Assessment of Risk Factors Associated with Severe Endometriosis and Establishment of Preoperative Prediction Model
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Sonography
2.3. Laboratory Tests
2.4. Stages of Laparoscopic Surgery for Endometriosis
2.5. Pathology
2.6. Statistical Analyses
3. Results
3.1. Baseline Characteristics of Subjects
3.2. Intraoperative and Pathological Findings
3.3. Preoperative Features of Severe Endometriosis
3.3.1. Sonographic Parameters
3.3.2. Laboratory Parameters
3.4. Consistency between Preoperative Ultrasonic Findings and Surgical Pathological Diagnosis
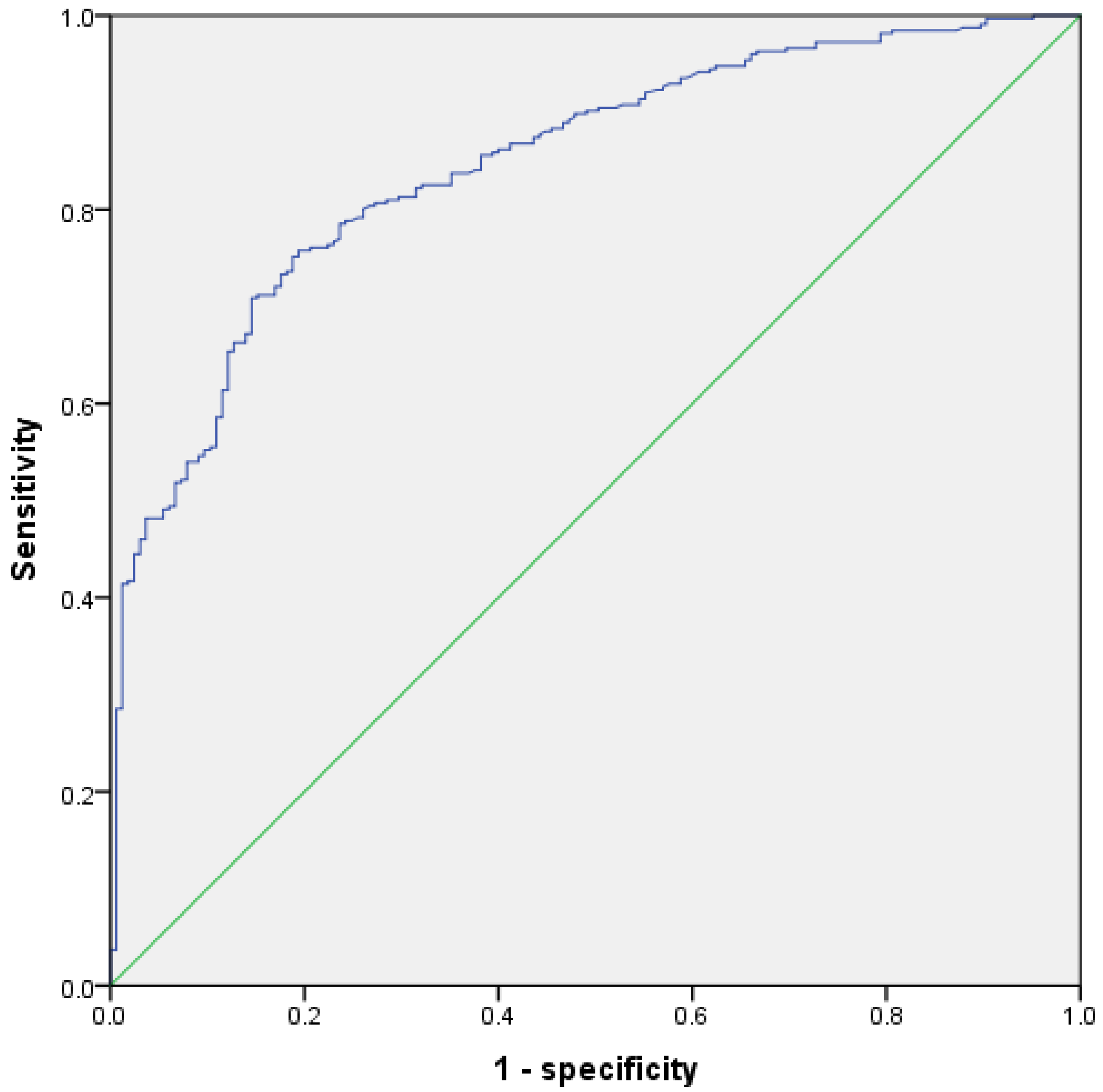
3.5. Receiver Operating Curve and Cutoff Value
3.6. Independent Related Factors and Predicting Model of Severe Endometriosis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Becker, C.M.; Bokor, A.; Heikinheimo, O.; Horne, A.; Jansen, F.; Kiesel, L.; King, K.; Kvaskoff, M.; Nap, A.; Petersen, K.; et al. ESHRE guideline: Endometriosis. Hum. Reprod. Open 2022, 2022, hoac009. [Google Scholar] [CrossRef]
- Horne, A.W.; Saunders, P.T.K. SnapShot: Endometriosis. Cell 2019, 179, 1677–1677.e1. [Google Scholar] [CrossRef] [PubMed]
- Taylor, H.S.; Kotlyar, A.M.; Flores, V.A. Endometriosis is a chronic systemic disease: Clinical challenges and novel innovations. Lancet 2021, 397, 839–852. [Google Scholar] [CrossRef]
- Škegro, B.; Bjedov, S.; Mikuš, M.; Mustač, F.; Lešin, J.; Matijević, V.; Ćorić, M.; Elveđi Gašparović, V.; Medić, F.; Sokol Karadjole, V. Endometriosis, Pain and Mental Health. Psychiatr. Danub. 2021, 33, 632–636. [Google Scholar]
- Mikuš, M.; Matak, L.; Vujić, G.; Škegro, B.; Škegro, I.; Augustin, G.; Lagana, A.S.; Ćorić, M. The short form endometriosis health profile questionnaire (EHP-5): Psychometric validity assessment of a Croatian version. Arch. Gynecol. Obs. 2022. [Google Scholar] [CrossRef] [PubMed]
- Horne, A.W.; Saunders, P.T.K.; Abokhrais, I.M.; Hogg, L. Top ten endometriosis research priorities in the UK and Ireland. Lancet 2017, 389, 2191–2192. [Google Scholar] [CrossRef]
