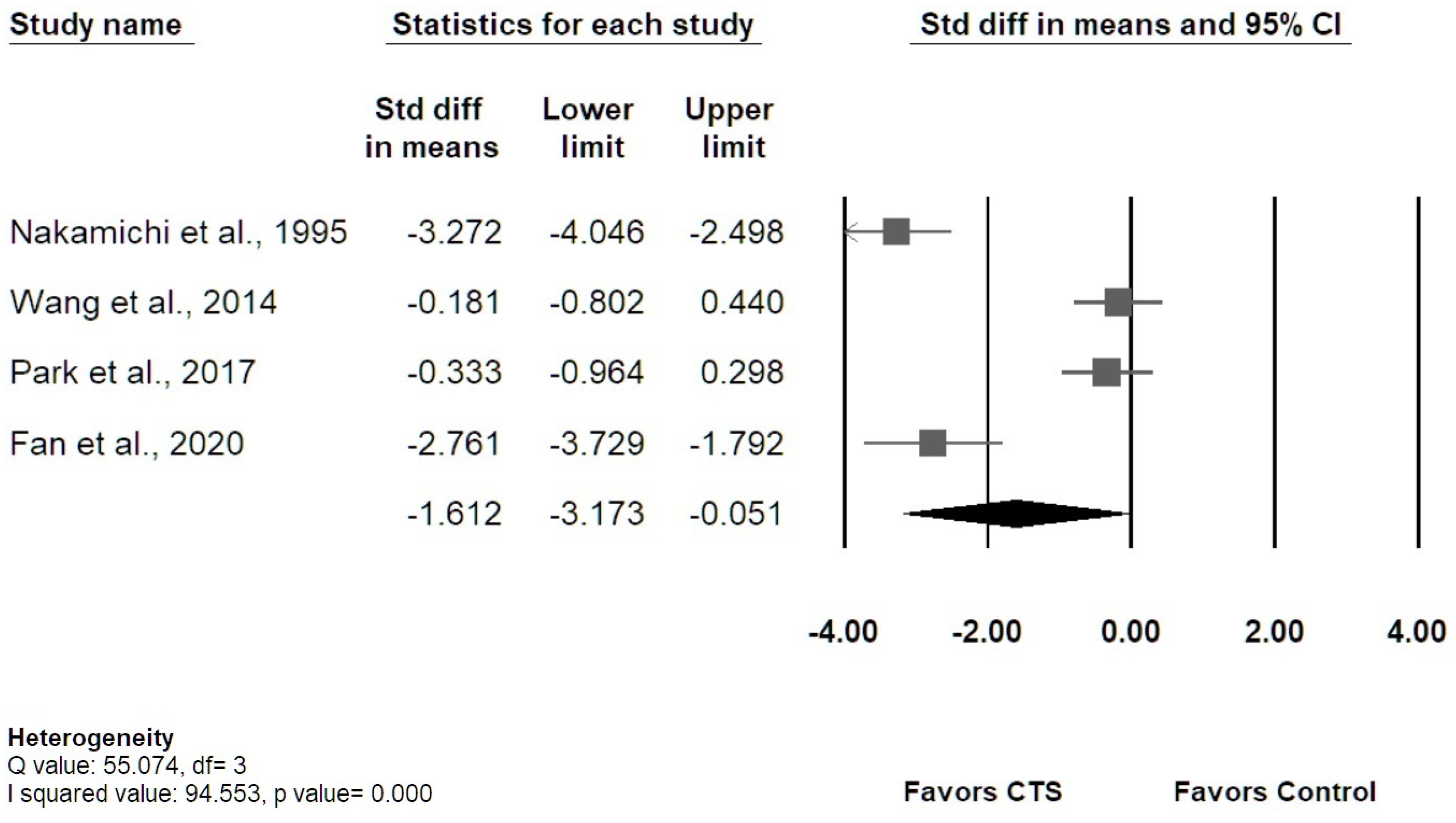
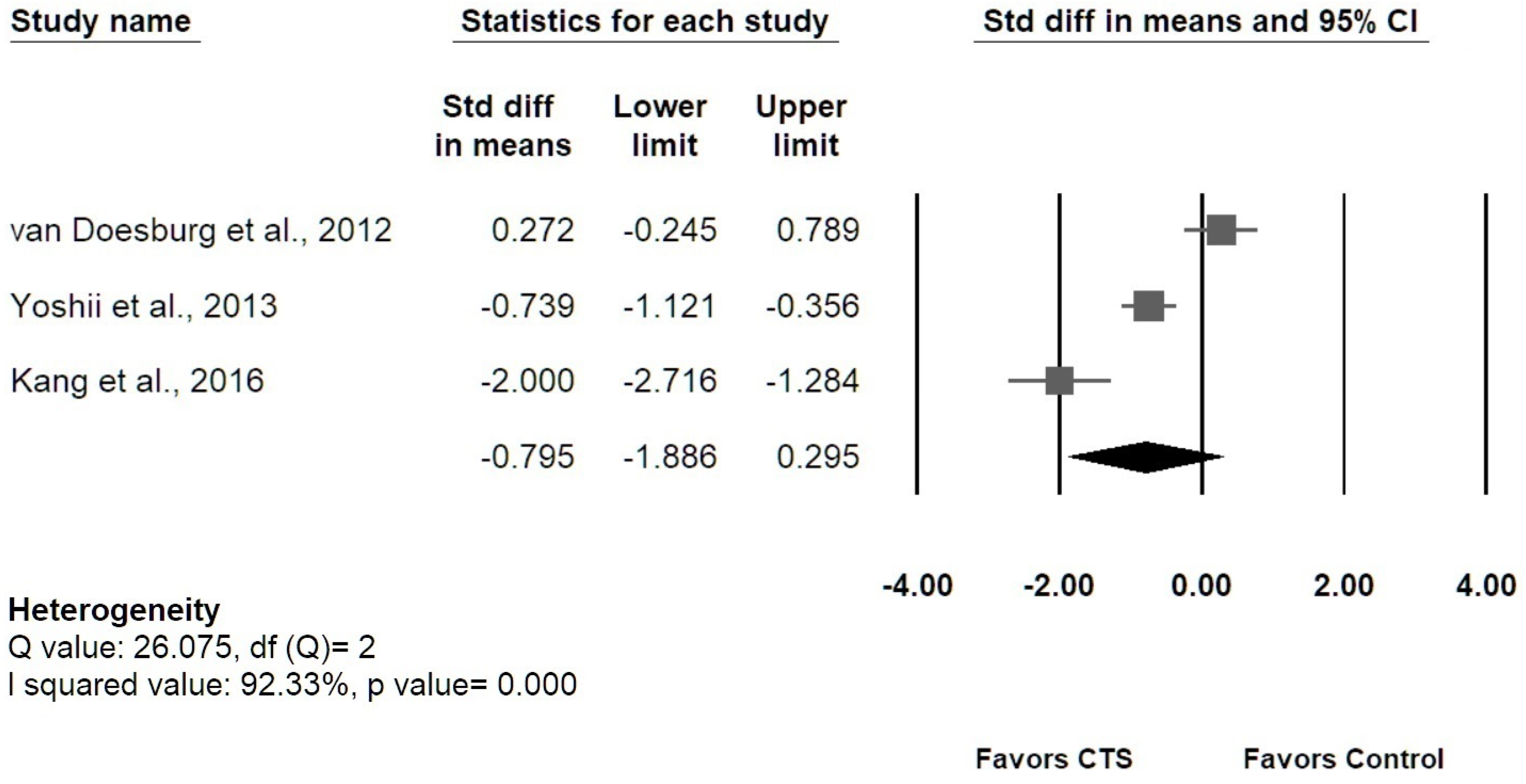
3.5. Results of Meta-Analysis
3.5.1. Transverse MN Displacement
3.5.2. Longitudinal MN Gliding
3.5.3. Sensitivity Analysis
4. Discussion
4.1. Summary of Evidence
4.2. Risk Assessment of Evidence
4.3. Heterogeneity between Studies
4.4. Variability of Measuring Methods
4.5. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CTS | carpal tunnel syndrome |
| MN | median nerve |
| US | ultrasound |
| SSCT | subsynovial connective tissue |
| FDS | flexor digitorum superficialis |
| FDP | flexor digitorum profundus |
| FPL | flexor pollicis longus |
| MCV | maximal change value |
| Tm | trapezium |
| NCSs | nerve conduction studies |
| EMG | electromyography |
| CSA | cross-sectional area |
| DCT | the depth of the carpal tunnel |
| WFR | wrist-forearm ratio |
| WFD | wrist-forearm difference |
| MUR | median-to-ulnar nerve ratio |
| MUD | median-to-ulnar nerve difference |
| DP | dorsopalmar |
| RU | radioulnar |
| DDR | radial deviation ratio |
| RDR | dorsal deviation ratio |
| MAMn | the summed motion area of the MN |
| RMMn | real motion area |
| MR | motion ratio |
| BSP | multilevel block-sum pyramid integrate algorithm |
| CC | cross-correlation algorithm |
| NOS | Newcastle–Ottawa Quality Assessment Scale |
| VTI | velocity-time integral |
| ROI | region of interest |
| CoM | center of mass |
References
- Yoshii, Y.; Villarraga, H.R.; Henderson, J.; Zhao, C.; An, K.-N.; Amadio, P.C. Ultrasound Assessment of the Displacement and Deformation of the Median Nerve in the Human Carpal Tunnel with Active Finger Motion. J. Bone Jt. Surg. 2009, 91, 2922–2930. [Google Scholar] [CrossRef] [PubMed]
- Festen-Schrier, V.; Amadio, P. The biomechanics of subsynovial connective tissue in health and its role in carpal tunnel syndrome. J. Electromyogr. Kinesiol. 2017, 38, 232–239. [Google Scholar] [CrossRef] [PubMed]
- Redmond, M.D.; Rivner, M.H. False positive electrodiagnostic tests in carpal tunnel syndrome. Muscle Nerve 1988, 11, 511–518. [Google Scholar] [CrossRef] [PubMed]
- Grundberg, A.B. Carpal tunnel decompression in site of normal electromyography. J. Hand Surg. 1983, 8, 348–349. [Google Scholar] [CrossRef]
- Wright, S.A. Nerve conduction studies as a routine diagnostic aid in carpal tunnel syndrome. Rheumatology 2003, 42, 602–603. [Google Scholar] [CrossRef] [PubMed][Green Version]
- AAEM Quality Assurance Committee; Jablecki, C.K.; Andary, C.M.T.; So, Y.T.; Wilkins, D.E.; Williams, F.H. Literature review of the usefulness of nerve conduction studies and electromyography for the evaluation of patients with carpal tunnel syndrome. Muscle Nerve 1993, 16, 1392–1414. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-T.; Williams, L.; Zak, M.J.; Fredericson, M. Review of Ultrasonography in the Diagnosis of Carpal Tunnel Syndrome and a Proposed Scanning Protocol. J. Ultrasound Med. 2016, 35, 2311–2324. [Google Scholar] [CrossRef]
- Visser, L.H.; Smidt, M.H.; Lee, M.L. High-resolution sonography versus EMG in the diagnosis of carpal tunnel syndrome. J. Neurol. Neurosurg. Psychiatry 2008, 79, 63–67. [Google Scholar] [CrossRef]
- Buchberger, W.; Judmaier, W.; Birbamer, G.; Lener, M.; Schmidauer, C. Carpal tunnel syndrome: Diagnosis with high-resolution sonography. Am. J. Roentgenol. 1992, 159, 793–798. [Google Scholar] [CrossRef]
