Positive Effect of Cognitive Training in Older Adults with Different APOE Genotypes and COVID-19 History: A 1-Year Follow-Up Cohort Study
Abstract
1. Introduction
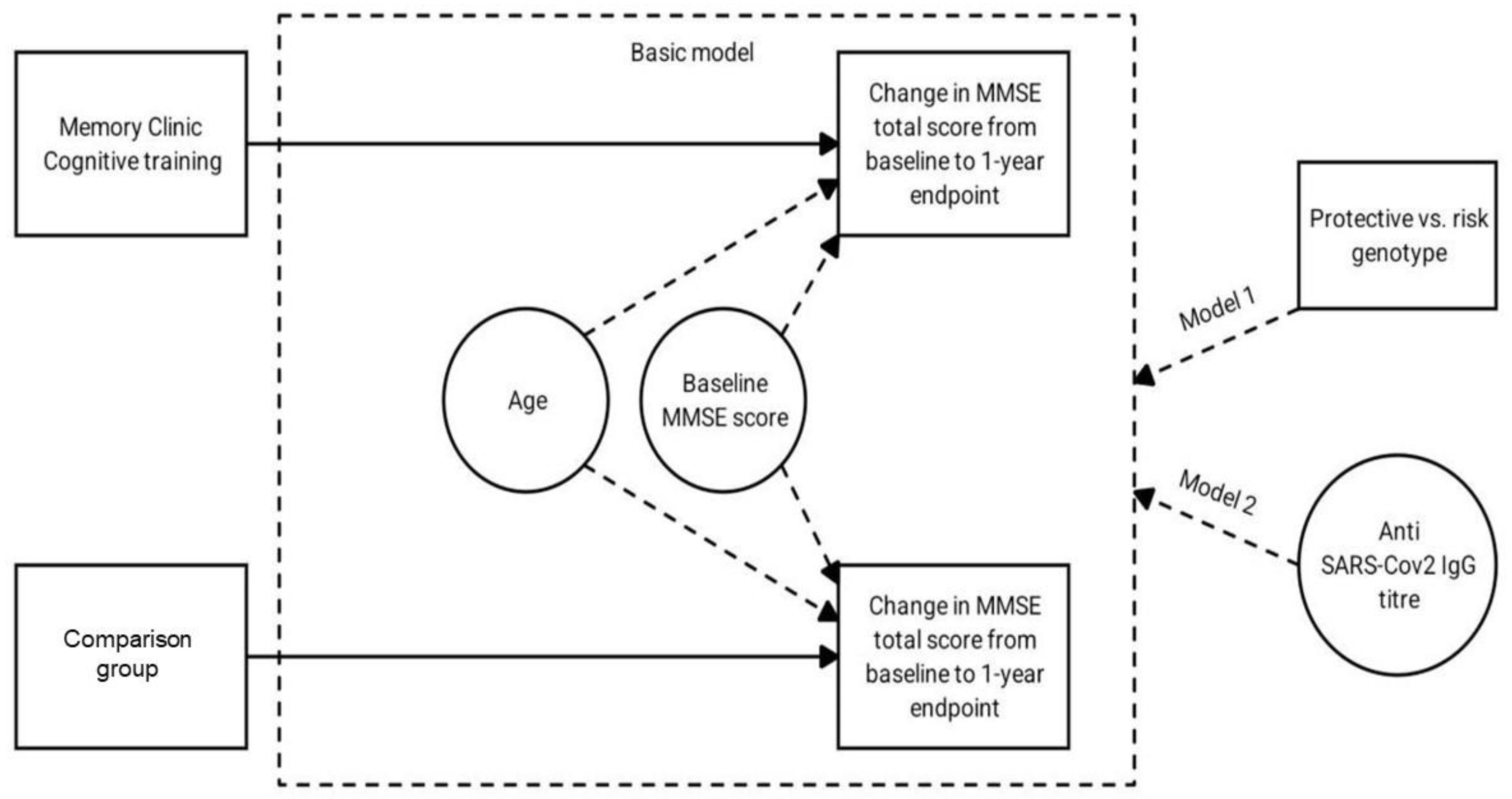
2. Materials and Methods
2.1. Subjects
- People who came to the “Memory Clinic” of Mental-health Clinic No. 1, named after N.A. Alekseev of the Moscow Healthcare Department, with subjective cognitive decline. These subjects received cognitive training. The study included individuals with complaints of forgetfulness, lack of attention and concentration (for example, when talking or reading a book), episodic difficulties in finding their way home, feeling if hard to articulate their thoughts, decreased professional and social productivity, impaired motor skills (writing, drawing) and experiencing problems in everyday life (paying bills, shopping).