- Peiris, A.N.; Chaljub, E.; Medlock, D. Endometriosis. JAMA 2018, 320, 2608. [Google Scholar] [CrossRef]
- Canis, M.; Donnez, J.G.; Guzick, D.S.; Halme, J.K.; Rock, J.A.; Schenken, R.S.; Vernon, M.W. Revised American Society for Reproductive Medicine classification of endometriosis: 1996. Fertil. Steril. 1997, 67, 817–821. [Google Scholar] [CrossRef]
- Horton, J.; Sterrenburg, M.; Lane, S.; Maheshwari, A.; Li, T.C.; Cheong, Y. Reproductive, obstetric, and perinatal outcomes of women with adenomyosis and endometriosis: A systematic review and meta-analysis. Hum. Reprod. Update 2019, 25, 592–632. [Google Scholar] [CrossRef]
- Moini, A.; Arabipoor, A.; Ashrafinia, N. Risk factors for recurrence rate of ovarian endometriomas following a laparoscopic cystectomy. Minerva Med. 2014, 105, 295–301. [Google Scholar]
- Leonardi, M.; Espada, M.; Choi, S.; Chou, D.; Chang, T.; Smith, C.; Rowan, K.; Condous, G. Transvaginal Ultrasound Can Accurately Predict the American Society of Reproductive Medicine Stage of Endometriosis Assigned at Laparoscopy. J. Minim. Invasive Gynecol. 2020, 27, 1581–1587.e1. [Google Scholar] [CrossRef] [PubMed]
- Collins, B.G.; Ankola, A.; Gola, S.; McGillen, K.L. Transvaginal US of Endometriosis: Looking beyond the Endometrioma with a Dedicated Protocol. Radiographics 2019, 39, 1549–1568. [Google Scholar] [CrossRef]
- Guerriero, S.; Condous, G.; van den Bosch, T.; Valentin, L.; Leone, F.P.; Van Schoubroeck, D.; Exacoustos, C.; Installé, A.J.; Martins, W.P.; Abrao, M.S.; et al. Systematic approach to sonographic evaluation of the pelvis in women with suspected endometriosis, including terms, definitions and measurements: A consensus opinion from the International Deep Endometriosis Analysis (IDEA) group. Ultrasound Obstet. Gynecol. Off. J. Int. Soc. Ultrasound Obstet. Gynecol. 2016, 48, 318–332. [Google Scholar] [CrossRef]
- Timmerman, D.; Valentin, L.; Bourne, T.H.; Collins, W.P.; Verrelst, H.; Vergote, I. Terms, definitions and measurements to describe the sonographic features of adnexal tumors: A consensus opinion from the International Ovarian Tumor Analysis (IOTA) Group. Ultrasound Obstet. Gynecol. Off. J. Int. Soc. Ultrasound Obstet. Gynecol. 2000, 16, 500–505. [Google Scholar] [CrossRef]