- Elsaman, A.M.M.Y.; Thabit, M.N.; Radwan, A.R.A.-A.; Ohrndorf, S. Idiopathic Carpal Tunnel Syndrome: Evaluation of the Depth of the Carpal Tunnel by Ultrasonography. Ultrasound Med. Biol. 2015, 41, 2827–2835. [Google Scholar] [CrossRef]
- Gonzalez-Suarez, C.B.; Fidel, B.C.; Cabrera, J.T.C.; Cruz, F.C.D.; Gesmundo, M.V.T.; Regala, C.F.G.; Saratan, R.; Suarez, C.G.; Grimmer, K. Diagnostic Accuracy of Ultrasound Parameters in Carpal Tunnel Syndrome: Additional Criteria for Diagnosis. J. Ultrasound Med. 2019, 38, 3043–3052. [Google Scholar] [CrossRef]
- Hunderfund, A.N.L.; Boon, A.J.; Mandrekar, J.N.; Sorenson, E.J. Sonography in carpal tunnel syndrome. Muscle Nerve 2011, 44, 485–491. [Google Scholar] [CrossRef]
- Elhabashy, H.; Elhadidy, R.; Ahmed, S.; El Sayed, B.; Ahmed, A.S. Carpal Tunnel Syndrome Grading Using High-Resolution Ultrasonography. J. Clin. Neurophysiol. 2017, 34, 353–358. [Google Scholar] [CrossRef]
- Billakota, S.; Hobson-Webb, L.D. Standard median nerve ultrasound in carpal tunnel syndrome: A retrospective review of 1,021 cases. Clin. Neurophysiol. Pr. 2017, 2, 188–191. [Google Scholar] [CrossRef]
- Chang, Y.-W.; Hsieh, T.-C.; Tzeng, I.-S.; Chiu, V.; Huang, P.-J.; Horng, Y.-S. Ratio and difference of the cross-sectional area of median nerve to ulnar nerve in diagnosing carpal tunnel syndrome: A case control study. BMC Med. Imaging 2019, 19, 52. [Google Scholar] [CrossRef]
- Kluge, S.; Langer, M.; Schelle, T. Sonographic Diagnosis of Carpal Tunnel Syndrome. Hand Clin. 2021, 38, 35–53. [Google Scholar] [CrossRef]
- Chang, Y.-W.; Chen, C.-J.; Wang, Y.-W.; Chiu, V.; Lin, S.-K.; Horng, Y.-S. Influence of temperature on sonographic images of the median nerve for the diagnosis of carpal tunnel syndrome: A case control study. BMC Med. Imaging 2021, 21, 163. [Google Scholar] [CrossRef]
- Abdelraouf, M.M.; Ahmed, A.I.; Elghitany, N.A. Sono-Elastography in evaluation of the median nerve in carpal tunnel syndrome patients. QJM Int. J. Med. 2021, 114. [Google Scholar] [CrossRef]
- Lee, C.-H.; Lin, Y.-T.; Wu, C.-T.; Lin, C.-H.; Chen, S.-H.; Hsu, C.-C. Sonoelastography in the Diagnosis of Carpal Tunnel Syndrome. Ann. Plast. Surg. 2021, 86, S299–S311. [Google Scholar] [CrossRef]
- Kuo, T.-T.; Lee, M.-R.; Liao, Y.-Y.; Chen, J.-P.; Hsu, Y.-W.; Yeh, C.-K. Assessment of Median Nerve Mobility by Ultrasound Dynamic Imaging for Diagnosing Carpal Tunnel Syndrome. PLoS ONE 2016, 11, e0147051. [Google Scholar] [CrossRef]
- Ellis, R.; Blyth, R.; Arnold, N. Is there a relationship between impaired median nerve excursion and carpal tunnel syndrome? A systematic review. Man. Ther. 2016, 25, e75. [Google Scholar] [CrossRef]
- Park, G.-Y.; Kwon, D.R.; Seok, J.I.; Park, D.-S.; Cho, H.K. Usefulness of ultrasound assessment of median nerve mobility in carpal tunnel syndrome. Acta Radiol. 2018, 59, 1494–1499. [Google Scholar] [CrossRef]
- Uchiyama, S.; Itsubo, T.; Nakamura, K.; Kato, H.; Yasutomi, T.; Momose, T. Current concepts of carpal tunnel syndrome: Pathophysiology, treatment, and evaluation. J. Orthop. Sci. 2010, 15, 1–13. [Google Scholar] [CrossRef]
- Armstrong, T.J.; A Castelli, W.; Evans, F.G.; Diaz-Perez, R. Some histological changes in carpal tunnel contents and their biomechanical implications. J. Occup. Med. Off. Publ. Ind. Med Assoc. 1984, 26, 197–201. [Google Scholar]