- People who came to outpatient clinic No 121 (Moscow) to receive COVID-19 vaccination. This group of subjects did not complain of cognitive decline. No cognitive training was conducted with this group (comparison group).
2.2. Neurocognitive Training
2.3. APOE Genotyping
2.4. ELISA
2.5. Statistical Processing
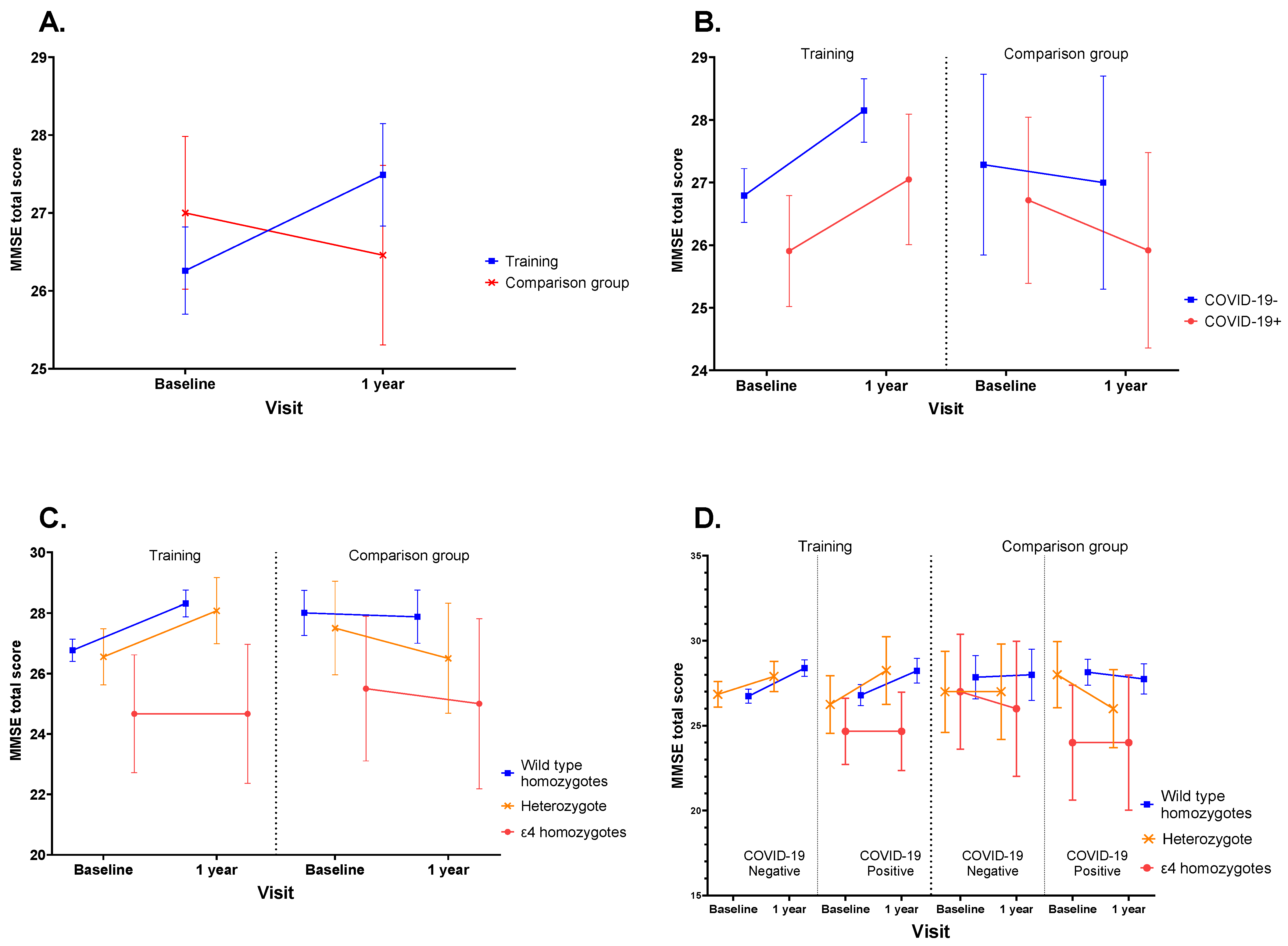
3. Results
4. Discussion
5. Conclusions
- Cognitive training is a positive factor in reducing cognitive impairment.