- Van Calster, B.; Van Hoorde, K.; Valentin, L.; Testa, A.C.; Fischerova, D.; Van Holsbeke, C.; Savelli, L.; Franchi, D.; Epstein, E.; Kaijser, J.; et al. Evaluating the risk of ovarian cancer before surgery using the ADNEX model to differentiate between benign, borderline, early and advanced stage invasive, and secondary metastatic tumours: Prospective multicentre diagnostic study. BMJ (Clin. Res. Ed.) 2014, 349, g5920. [Google Scholar] [CrossRef] [PubMed]
- Naess, I.A.; Christiansen, S.C.; Romundstad, P.; Cannegieter, S.C.; Rosendaal, F.R.; Hammerstrøm, J. Incidence and mortality of venous thrombosis: A population-based study. J. Thromb. Haemost. 2007, 5, 692–699. [Google Scholar] [CrossRef]
- Chinese Obstetricians and Gynecologists Association; Cooperative Group of Endometriosis; Chinese Society of Obstetrics and Gynecology; Chinese Medical Association. Guideline for the diagnosis and treatment of endometriosis (Third edition). Chin. J. Obstet. Gynecol. 2021, 56, 812–824. [Google Scholar] [CrossRef]
- Shafrir, A.L.; Farland, L.V.; Shah, D.K.; Harris, H.R.; Kvaskoff, M.; Zondervan, K.; Missmer, S.A. Risk for and consequences of endometriosis: A critical epidemiologic review. Best Pract. Res. Clin. Obstet. Gynaecol. 2018, 51, 1–15. [Google Scholar] [CrossRef]
- Agarwal, S.K.; Chapron, C.; Giudice, L.C.; Laufer, M.R.; Leyland, N.; Missmer, S.A.; Singh, S.S.; Taylor, H.S. Clinical diagnosis of endometriosis: A call to action. Am. J. Obstet. Gynecol. 2019, 220, e351–e354. [Google Scholar] [CrossRef]
- Nisenblat, V.; Bossuyt, P.M.; Farquhar, C.; Johnson, N.; Hull, M.L. Imaging modalities for the non-invasive diagnosis of endometriosis. Cochrane Database Syst. Rev. 2016, 2, Cd009591. [Google Scholar] [CrossRef] [PubMed]
- Exacoustos, C.; Malzoni, M.; Di Giovanni, A.; Lazzeri, L.; Tosti, C.; Petraglia, F.; Zupi, E. Ultrasound mapping system for the surgical management of deep infiltrating endometriosis. Fertil. Steril. 2014, 102, 143–150.e2. [Google Scholar] [CrossRef]