- Ettema, A.M.; Amadio, P.C.; Zhao, C.; Wold, L.E.; An, K.-N. A histological and immunohistochemical study of the subsynovial connective tissue in idiopathic carpal tunnel syndrome. JBJS 2004, 86, 1458–1466. [Google Scholar] [CrossRef]
- Oh, J.; Zhao, C.; Zobitz, M.E.; Wold, L.E.; An, K.-N.; Amadio, P.C. Morphological Changes of Collagen Fibrils in the Subsynovial Connective Tissue in Carpal Tunnel Syndrome. J. Bone Jt. Surg. 2006, 88, 824–831. [Google Scholar] [CrossRef]
- Osamura, N.; Zhao, C.; Zobitz, M.E.; An, K.-N.; Amadio, P.C. Evaluation of the material properties of the subsynovial connective tissue in carpal tunnel syndrome. Clin. Biomech. 2007, 22, 999–1003. [Google Scholar] [CrossRef]
- Robben, E.; Dever, J.; De Groef, A.; Degreef, I.; Peers, K. Subsynovial connective tissue thickness in carpal tunnel syndrome: A systematic review. Clin. Biomech. 2020, 75, 105002. [Google Scholar] [CrossRef] [PubMed]
- Erel, E.; Dilley, A.; Greening, J.; Morris, V.; Cohen, B.; Lynn, B. Longitudinal sliding of the median nerve in patients with carpal tunnel syndrome. J. Hand Surg. 2003, 28, 439–443. [Google Scholar] [CrossRef]
- Hough, A.D.; Moore, A.P.; Jones, M.P. Reduced Longitudinal Excursion of the Median Nerve in Carpal Tunnel Syndrome. Arch. Phys. Med. Rehabilitation 2007, 88, 569–576. [Google Scholar] [CrossRef] [PubMed]
- Filius, A.; Scheltens, M.; Bosch, H.G.; Van Doorn, P.A.; Stam, H.J.; Hovius, S.E.R.; Amadio, P.C.; Selles, R.W. Multidimensional ultrasound imaging of the wrist: Changes of shape and displacement of the median nerve and tendons in carpal tunnel syndrome. J. Orthop. Res. 2015, 33, 1332–1340. [Google Scholar] [CrossRef]
- Filius, A.; Thoreson, A.R.; Wang, Y.; Passe, S.M.; Zhao, C.; An, K.-N.; Amadio, P.C. The effect of tendon excursion velocity on longitudinal median nerve displacement: Differences between carpal tunnel syndrome patients and controls. J. Orthop. Res. 2015, 33, 483–487. [Google Scholar] [CrossRef]
- Liu, C.-T.; Liu, D.-H.; Chen, C.-J.; Wang, Y.-W.; Wu, P.-S.; Horng, Y.-S. Effects of wrist extension on median nerve and flexor tendon excursions in patients with carpal tunnel syndrome: A case control study. BMC Musculoskelet. Disord. 2021, 22, 477. [Google Scholar] [CrossRef]
- Msc, J.H.K.; Boer, M.S.-D.; Blok, J.H.; Amadio, P.C.; Hovius, S.E.; Stam, H.J.; Selles, R.W. Ultrasonographic assessment of longitudinal median nerve and hand flexor tendon dynamics in carpal tunnel syndrome. Muscle Nerve 2011, 45, 721–729. [Google Scholar] [CrossRef]
- Lopes, M.M.; Lawson, W.; Scott, T.; Keir, P. Tendon and nerve excursion in the carpal tunnel in healthy and CTD wrists. Clin. Biomech. 2011, 26, 930–936. [Google Scholar] [CrossRef]
- Wang, Y.; Filius, A.; Zhao, C.; Passe, S.M.; Thoreson, A.R.; An, K.-N.; Amadio, P.C. Altered Median Nerve Deformation and Transverse Displacement during Wrist Movement in Patients with Carpal Tunnel Syndrome. Acad. Radiol. 2014, 21, 472–480. [Google Scholar] [CrossRef]
- Nakamichi, K.; Tachibana, S. Restricted Motion of the Median Nerve in Carpal Tunnel Syndrome. J. Hand Surg. 1995, 20, 460–464. [Google Scholar] [CrossRef]
- Van Doesburg, M.H.M.; Henderson, J.; Van Der Molen, A.B.M.; An, K.-N.; Amadio, P.C. Transverse Plane Tendon and Median Nerve Motion in the Carpal Tunnel: Ultrasound Comparison of Carpal Tunnel Syndrome Patients and Healthy Volunteers. PLoS ONE 2012, 7, e37081. [Google Scholar] [CrossRef]