- COVID-19 infection had no effect on cognitive function during the 1-year follow-up in older adults.
- Older adults with APOE-ε4 genotype showed no positive effect of cognitive training.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviation
| AD | Alzheimer’s disease |
| APOE | apolipoprotein E |
| HADS | Hospital Anxiety and Depression Scale |
| MCI | Mild cognitive impairment |
| MMSE | Mini-Mental State Examination |
References
- Pais, R.; Ruano, L.; Moreira, C.; Carvalho, O.P.; Barros, H. Prevalence and incidence of cognitive impairment in an elderly Portuguese population (65–85 years old). BMC Geriatr. 2020, 20, 470. [Google Scholar] [CrossRef] [PubMed]
- Petersen, R.C.; Roberts, R.O.; Knopman, D.S.; Boeve, B.F.; Geda, Y.E.; Ivnik, R.J.; Smith, G.E.; Jack, C.R., Jr. Mild cognitive impairment: Ten years later. Arch. Neurol. 2009, 66, 1447–1455. [Google Scholar] [CrossRef] [PubMed]
- Jongsiriyanyong, S.; Limpawattana, P. Mild Cognitive Impairment in Clinical Practice: A Review Article. Am. J. Alzheimers Dis. Other Demen. 2018, 33, 500–507. [Google Scholar] [CrossRef] [PubMed]
- Plassman, B.L.; Langa, K.M.; Fisher, G.G.; Heeringa, S.G.; Weir, D.R.; Ofstedal, M.B.; Burke, J.R.; Hurd, M.D.; Potter, G.G.; Rodgers, W.L.; et al. Prevalence of cognitive impairment without dementia in the United States. Ann. Intern. Med. 2008, 148, 427–434. [Google Scholar] [CrossRef]
- Albert, M.S.; DeKosky, S.T.; Dickson, D.; Dubois, B.; Feldman, H.H.; Fox, N.C.; Gamst, A.; Holtzman, D.M.; Jagust, W.J.; Petersen, R.C.; et al. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011, 7, 270–279. [Google Scholar] [CrossRef] [PubMed]
- Schmidtke, K.; Hermeneit, S. High rate of conversion to Alzheimer’s disease in a cohort of amnestic MCI patients. Int. Psychogeriatr. 2007, 20, 96–108. [Google Scholar] [CrossRef]
- Serrano-Pozo, A.; Das, S.; Hyman, B.T. APOE and Alzheimer’s disease: Advances in genetics, pathophysiology, and therapeutic approaches. Lancet Neurol. 2021, 20, 68–80. [Google Scholar] [CrossRef]
- Karch, C.M.; Cruchaga, C.; Goate, A.M. Alzheimer’s disease genetics: From the bench to the clinic. Neuron 2014, 83, 11–26. [Google Scholar] [CrossRef]
- Elias-Sonnenschein, L.S.; Viechtbauer, W.; Ramakers, I.H.; Verhey, F.R.; Visser, P.J. Predictive value of APOE-ε4 allele for progression from MCI to AD-type dementia: A meta-analysis. J. Neurol. Neurosurg. Psychiatry 2011, 82, 1149–1156. [Google Scholar] [CrossRef]
- Ma, C.; Wang, J.; Zhang, J.; Chen, K.; Li, X.; Shu, N.; Chen, Y.; Liu, Z.; Zhang, Z. Disrupted Brain Structural Connectivity: Pathological Interactions Between Genetic APOE ε4 Status and Developed MCI Condition. Mol. Neurobiol. 2017, 54, 6999–7007. [Google Scholar] [CrossRef]
- Erickson, M.A.; Rhea, E.M.; Knopp, R.C.; Banks, W.A. Interactions of SARS-CoV-2 with the Blood–Brain Barrier. Int. J. Mol. Sci. 2021, 22, 2681. [Google Scholar] [CrossRef] [PubMed]