- Van den Bosch, T.; Van Schoubroeck, D. Ultrasound diagnosis of endometriosis and adenomyosis: State of the art. Best Pract. Res. Clin. Obstet. Gynaecol. 2018, 51, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Ban Frangež, H.; Vrtacnik Bokal, E.; Štimpfel, M.; Divjak Budihna, T.; Gulino, F.A.; Garzon, S.; Ghezzi, F.; Alkatout, I.; Gitas, G.; Laganà, A.S. Reproductive outcomes after laparoscopic surgery in infertile women affected by ovarian endometriomas, with or without in vitro fertilisation: Results from the SAFE (surgery and ART for endometriomas) trial. J. Obstet. Gynaecol. J. Inst. Obstet. Gynaecol. 2022, 42, 1293–1300. [Google Scholar] [CrossRef]
- D’Alterio, M.N.; Saponara, S.; D’Ancona, G.; Russo, M.; Laganà, A.S.; Sorrentino, F.; Nappi, L.; Angioni, S. Role of surgical treatment in endometriosis. Minerva Obs. Gynecol. 2021, 73, 317–332. [Google Scholar] [CrossRef] [PubMed]
- Moustafa, S.; Burn, M.; Mamillapalli, R.; Nematian, S.; Flores, V.; Taylor, H.S. Accurate diagnosis of endometriosis using serum microRNAs. Am. J. Obstet. Gynecol. 2020, 223, e551–e557. [Google Scholar] [CrossRef]
- Vanhie, A.; Peterse, D.; Beckers, A.; Cuéllar, A.; Fassbender, A.; Meuleman, C.; Mestdagh, P.; D’Hooghe, T. Plasma miRNAs as biomarkers for endometriosis. Hum. Reprod. 2019, 34, 1650–1660. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Leng, J.; Shi, J.; Jia, S.; Lang, J. Effects of endometriosis types and related factors on serum CA125 level. Chin. J. Obstet. Gynecol. 2011, 46, 940–942. [Google Scholar] [CrossRef]
- Muyldermans, M.; Cornillie, F.J.; Koninckx, P.R. CA125 and endometriosis. Hum. Reprod. Update 1995, 1, 173–187. [Google Scholar] [CrossRef] [PubMed]
- Guralp, O.; Kaya, B.; Tüten, N.; Kucur, M.; Malik, E.; Tüten, A. Non-invasive diagnosis of endometriosis and moderate-severe endometriosis with serum CA125, endocan, YKL-40, and copeptin quadruple panel. J. Obstet. Gynaecol. J. Inst. Obstet. Gynaecol. 2021, 41, 927–932. [Google Scholar] [CrossRef]
- Li, R.; Wang, C. Clinical Diagnostic Significance of Coagulation and Inflammatory Factors in Moderate and Severe Ovarian Endometriosis. J. Chin. J. Thromb. Hemost. 2022, 28, 79–80. [Google Scholar] [CrossRef]