- Yoshii, Y.; Ishii, T.; Tung, W.-L.; Sakai, S.; Amadio, P.C. Median nerve deformation and displacement in the carpal tunnel during finger motion. J. Orthop. Res. 2013, 31, 1876–1880. [Google Scholar] [CrossRef]
- Yoshii, Y.; Ishii, T.; Sakai, S. Median nerve deformation during finger motion in carpal tunnel syndrome: Correlation between nerve conduction and ultrasonographic indices. Hand Surg. 2013, 18, 203–208. [Google Scholar] [CrossRef] [PubMed]
- Nanno, M.; Sawaizumi, T.; Kodera, N.; Tomori, Y.; Takai, S. Transverse Movement of the Median Nerve in the Carpal Tunnel during Wrist and Finger Motion in Patients with Carpal Tunnel Syndrome. Tohoku J. Exp. Med. 2015, 236, 233–240. [Google Scholar] [CrossRef]
- Kang, H.J.; Yoon, J.S. Effect of finger motion on transverse median nerve movement in the carpal tunnel. Muscle Nerve 2016, 54, 738–742. [Google Scholar] [CrossRef] [PubMed]
- Park, D. Ultrasonography of the Transverse Movement and Deformation of the Median Nerve and Its Relationships With Electrophysiological Severity in the Early Stages of Carpal Tunnel Syndrome. PM&R 2017, 9, 1085–1094. [Google Scholar] [CrossRef]
- Fan, C.; Fede, C.; Pirri, C.; Guidolin, D.; Biz, C.; Macchi, V.; De Caro, R.; Stecco, C. Quantitative Evaluation of the Echo Intensity of Paraneural Area and Myofascial Structure around Median Nerve in Carpal Tunnel Syndrome. Diagnostics 2020, 10, 914. [Google Scholar] [CrossRef] [PubMed]
- Nakamichi, K.; Tachibana, S. Transverse Sliding of the Median Nerve Beneath the Flexor Retinaculum. J. Hand Surg. 1992, 17, 213–216. [Google Scholar] [CrossRef]
- Toge, Y.; Nishimura, Y.; Basford, J.R.; Nogawa, T.; Yamanaka, M.; Nakamura, T.; Yoshida, M.; Nagano, A.; Tajima, F. Comparison of the Effects of Flexion and Extension of the Thumb and Fingers on the Position and Cross-Sectional Area of the Median Nerve. PLoS ONE 2013, 8, e83565. [Google Scholar] [CrossRef][Green Version]
- Ugbolue, U.C.; Hsu, W.-H.; Goitz, R.J.; Li, Z.-M. Tendon and nerve displacement at the wrist during finger movements. Clin. Biomech. 2005, 20, 50–56. [Google Scholar] [CrossRef]
- Stecco, C.; Giordani, F.; Fan, C.; Biz, C.; Pirri, C.; Frigo, A.C.; Fede, C.; Macchi, V.; Masiero, S.; De Caro, R. Role of fasciae around the median nerve in pathogenesis of carpal tunnel syndrome: Microscopic and ultrasound study. J. Anat. 2019, 236, 660–667. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, C.; Passe, S.M.; Filius, A.; Thoreson, A.R.; An, K.-N.; Amadio, P.C. Transverse Ultrasound Assessment of Median Nerve Deformation and Displacement in the Human Carpal Tunnel during Wrist Movements. Ultrasound Med. Biol. 2013, 40, 53–61. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Cumpston, M.; Li, T.; Page, M.J.; Chandler, J.; Welch, V.A.; Higgins, J.P.; Thomas, J. Updated guidance for trusted systematic reviews: A new edition of the Cochrane Handbook for Systematic Reviews of Interventions. Cochrane Database Syst. Rev. 2019, 10, Ed000142. [Google Scholar] [CrossRef]
- Korstanje, J.-W.H.; Selles, R.W.; Stam, H.J.; Hovius, S.E.; Bosch, J.G. Development and validation of ultrasound speckle tracking to quantify tendon displacement. J. Biomech. 2010, 43, 1373–1379. [Google Scholar] [CrossRef]
- Li, P.-C.; Lee, W.-N. An efficient speckle tracking algorithm for ultrasonic imaging. Ultrason. Imaging 2002, 24, 215–228. [Google Scholar] [CrossRef]