- Tremblay, M.E.; Madore, C.; Bordeleau, M.; Tian, L.; Verkhratsky, A. Neuropathobiology of COVID-19: The Role for Glia. Front. Cell. Neurosci. 2020, 14, 592214. [Google Scholar] [CrossRef] [PubMed]
- Raony, Í.; de Figueiredo, C.S.; Pandolfo, P.; Giestal-de-Araujo, E.; Oliveira-Silva Bomfim, P.; Savino, W. Psycho-Neuroendocrine-Immune Interactions in COVID-19: Potential Impacts on Mental Health. Front. Immunol. 2020, 11, 1170. [Google Scholar] [CrossRef]
- Llach, C.D.; Vieta, E. Mind long COVID: Psychiatric sequelae of SARS-CoV-2 infection. Eur. Neuropsychopharmacol. 2021, 49, 119–121. [Google Scholar] [CrossRef]
- Ceban, F.; Ling, S.; Lui, L.M.W.; Lee, Y.; Gill, H.; Teopiz, K.M.; Rodrigues, N.B.; Subramaniapillai, M.; Di Vincenzo, J.D.; Cao, B.; et al. Fatigue and cognitive impairment in Post-COVID-19 Syndrome: A systematic review and meta-analysis. Brain Behav. Immun. 2022, 101, 93–135. [Google Scholar] [CrossRef] [PubMed]
- Miskowiak, K.W.; Johnsen, S.; Sattler, S.M.; Nielsen, S.; Kunalan, K.; Rungby, J.; Lapperre, T.; Porsberg, C.M. Cognitive impairments four months after COVID-19 hospital discharge: Pattern, severity and association with illness variables. Eur. Neuropsychopharmacol. 2021, 46, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Taquet, M.; Geddes, J.R.; Husain, M.; Luciano, S.; Harrison, P.J. 6-month neurological and psychiatric outcomes in 236 379 survivors of COVID-19: A retrospective cohort study using electronic health records. Lancet Psychiatry 2021, 8, 416–427. [Google Scholar] [CrossRef]
- Houben, S.; Bonnechère, B. The Impact of COVID-19 Infection on Cognitive Function and the Implication for Rehabilitation: A Systematic Review and Meta-Analysis. Int. J. Environ. Res. Public Health 2022, 19, 7748. [Google Scholar] [CrossRef]
- Tondo, G.; Aprile, D.; Tesser, F.; Comi, C. Increased Prevalence of Neuropsychiatric Disorders during COVID-19 Pandemic in People Needing a Non-Deferrable Neurological Evaluation. J. Clin. Med. 2021, 10, 5169. [Google Scholar] [CrossRef]
- Cagnin, A.; Di Lorenzo, R.; Marra, C.; Bonanni, L.; Cupidi, C.; Laganà, V.; Rubino, E.; Vacca, A.; Provero, P.; Isella, V.; et al. SINdem COVID-19 Study Group. Behavioral and Psychological Effects of Coronavirus Disease-19 Quarantine in Patients with Dementia. Front. Psychiatry 2020, 11, 578015. [Google Scholar] [CrossRef]
- Kurki, S.N.; Kantonen, J.; Kaivola, K.; Hokkanen, L.; Mäyränpää, M.I.; Puttonen, H.; Martola, J.; Pöyhönen, M.; Kero, M.; Tuimala, J.; et al. APOE ε4 associates with increased risk of severe COVID-19, cerebral microhaemorrhages and post-COVID mental fatigue: A Finnish biobank, autopsy and clinical study. Acta Neuropathol. Commun. 2021, 9, 199. [Google Scholar] [CrossRef] [PubMed]