- Wu, Q.; Ding, D.; Liu, X.; Guo, S.W. Evidence for a Hypercoagulable State in Women with Ovarian Endometriomas. Reprod. Sci. 2015, 22, 1107–1114. [Google Scholar] [CrossRef] [PubMed]
- Ding, S.; Lin, Q.; Zhu, T.; Li, T.; Zhu, L.; Wang, J.; Zhang, X. Is there a correlation between inflammatory markers and coagulation parameters in women with advanced ovarian endometriosis? BMC Womens Health 2019, 19, 169. [Google Scholar] [CrossRef]
- Zondervan, K.T.; Becker, C.M.; Koga, K.; Missmer, S.A.; Taylor, R.N.; Viganò, P. Endometriosis. Nat. Rev. Dis. Primers 2018, 4, 9. [Google Scholar] [CrossRef] [PubMed]
- Ding, D.; Liu, X.; Guo, S.W. Further Evidence for Hypercoagulability in Women with Ovarian Endometriomas. Reprod. Sci. 2018, 25, 1540–1548. [Google Scholar] [CrossRef]
- Mu, F.; Rich-Edwards, J.; Rimm, E.B.; Spiegelman, D.; Missmer, S.A. Endometriosis and Risk of Coronary Heart Disease. Circ. Cardiovasc. Qual. Outcomes 2016, 9, 257–264. [Google Scholar] [CrossRef]
- Watrowski, R.; Heinze, G.; Jäger, C.; Forster, J.; Zeillinger, R. Usefulness of the preoperative platelet count in the diagnosis of adnexal tumors. Tumour Biol. 2016, 37, 12079–12087. [Google Scholar] [CrossRef]
- Watrowski, R.; Zeillinger, R. Simple laboratory score improves the preoperative diagnosis of adnexal mass. Tumour Biol. 2016, 37, 4343–4349. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Fu, Z.; Liu, G.; Xu, P.; Xu, J.; Jia, X. Clinical significance of plasma D-dimer in ovarian cancer: A meta-analysis. Medicine 2017, 96, e7062. [Google Scholar] [CrossRef]
- Di Donato, N.; Montanari, G.; Benfenati, A.; Leonardi, D.; Bertoldo, V.; Monti, G.; Raimondo, D.; Seracchioli, R. Prevalence of adenomyosis in women undergoing surgery for endometriosis. Eur. J. Obstet. Gynecol. Reprod. Biol. 2014, 181, 289–293. [Google Scholar] [CrossRef]
- Haas, D.; Oppelt, P.; Shebl, O.; Shamiyeh, A.; Schimetta, W.; Mayer, R. Enzian classification: Does it correlate with clinical symptoms and the rASRM score? Acta Obstet. Et Gynecol. Scand. 2013, 92, 562–566. [Google Scholar] [CrossRef] [PubMed]