- Hara, Y.; Tajiri, Y.; Kawano, K.; Hoshikawa, S. Evaluation of Restricted Motion Area of the Median Nerve in Patients with Carpal Tunnel Syndrome: A New Measurement Method Using an Ultrasonographic Video Image. J. Hand Surg. (Asian-Pac. Vol.) 2021, 26, 635–643. [Google Scholar] [CrossRef]
- Hough, A.; Moore, A.; Jones, M. Measuring longitudinal nerve motion using ultrasonography. Man. Ther. 2000, 5, 173–180. [Google Scholar] [CrossRef]
- Ettema, A.M.; An, K.-N.; Zhao, C.; O’Byrne, M.M.; Amadio, P.C. Flexor tendon and synovial gliding during simultaneous and single digit flexion in idiopathic carpal tunnel syndrome. J. Biomech. 2008, 41, 292–298. [Google Scholar] [CrossRef]
- Ortiz SH, C.; Chiu, T.; Fox, M.D. Ultrasound image enhancement: A review. Biomed. Signal Process. Control 2012, 7, 419–428. [Google Scholar] [CrossRef]
- Karaoğlu, O.; Bilge, H.; Uluer, I. Removal of speckle noises from ultrasound images using five different deep learning networks. Eng. Sci. Technol. Int. J. 2022, 29, 101030. [Google Scholar] [CrossRef]
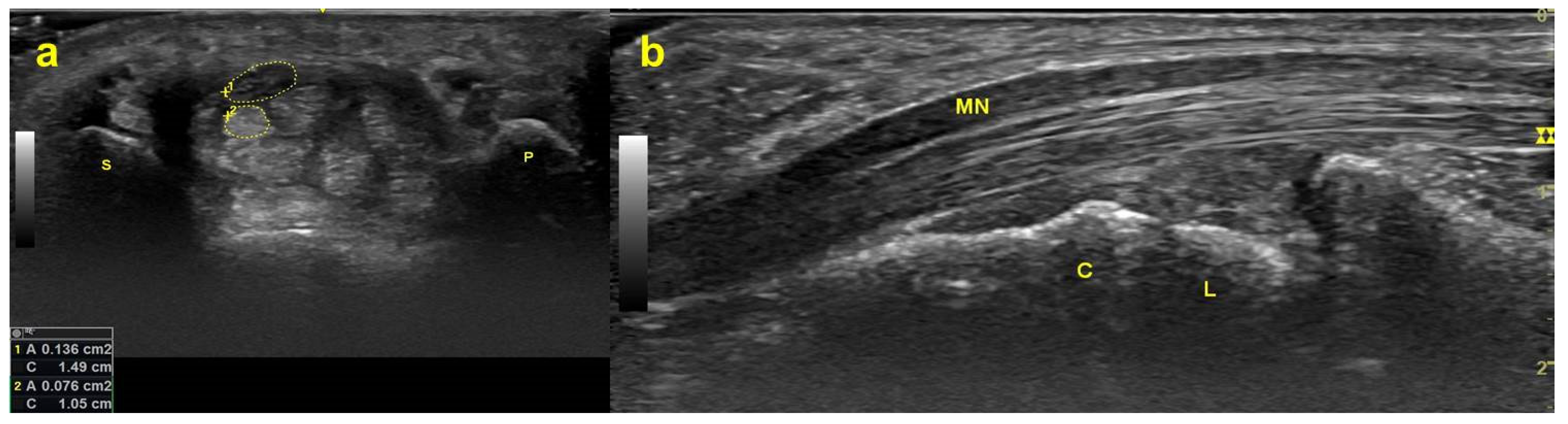
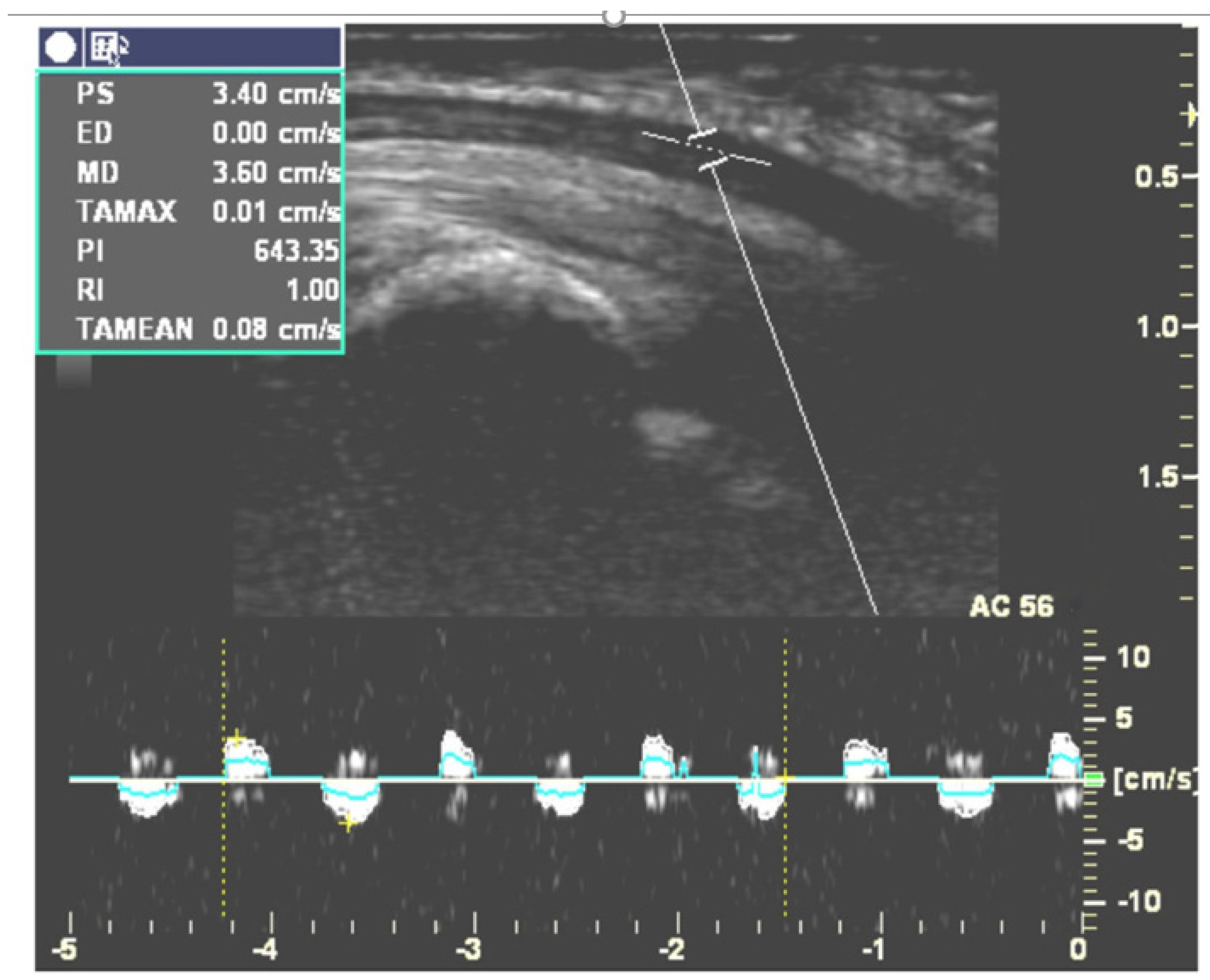

| Study | Wrists (CTS/Control) | Age (mean ± SD) | Gender (M, F) | Measuring Site (Transverse/Longitudinal) | US Image Analysis | Postures | Outcome |
|---|---|---|---|---|---|---|---|
| Nakamichi et al. [37] | 30/30 | 54.15 ± 2.02 | female only | proximal CT | Initial and final recorded frames of motion cycle | Passive/2nd finger/PIP and DIP joints/full flexion-extension | Transverse MN displacement |
| Erel et al. [29] | 17/19 | 42.95 ± 9.56 | 5, 31 | proximal CT/5–15 cm from the distal wrist crease | Transverse plane: Initial and final recorded frames of motion cycle Longitudinal plane: CC algorithm | Passive/MCP joint/90 degree flexion to neutral | Longitudinal MN gliding Transverse displacement of radial nerve, CSA, and AR |
| Hough et al. [30] | 19/37 | 51.19 ± 12.62 | 16, 40 | lunate-capitate intercarpal joint | Duplex Doppler | Active/all fingers/full flexion-extension | Longitudinal MN and FDS gliding with elbow flexion and extension |
| van Doesburg et al. [38] | 29/29 | 43.3 ± 13.59 | 25, 33 | proximal CT | Initial and final recorded frames of motion cycle | Active/ 4 motions: 1st; 2nd; 3rd finger flexion; full flexion of four fingers | Transverse MN displacement along DP and RU axis, the distance between MN and the tendons |
| Yoshii et al. [39] | 51/62 | 51.39 ± 13.85 | 11, 48 | proximal CT | Initial and final recorded frames of motion cycle | Active/all fingers/full extension to flexion | Transverse MN displacement along DP and RU axis, CSA, perimeter, AR, and circularity |
| Wang et al. [36] | 20/20 | 45.77 ± 8.55 | 10, 13 | proximal CT | Initial and final recorded frames of motion cycle | Active/6 motions: finger flexion, wrist flexion with fingers extended, wrist flexion with fingers flexed, wrist extension with fingers extended, wrist extension with fingers flexed, and wrist ulnar deviation with fingers extended | Transverse DR and MN displacement (described as vector and magnitude) normalized to hand length (tip of middle finger to midline of distal wrist crease) |
| Nanno et al. [41] | 21/21 | 69 ± 12.25 | 5, 16 | proximal CT | Initial and final recorded frames of motion cycle | Active/all five fingers/with 5 wrist position (neutral, dorsal flexion, palmar flexion, ulnar deviation and radial deviation)/full extension-flexion | Transverse RDR and DDR |