- Espinosa-Salinas, I.; Colmenarejo, G.; Fernández-Díaz, C.M.; Gómez de Cedrón, M.; Martinez, J.A.; Reglero, G.; Ramírez de Molina, A. Potential protective effect against SARS-CoV-2 infection by APOE rs7412 polymorphism. Sci. Rep. 2022, 12, 7247. [Google Scholar] [CrossRef] [PubMed]
- Kuo, C.L.; Pilling, L.C.; Atkins, J.L.; Masoli, J.A.H.; Delgado, J.; Kuchel, G.A.; Melzer, D. APOE e4 Genotype Predicts Severe COVID-19 in the UK Biobank Community Cohort. J. Gerontol. A Biol. Sci. Med. Sci. 2020, 75, 2231–2232. [Google Scholar] [CrossRef]
- Manzo, C.; Serra-Mestres, J.; Isetta, M.; Castagna, A. Could COVID-19 anosmia and olfactory dysfunction trigger an increased risk of future dementia in patients with ApoE4? Med. Hypotheses 2021, 147, 110479. [Google Scholar] [CrossRef]
- Miners, S.; Kehoe, P.G.; Love, S. Cognitive impact of COVID-19: Looking beyond the short term. Alzheimers Res. Ther. 2020, 12, 170. [Google Scholar] [CrossRef]
- Peng, Z.; Jiang, H.; Wang, X.; Huang, K.; Zuo, Y.; Wu, X.; Abdullah, A.S.; Yang, L. The Efficacy of Cognitive Training for Elderly Chinese Individuals with Mild Cognitive Impairment. BioMed Res. Int. 2019, 2019, 4347281. [Google Scholar] [CrossRef] [PubMed]
- Karssemeijer, E.G.A.; Aaronson, J.A.; Bossers, W.J.; Smits, T.; Olde Rikkert, M.G.M.; Kessels, R.P.C. Positive effects of combined cognitive and physical exercise training on cognitive function in older adults with mild cognitive impairment or dementia: A meta-analysis. Ageing Res. Rev. 2017, 40, 75–83. [Google Scholar] [CrossRef]
- Sherman, D.S.; Mauser, J.; Nuno, M.; Sherzai, D. The Efficacy of Cognitive Intervention in Mild Cognitive Impairment (MCI): A Meta-Analysis of Outcomes on Neuropsychological Measures. Neuropsychol. Rev. 2017, 27, 440–484. [Google Scholar] [CrossRef]
- Basak, C.; Qin, S.; O’Connell, M.A. Differential effects of cognitive training modules in healthy aging and mild cognitive impairment: A comprehensive meta-analysis of randomized controlled trials. Psychol. Aging 2020, 35, 220–249. [Google Scholar] [CrossRef]
- Law, C.K.; Lam, F.M.; Chung, R.C.; Pang, M.Y. Physical exercise attenuates cognitive decline and reduces behavioural problems in people with mild cognitive impairment and dementia: A systematic review. J. Physiother. 2020, 66, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Biazus-Shen, L.F.; Schuch, F.B.; Firth, J.; Stigger, F.S. Effects of physical exercise on cognitive function of older adults with mild cognitive impairment: A systematic review and meta-analysis. Arch. Gerontol. Geriatr. 2020, 89, 104048. [Google Scholar] [CrossRef] [PubMed]
- Folstein, M.F.; Folstein, S.E.; McHugh, P.R. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J. Psychiatr. Res. 1975, 12, 189–198. [Google Scholar] [CrossRef]
- Cosco, T.D.; Doyle, F.; Ward, M.; McGee, H. Latent structure of the Hospital Anxiety and Depression Scale: A 10-year systematic review. J. Psychosom. Res. 2012, 72, 180–184. [Google Scholar] [CrossRef] [PubMed]