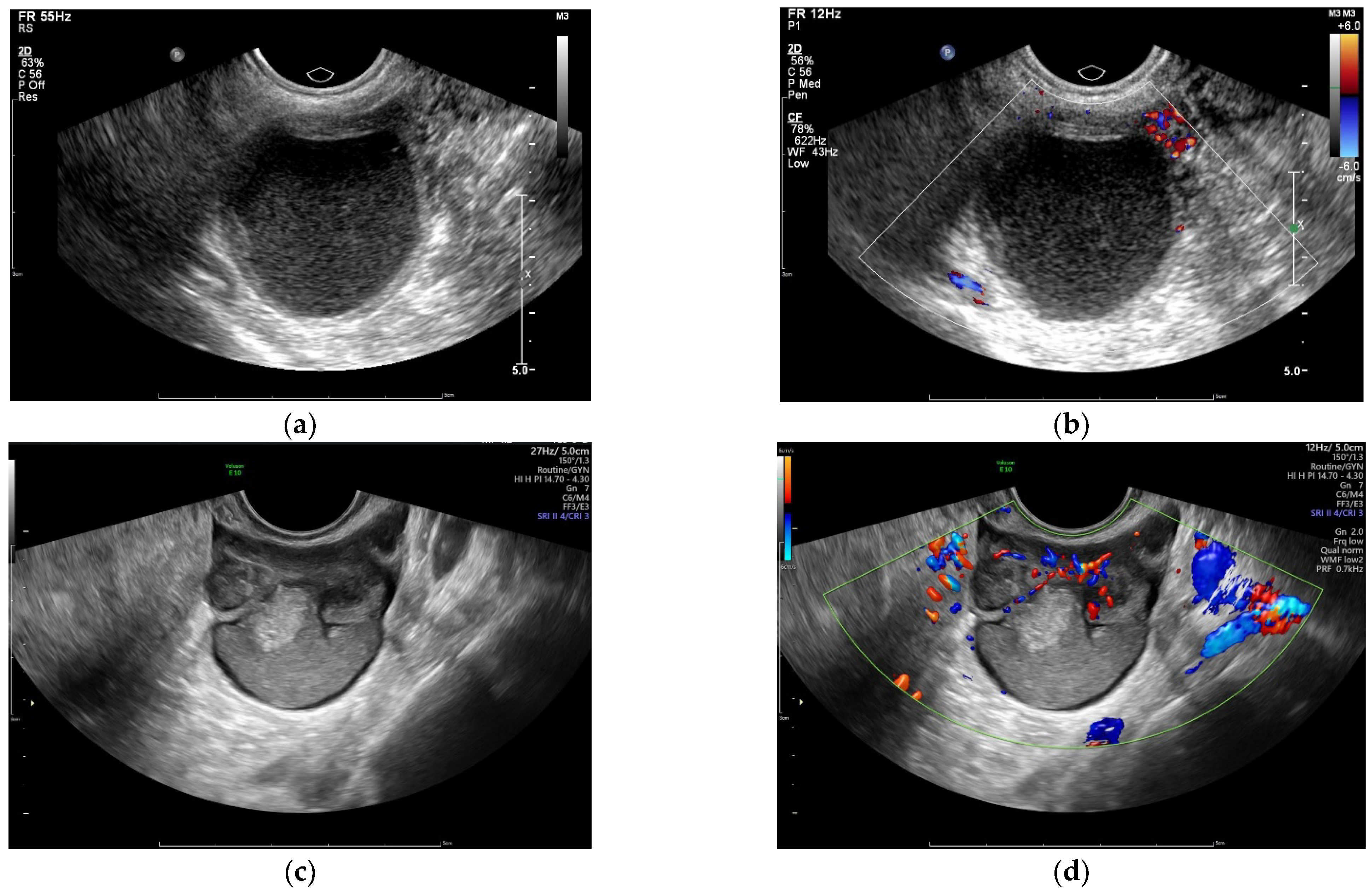
| Baseline Characteristics | Non-Severe Endometriosis (Group A, n = 165) | Severe Endometriosis (Group B, n = 326) | p-Value | |
|---|---|---|---|---|
| Age * (years old) | 34 (29~39) | 37 (31~42) | <0.001 | |
| Age | ≥40 years old | 37 (22.4%) | 134 (41.1%) | <0.001 |
| <40 years old | 128 (77.6%) | 192 (58.9%) | ||
| Number of gravidities * | 1 (0~1) | 1 (0~2) | 0.013 | |
| Number of parities * | 0 (0~1) | 1 (0~1) | 0.009 | |
| History of ovarian cystectomy | Yes | 14 (8.5%) | 55 (16.9%) | 0.012 |
| No | 151 (91.5%) | 271 (83.1%) | ||
| Features | Non-Severe Endometriosis (Group A, n = 165) | Severe Endometriosis (Group B, n = 326) | p-Value | |
|---|---|---|---|---|
| Endometrial cysts ** | Unilateral | 151 (91.5%) | 177 (54.3%) | <0.001 |
| Bilateral | 14 (8.5%) | 149 (45.7%) | ||
| Adenomyosis ** | 23 (13.9%) | 145 (44.5%) | <0.001 | |
| 142 (86.1%) | 181 (55.5%) | |||
| Pelvic endometriosis nodules ** | Yes | 14 (8.5%) | 96 (29.4%) | <0.001 |
| No | 151 (91.5%) | 230 (70.6%) | ||
| Endometrial cysts *** | Unilateral | 146 (88.5%) | 158 (48.5%) | <0.001 |
| Bilateral | 19 (11.5%) | 168 (51.5%) | ||
| Adenomyosis *** | Yes | 17 (10.3%) | 98 (30.1%) | <0.001 |
| No | 148 (89.7%) | 228 (69.9%) | ||
| Intraoperative finding of peritoneal and deep endometriosis | Yes | 76 (46.1%) | 234 (71.8%) | <0.001 |
| No | 89 (53.9%) | 92 (28.2%) | ||
| Maximum cyst diameter | ≥40 mm | 126 (76.4%) | 269 (82.5%) | 0.104 |
| <40 mm | 39 (23.6%) | 57 (17.5%) | ||
| Maximum cyst diameter | ≥58 mm | 57 (34.5%) | 160 (49.1%) | 0.002 |
| <58 mm | 108 (65.5%) | 166 (50.9%) | ||
| Unilocular cyst | Yes | 103 (62.4%) | 162 (49.7%) | 0.008 |
| No | 62 (37.6%) | 164 (50.3%) | ||
| Color Doppler flow | Without | 102 (61.8%) | 199 (61.0%) | 0.868 |
| With | 63 (38.2%) | 127 (39.0%) | ||
| Number of cavities * | 1 (1~2) | 2 (1~3) | 0.007 | |
| Maximum cyst diameter * (mm) | 52 (40~67) | 57 (43~72) | 0.013 | |
| CA125 * (U/mL) | 28.4 (17.8~53.3) | 46.1 (25.4~84.6) | <0.001 | |