| Filius et al. (a) [31] | 113/42 | 41.71 ± 12.18 | 41, 114 | proximal CT | Speckle-tracking algorithm | Active/all five fingers/full flexion in 8 s | Longitudinal gliding of MN, FDS3, FDP3, SSCT, displacement ratios of the MN and tendons Transverse plane: area, perimeter, circularity, DR, CoM |
| Filius et al. (b) [32] | 25/14 | 46.5 ± 12.64 | 13, 19 | proximal CT l | Speckle-tracking algorithm | Active/all five fingers/full extension to flexion/within 8, 4, and 2 s | Longitudinal gliding of MN, FDS3, FDP3 in low, medium, and high velocity |
| Kuo et al. [20] | 40/32 | - | - | proximal CT | Speckle-tracking algorithm (BSP) | Active/all five fingers/flexion- extension cycles | Transverse plane: R square, curvature, amplitude of MN displacement |
| Kang et al. [42] | 22/23 | 55.82 ± 2.30 | female only | proximal CT | Initial and final recorded frames of motion cycle | Active/4 motions: First, second, third finger full flexion and grip motion | Transverse MN displacement along DP and RU axis |
| Park et al. [43] | 39/13 | 60.50 ± 11.57 | 15, 22 | proximal CT | Initial and final recorded frames of motion cycle | Active/ Maximal voluntary motion of 6 motions (1st, 2nd, 3rd finger flexion, grasp, wrist ulnar deviation with finger extension, wrist radial deviation with finger extension) | Transverse MN MCV and relative to wrist width, CSA, and AR |
| Fan et al. [44] | 16/16 | 61.29 ± 13.71 | 14, 18 | proximal CT and mid-forearm | Initial and final recorded frames of motion cycle | Active/all five fingers/full extension to flexion | Transverse plane: echo intensity of the paraneural area, MN and myofascial structure; MN displacement |
| Liu et al. [33] | 49/48 | 49.70 ± 9.46 | 11, 86 | pisiform level/lunate- capitate intercarpal joint | Duplex Doppler | Active/2nd finger/MCP and proximal IP joints/full extension to flexion/in the neutral and 30 degree extension of wrist/at speed of 1 cycle per sec | Longitudinal MN gliding Transverse MN CSA, FR |
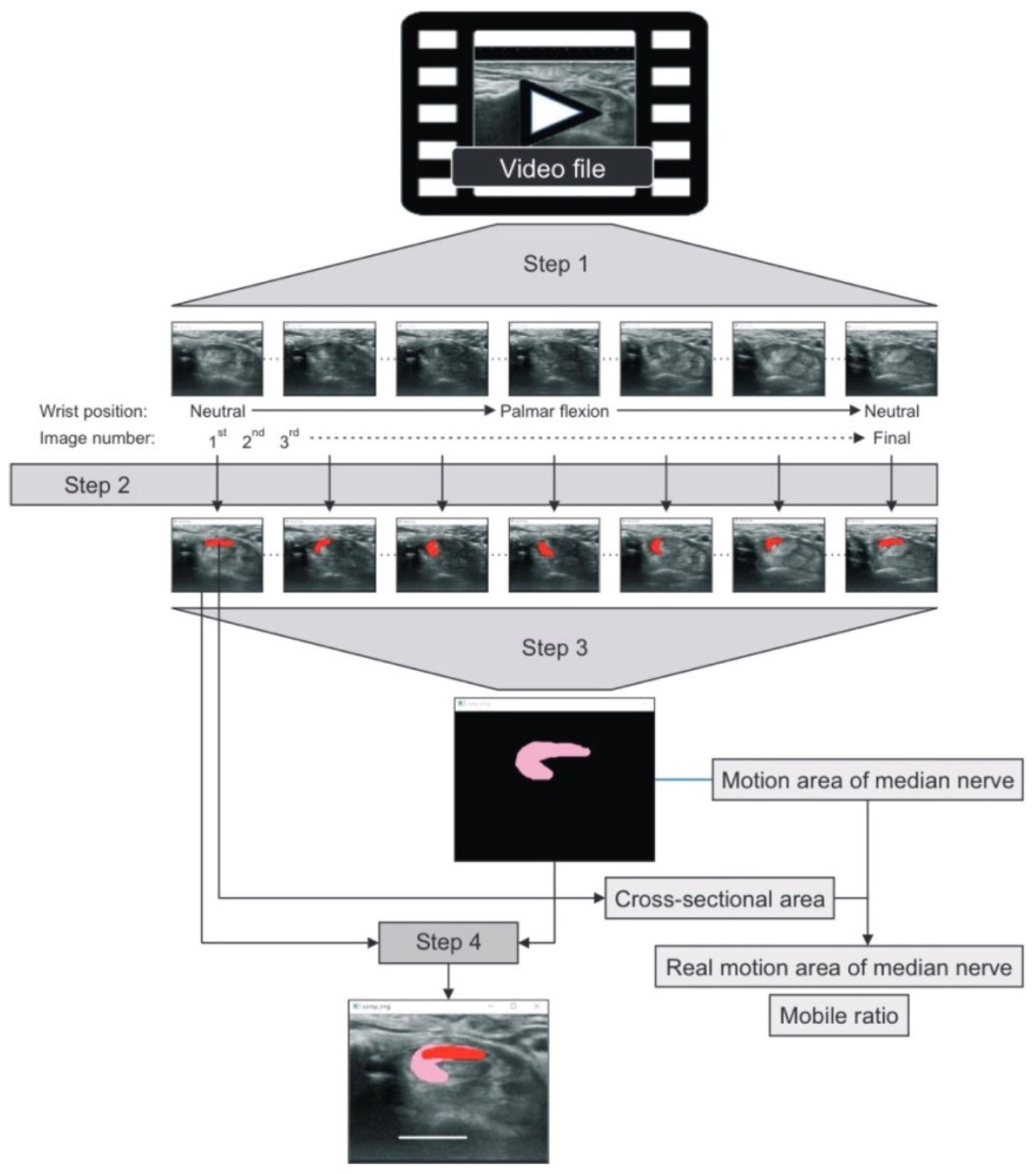
| Hara et al. [54] | 6/6 | 68.42 ± 11.88 | 1, 11 | tubercle of Tm | Composite image created from audio-video interleave file | Passive/ wrist joint/ neutral to full palmar flexion | Transverse plane: CSA, MAMn, RMMn, MR |
| Study | Selection | Comparability Control for Important Factor | Exposure | Score | |||||
|---|---|---|---|---|---|---|---|---|---|
| Adequate Definition of Cases | Representativeness of the Cases | Selection of Controls | Definition of Controls | Ascertainment of Exposure | Same Method of Ascertainment for Cases & Controls | Non-Response Rate | |||
| Nakamichi et al. [37] | ✩ | ✩ | ✩ | ✩ | ✩✩ | ✩ | ✩ | ✩ | 9 |
| Erel et al. [29] | ✩ | ✩ | ✩ | ✩ | ✩✩ | - | ✩ | ✩ | 8 |
| Hough et al. [30] | ✩ | ✩ | ✩ | ✩ | ✩ | ✩ | ✩ | ✩ | 8 |
| van Doesburg et al. [38] | ✩ | ✩ | ✩ | ✩ | ✩ | - | ✩ | ✩ | 7 |
| Yoshii et al. [39] | ✩ | ✩ | ✩ | ✩ | ✩ | - | ✩ | ✩ | 7 |
| Wang et al. [36] | ✩ | ✩ | ✩ | ✩ | ✩ | - | ✩ | ✩ | 7 |
| Nanno et al. [41] | ✩ | ✩ | ✩ | ✩ | ✩ | - | ✩ | ✩ | 7 |
| Filius et al. (a) [31] | ✩ | ✩ | ✩ | ✩ | ✩ | - | ✩ | ✩ | 7 |
| Filius et al. (b) [32] | ✩ | ✩ | ✩ | ✩ | ✩ | - | ✩ | ✩ | 7 |
| Kuo et al. [20] | ✩ | ✩ | ✩ | ✩ | ✩ | - | ✩ | ✩ | 7 |
| Kang et al. [42] | ✩ | ✩ | ✩ | ✩ | ✩✩ | - | ✩ | ✩ | 8 |
| Park et al. [43] | ✩ | ✩ | ✩ | ✩ | ✩ | - | ✩ | ✩ | 7 |
| Fan et al. [44] | ✩ | ✩ | ✩ | ✩ | ✩✩ | - | ✩ | ✩ | 8 |