- Kostyuk, G.P.; Kourmyshev, M.V.; Savilov, V.B.; Efremova, D.N.; Pak, M.V.; Burigina, L.A. Recovery of cognitive function in elderly persons in a special medico-rehabilitation unit ‘the Memory clinic’. Soc. Clin. Psychiatry 2017, 27, 4. [Google Scholar]
- Yi, L.; Wu, T.; Luo, W.; Zhou, W.; Wu, J. A non-invasive, rapid method to genotype late-onset Alzheimer’s disease-related apolipoprotein E gene polymorphisms. Neural Regen. Res. 2014, 9, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Burigina, L.A.; Gavrilova, S.I.; Kostyuk, G.P.; Pak, M.V.; Kurmishev, M.V.; Savilov, V.B.; Starodubcev, S.V.; Urchenko, I.E. Psychosocial therapy and neurocognitive rehabilitation of elderly patients with cognitive disorders. In Structural and Functional Model of Rehabilitation Program “Memory Clinic”; Kostyuk, G.P., Ed.; Scientific Research Institute of Health Organization and Medical Management of the Department of Health of the City of Moscow: Moscow, Russia, 2019. (In Russian) [Google Scholar]
- Del Brutto, O.H.; Wu, S.; Mera, R.M.; Costa, A.F.; Recalde, B.Y.; Issa, N.P. Cognitive decline among individuals with history of mild symptomatic SARS-CoV-2 infection: A longitudinal prospective study nested to a population cohort. Eur. J. Neurol. 2021, 28, 3245–3253. [Google Scholar] [CrossRef]
- Alemanno, F.; Houdayer, E.; Parma, A.; Spina, A.; Del Forno, A.; Scatolini, A.; Angelone, S.; Brugliera, L.; Tettamanti, A.; Beretta, L.; et al. COVID-19 cognitive deficits after respiratory assistance in the subacute phase: A COVID-rehabilitation unit experience. PLoS ONE 2021, 16, e0246590. [Google Scholar] [CrossRef]
- Rass, V.; Beer, R.; Schiefecker, A.J.; Kofler, M.; Lindner, A.; Mahlknecht, P.; Heim, B.; Limmert, V.; Sahanic, S.; Pizzini, A.; et al. Neurological outcome and quality of life 3 months after COVID-19: A prospective observational cohort study. Eur. J. Neurol. 2021, 28, 3348–3359. [Google Scholar] [CrossRef]
- Alonso-Lana, S.; Marquié, M.; Ruiz, A.; Boada, M. Cognitive and Neuropsychiatric Manifestations of COVID-19 and Effects on Elderly Individuals with Dementia. Front. Aging Neurosci. 2020, 12, 588872. [Google Scholar] [CrossRef]
- Liu, Y.H.; Wang, Y.R.; Wang, Q.H.; Chen, Y.; Chen, X.; Li, Y.; Cen, Y.; Xu, C.; Hu, T.; Liu, X.D.; et al. Post-infection cognitive impairments in a cohort of elderly patients with COVID-19. Mol. Neurodegener. 2021, 16, 48. [Google Scholar] [CrossRef]
- Wang, Y.; Li, M.; Kazis, L.E.; Xia, W. Clinical outcomes of COVID-19 infection among patients with Alzheimer’s disease or mild cognitive impairment. Alzheimers Dement. 2022, 18, 911–923. [Google Scholar] [CrossRef] [PubMed]
- Kim, N.; Son, S.; Kim, S.; Lee, J.; Ahn, Y.M.; Yon, D.K.; Hahm, B.J. Association Between Dementia Development and COVID-19 among Individuals Who Tested Negative for COVID-19 in South Korea: A Nationwide Cohort Study. Am. J. Alzheimers Dis. Other Demen. 2022, 37, 15333175211072387. [Google Scholar] [CrossRef] [PubMed]