| CA125 | ≥34.5 U/mL | 67 (40.6%) | 208 (63.8%) | <0.001 |
| <34.5 U/mL | 98 (59.4%) | 118 (36.2%) | ||
| APTT * (s) | 30.2 (28.4~32.7) | 29.7 (28.2~31.6) | 0.015 | |
| PT * (s) | 11.5 (11.0~12.1) | 11.4 (11.0~12.0) | 0.481 | |
| INR * | 0.97 (0.93~1.03) | 0.96 (0.93~1.02) | 0.502 | |
| TT * (s) | 18.5 (17.7~19.2) | 18.5 (17.6~19.0) | 0.607 | |
| Fg * (g/L) | 2.5 (2.2~2.8) | 2.5 (2.3~2.9) | 0.295 | |
| FDP * (mg/L) | 1.3 (1.2~1.7) | 1.5 (1.2~2.1) | 0.050 | |
| D-dimer * (mg/L) | 0.28 (0.21~0.33) | 0.31 (0.24~0.39) | <0.001 | |
| D-dimer | ≥0.34 mg/L | 38 (23.0%) | 133 (40.8%) | <0.001 |
| <0.34 mg/L | 127 (77.0%) | 193 (59.2%) | ||
| PLT * (×109/L) | 235 (194~277) | 255 (216~293) | 0.005 | |
| Hb * (g/L) | 124 (116~131) | 121 (112~128) | 0.015 | |
| Parameter | Minimum | Maximum | P25 | P50 | P75 |
|---|---|---|---|---|---|
| Ca125 (U/mL) | 7.1 | 2657.2 | 22.0 | 38.8 | 72.2 |
| APTT (s) | 23.8 | 39.1 | 28.3 | 29.8 | 32.1 |
| PT (s) | 10.0 | 15.1 | 11.0 | 11.4 | 12.0 |
| INR | 0.84 | 1.29 | 0.93 | 0.96 | 1.02 |
| TT (s) | 15.00 | 21.10 | 17.60 | 18.50 | 19.20 |
| Fg (g/L) | 1.6 | 6.3 | 2.2 | 2.5 | 2.9 |
| FDP (mg/L) | 0.5 | 34.1 | 1.2 | 1.5 | 1.9 |
| D-dimer (mg/L) | 0.10 | 9.30 | 0.23 | 0.29 | 0.37 |
| PLT (×109/L) | 89 | 564 | 206 | 247 | 287 |
| Hb (g/L) | 73 | 156 | 113 | 122 | 129 |
| Variables | AUC | p-Value | 95% CI |
|---|---|---|---|
| Age | 0.604 | <0.001 | 0.552~0.657 |
| Maximum cyst diameter | 0.568 | 0.014 | 0.514~0.622 |
| CA125 | 0.638 | <0.001 | 0.586~0.690 |
| Number of gravidities | 0.565 | 0.019 | 0.512~0.618 |
| Number of parities | 0.565 | 0.014 | 0.512~0.617 |
| Number of cavities | 0.568 | 0.013 | 0.516~0.621 |
| APTT | 0.433 | 0.015 | 0.378~0.487 |
| D-dimer | 0.604 | <0.001 | 0.552~0.656 |
| PLT | 0.577 | 0.005 | 0.523~0.632 |
| Hb | 0.433 | 0.015 | 0.380~0.486 |
| Variables | B | p-Value | OR | 95% CI |
|---|---|---|---|---|
| Age ≥ 40 yrs | 0.802 | 0.005 | 2.231 | 1.270~3.918 |
| Bilateral endometrial cysts * | 1.027 | 0.040 | 2.792 | 1.045~7.457 |
| Pelvic endometriosis nodules * | 1.131 | 0.002 | 3.097 | 1.505~6.375 |