| Liu et al. [33] | ✩ | ✩ | ✩ | ✩ | ✩ | ✩ | ✩ | ✩ | 8 |
| Hara et al. [54] | ✩ | ✩ | ✩ | ✩ | ✩ | - | ✩ | ✩ | 7 |
| Study | CTS (Mean ± SD) | Control (Mean ± SD) | p Value |
|---|---|---|---|
| Nakamichi et al. [37] | |||
| Index finger flexion (mm) | 0.37 ± 0.34 | 1.75 ± 0.49 | 0.0001 |
| Erel et al. [28] | RU | RU | |
| MCP joint flexion (mm) | 0.89 ± 1.15 | 1.55 ± 1.04 | >0.08 |
| van Doesburg et al. [38] | DP*; RU* | DP*; RU* | |
| First finger flexion (mm) | −0.10 ± 0.21;-0.63 ± 0.76 | 0.02 ± 0.23; 0.17 ± 0.84 | <0.05; <0.05 |
| Second finger flexion (mm) | 0.13 ± 0.31; 1.25 ± 1.43 | 0.04 ± 0.35; 0.49 ± 1.61 | >0.05; <0.038 |
| Third finger flexion (mm) | 0.19 ± 0.33; 1.90 ± 1.64 | 0.09 ± 0.38; 1.13 ± 2.13 | >0.05; <0.0001 |
| Four fingers flexion (mm) | 0.09 ± 0.39; 1.63 ± 2.29 | 0.18 ± 0.39; 1.40 ± 1.95 | >0.05; >0.05 |
| Yoshii et al. [39] | DP*; RU* | DP*; RU* | |
| All fingers flexion (mm) | 0.069 ± 0.438; 2.05 ± 2.82 | 0.377 ± 0.399; 2.45 ± 1.76 | 0.06; <0.01 |
| Wang et al. [36] | |||
| Fingers flex. with neutral wrist postion (NU) | Vector; Magnitude | Vector; Magnitude | |
| Wrist flex. with fingers ext. (NU) | 0.1; 0.75 ± 0.44 | 0.2; 0.82 ± 0.33 | 0.217; >0.05 |
| Wrist flex. with fingers flex. (NU) | 0.8; 1.74 ± 0.78 | 1.5; 2.36 ± 0.79 | <0.01; 0.016 |
| Wrist ext. with fingers ext. (NU) | 1.0; 1.71 ± 0.90 | 1.8; 2.46 ± 0.84 | <0.01; 0.010 |
| Wrist extension with fingers flexed. (NU) | 0.2; 0.90 ± 0.68 | 0.4; 0.77 ± 0.46 | <0.05; >0.05 |
| Wrist UD with fingers ext. (NU) | 0.6; 0.85 ± 0.56 | 0.5; 0.81 ± 0.58 | 0.106; >0.05 |
| (normalized to hand length; 1NU = 1.8 mm) | 1.8; 1.93 ± 1.23 | 2.8; 2.86 ± 0.51 | <0.01; 0.005 |
| Nanno et al. [41] | <0.05 | ||
| All fingers flexion, | RDR; DDR | RDR; DDR | |
| with neutral wrist position | ext.: 3.54 ± 0.51; 6.43 ± 1.37; flex.: 4.81 ± 0.64: 5.42 ± 0.86 | ext.: 7.89 ± 0.84; 9.09 ± 0.92; flex.: 9.75 ± 0.84: 7.53 ± 0.68 | |
| wrist dorsal flexion | ext.: 4.77 ± 1.04; 7.8 ± 1.06; flex.: 7.02 ± 1.56: 6.46 ± 4.61 | ext.: 8.56 ± 0.68; 10.42 ± 1.42; flex: 10.37 ± 1.34: 8.54 ± 1.72 | |
| wrist palmar flexion | ext.: −7.66 ± 2.47; 1.56 ± 1.44; flex.: −10.61 ± 2.7: 0.52 ± 1.32 | ext.: −1.54 ± 0.85; 6.54 ± 1.18; flex.: −6.98 ± 1.76: 4.8 ± 2.01 | |
| wrist ulnar deviation | ext.: 6.34 ± 1.69; 5.2 ± 0.96; flex.: 8.99 ± 2.53: 4.57 ± 3.95 | ext.: 9.32 ± 0.79; 8.26 ± 1.1; flex.: 12.2 ± 1.71: 7.03 ± 0.9 | |
| wrist radial deviation | ext.: −2.11 ± 1.37; 2.31 ± 1.18; flex.: −3.09 ± 0.92: 0.97 ± 1.27 | ext.: −0.65 ± 0.48; 7.73 ± 1.43; flex.: −2.19 ± 0.96: 5.95 ± 1.33 | |
| Filius et al. (a) [31] | relative to FDS3 | relative to FDS3 | |
| All fingers flexion | - | - | >0.05 |
| Kang et al. [42] | DP; RU | DP; RU | |
| First finger flexion (mm) | 0.22 ± 0.07; 0.29 ± 0.08 | 0.32 ± 0.06; 0.81 ± 0.18 | 0.195; 0.012 |
| Second finger flexion (mm) | 0.30 ± 0.10; 0.40 ± 0.12 | 0.50 ± 0.10; 0.98 ± 0.21 | 0.099; 0.013 |
| Third finger flexion (mm) | 0.36 ± 0.11; 0.55 ± 0.16 | 0.95 ± 0.14; 1.05 ± 0.27 | 0.017; 0.195 |
| Grip (mm) | 0.29 ± 0.08; 0.40 ± 0.13 | 0.64 ± 0.11; 0.84 ± 0.18 | 0.015; 0.021 |
| Kuo et al. [20] All five fingers flexion | R square; curvature; amplitude mild CTS: 0.77 ± 0.15; 0.25 ± 0.23; 0.57 ± 0.42 severe CTS: 0.56 ± 0.19; 0.12 ± 0.11; 0.35 ± 0.31 | R square; curvature; amplitude 0.94 (0.02); 0.69 (0.28); 1.27 (0.62) | <0.001; <0.001; <0.001 |
| Park et al. [43] MCV (mm; NU%) | Bland grade 1: 0.51 ± 0.17; 15.56 ± 5.08 Bland grade 2: 0.45 ± 0.09; 14.47 ± 3.03 Bland grade 3: 0.25 ± 0.08; 7.20 ± 2.19 | 0.5 ± 0.10; 15.27 ± 3.49 | >0.05; >0.05 >0.05; >0.05 <0.05; <0.001 |
| Fan et al. [44] | |||
| All five fingers flexion (proximal CT, mm) | 0.704 ± 0.159 | 2.346 ± 0.826 | <0.01 |
| All five fingers flexion (mid-forearm, mm) | 0.808 ± 0.242 | 2.050 ± 0.873 | <0.01 |
| Hara et al. [54] | |||
| Wrist flexion (MAMn, mm2) | 11.8 ± 4.23 | 23.1 ± 4.28 | <0.01 |
| Wrist flexion (RMMn, mm2) | 5.35 ± 2.32 | 16.35 ± 4.16 | <0.01 |
| Wrist flexion (MR) | 1.86 ± 0.27 | 3.52 ± 0.79 | <0.01 |
| Studies Using Speckle Tracking Method | CTS | Control | p Value |
|---|---|---|---|
| Erel et al. [29] | |||
| passive MCP from 90 degree flex. to neutral (mm) | 2.2 ± 0.93 | 2.62 ± 0.73 | >0.1 |
| Filius et al. (a) [31] | |||
| all fingers flex. (clinical grading, mm) | minimal: 3.9 ± 1.2; mild: 3.1 ± 1.6; moderate: 2.7 ± 1.5; severe: 3.1 ± 1.5 | 4.1 ± 1.9 | 0.019 (mild/moderate v.s control) |
| all fingers flex. (NCS grading, mm) | minimal: 3.1 ± 1.5; mild: 3.4 ±1.7; moderate: 2.6 ± 1.4; severe: 2.4 ± 1.0 | 4.1 ± 1.8 | 0.001 (moderate/severe v.s control) |
| Filius et al. (b) [32] | |||
| all fingers flex. (low/medium/high motion velocity, cm) | 0.18 ± 0.10; 0.21 ± 0.12; 0.23 ± 0.12 | 0.21 ± 0.07; 0.33 ± 0.10; 0.40 ± 0.11 | < 0.001; 0.002; <0.001 |
| all fingers flex. (low/medium/high motion velocity, NU*) | 0.10 ± 0.06; 0.12 ± 0.07; 0.13 ± 0.08 | 0.22 ± 0.07; 0.23 ± 0.07; 0.28 ± 0.11 | <0.001; <0.001; <0.001 |
| Studies using Duplex Doppler waveform | CTS | Control | p value |
| Hough et al. [30] | |||
| all fingers flex. with elbow flexion & extension (mm) | 10.2 ± 3.1; 8.3 ± 2.6 | 12.5 ± 2.5; 11.2 ± 2.8 | 0.089; 0.013 |
| all fingers flex. with elbow flexion & extension (NU*) | 0.28 ± 0.10; 0.23 ± 0.06 | 0.36 ± 0.06; 0.32 ± 0.07 | 0.019; <0.001 |
| Liu et al. [33] index finger flex. (neutral; 30 degree ext. wrist, mm) index finger flex. (neutral; 30 degree ext. wrist, NU*) | 18.5 ± 7.0; 21.3 ± 10.6 0.20 ± 0.11; 0.21 ± 0.11 | 23.7 ± 9.1; 25.6 ± 10.3 0.29 ± 0.15; 0.29 ± 0.14 | 0.0001; 0.02 0.0008; 0.005 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, Y.-T.; Chen, C.-J.; Wang, Y.-W.; Peng, P.-L.; Luo, Y.-T.; Horng, Y.-S. Ultrasonographical Evaluation of the Median Nerve Mobility in Carpal Tunnel Syndrome: A Systematic Review and Meta-Analysis. Diagnostics 2022, 12, 2349. https://doi.org/10.3390/diagnostics12102349
Huang Y-T, Chen C-J, Wang Y-W, Peng P-L, Luo Y-T, Horng Y-S. Ultrasonographical Evaluation of the Median Nerve Mobility in Carpal Tunnel Syndrome: A Systematic Review and Meta-Analysis. Diagnostics. 2022; 12(10):2349. https://doi.org/10.3390/diagnostics12102349
Chicago/Turabian StyleHuang, Yu-Ting, Chii-Jen Chen, You-Wei Wang, Po-Lin Peng, Yan-Ting Luo, and Yi-Shiung Horng. 2022. "Ultrasonographical Evaluation of the Median Nerve Mobility in Carpal Tunnel Syndrome: A Systematic Review and Meta-Analysis" Diagnostics 12, no. 10: 2349. https://doi.org/10.3390/diagnostics12102349
APA StyleHuang, Y.-T., Chen, C.-J., Wang, Y.-W., Peng, P.-L., Luo, Y.-T., & Horng, Y.-S. (2022). Ultrasonographical Evaluation of the Median Nerve Mobility in Carpal Tunnel Syndrome: A Systematic Review and Meta-Analysis. Diagnostics, 12(10), 2349. https://doi.org/10.3390/diagnostics12102349