| Adenomyosis * | 1.260 | <0.001 | 3.525 | 1.942~6.399 |
| Intraoperative finding of peritoneal and deep endometriosis | 0.881 | 0.001 | 2.412 | 1.454~4.004 |
| Bilateral endometrial cysts ** | 1.621 | <0.001 | 5.059 | 2.072~12.354 |
| APTT | −0.127 | 0.011 | 0.880 | 0.798~0.971 |
| CA125 ≥ 34.5 U/mL | 0.704 | 0.005 | 2.021 | 1.233~3.313 |
| D-dimer ≥ 0.34 mg/L | 0.840 | 0.002 | 2.317 | 1.371~3.918 |
| Maximum cyst diameter ≥ 58 mm | 0.634 | 0.012 | 1.885 | 1.153~3.083 |
| Constant | −2.542 | 0.119 | 0.079 |
| Variables | B | p-Value | OR | 95% CI |
|---|---|---|---|---|
| Age ≥ 40 yrs | 0.718 | 0.009 | 2.050 | 1.197~3.511 |
| Bilateral endometrial cysts * | 2.264 | <0.001 | 9.624 | 5.053~18.331 |
| Pelvic endometriosis nodules * | 1.251 | <0.001 | 3.494 | 1.768~6.906 |
| Adenomyosis * | 1.257 | <0.001 | 3.517 | 1.978~6.252 |
| APTT | −0.127 | 0.009 | 0.880 | 0.800~0.968 |
| CA125 ≥ 34.5 U/mL | 0.797 | 0.001 | 2.220 | 1.382~3.566 |
| D-dimer ≥ 0.34 mg/L | 0.793 | 0.002 | 2.210 | 1.334~3.661 |
| Maximum cyst diameter ≥ 58 mm | 0.571 | 0.018 | 1.770 | 1.104~2.839 |
| Constant | −1.525 | 0.329 | 0.218 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Y.; Li, J.; Chen, H.; Feng, W. Assessment of Risk Factors Associated with Severe Endometriosis and Establishment of Preoperative Prediction Model. Diagnostics 2022, 12, 2348. https://doi.org/10.3390/diagnostics12102348
Yang Y, Li J, Chen H, Feng W. Assessment of Risk Factors Associated with Severe Endometriosis and Establishment of Preoperative Prediction Model. Diagnostics. 2022; 12(10):2348. https://doi.org/10.3390/diagnostics12102348
Chicago/Turabian StyleYang, Yanhua, Jing Li, Hui Chen, and Weiwei Feng. 2022. "Assessment of Risk Factors Associated with Severe Endometriosis and Establishment of Preoperative Prediction Model" Diagnostics 12, no. 10: 2348. https://doi.org/10.3390/diagnostics12102348
APA StyleYang, Y., Li, J., Chen, H., & Feng, W. (2022). Assessment of Risk Factors Associated with Severe Endometriosis and Establishment of Preoperative Prediction Model. Diagnostics, 12(10), 2348. https://doi.org/10.3390/diagnostics12102348