- Soysal, P.; Smith, L.; Trott, M.; Alexopoulos, P.; Barbagallo, M.; Tan, S.G.; Koyanagi, A.; Shenkin, S.; Veronese, N. European Society of Geriatric Medicine Special Interest Group in Dementia and Systematic Reviews and Meta-Analyses. The Effects of COVID-19 lockdown on neuropsychiatric symptoms in patients with dementia or mild cognitive impairment: A systematic review and meta-analysis. Psychogeriatrics 2022, 22, 402–412. [Google Scholar] [CrossRef] [PubMed]
- Tsatali, M.; Moraitou, D.; Poptsi, E.; Sia, E.; Agogiatou, C.; Gialaouzidis, M.; Tabakis, I.M.; Avdikou, K.; Bakoglidou, E.; Batsila, G.; et al. Are There Any Cognitive and Behavioral Changes Potentially Related to Quarantine Due to the COVID-19 Pandemic in People with Mild Cognitive Impairment and AD Dementia? A Longitudinal Study. Brain Sci. 2021, 11, 1165. [Google Scholar] [CrossRef] [PubMed]
- Tavares-Júnior, J.W.L.; de Souza, A.C.C.; Borges, J.W.P.; Oliveira, D.N.; Siqueira-Neto, J.I.; Sobreira-Neto, M.A.; Braga-Neto, P. COVID-19 associated cognitive impairment: A systematic review. Cortex 2022, 152, 77–97. [Google Scholar] [CrossRef]
- Jiang, Y.; He, T.; Deng, W.; Sun, P. Association between apolipoprotein E gene polymorphism and mild cognitive impairment: A meta-analysis. Clin. Interv. Aging 2017, 12, 1941–1949. [Google Scholar] [CrossRef]
- Neu, S.C.; Pa, J.; Kukull, W.; Beekly, D.; Kuzma, A.; Gangadharan, P.; Wang, L.S.; Romero, K.; Arneric, S.P.; Redolfi, A.; et al. Apolipoprotein E Genotype and Sex Risk Factors for Alzheimer Disease: A Meta-analysis. JAMA Neurol. 2017, 74, 1178–1189. [Google Scholar] [CrossRef]
- Banning, L.C.P.; Ramakers, I.H.G.B.; Deckers, K.; Verhey, F.R.J.; Aalten, P. Apolipoprotein E and affective symptoms in mild cognitive impairment and Alzheimer’s disease dementia: A systematic review and meta-analysis. Neurosci. Biobehav. Rev. 2019, 96, 302–315. [Google Scholar] [CrossRef]
- Valero, S.; Marquié, M.; De Rojas, I.; Espinosa, A.; Moreno-Grau, S.; Orellana, A.; Montrreal, L.; Hernández, I.; Mauleón, A.; Rosende-Roca, M.; et al. Interaction of neuropsychiatric symptoms with APOE ε4 and conversion to dementia in MCI patients in a Memory Clinic. Sci. Rep. 2020, 10, 20058. [Google Scholar] [CrossRef]
- Whitehair, D.C.; Sherzai, A.; Emond, J.; Raman, R.; Aisen, P.S.; Petersen, R.C.; Fleisher, A.S. Alzheimer’s Disease Cooperative Study. Influence of apolipoprotein E varepsilon4 on rates of cognitive and functional decline in mild cognitive impairment. Alzheimers Dement. 2010, 6, 412–419. [Google Scholar] [CrossRef]
- Caselli, R.J.; Reiman, E.M.; Osborne, D.; Hentz, J.G.; Baxter, L.C.; Hernandez, J.L.; Alexander, G.G. Longitudinal changes in cognition and behavior in asymptomatic carriers of the APOE e4 allele. Neurology 2004, 62, 1990–1995. [Google Scholar] [CrossRef] [PubMed]
- Alegret, M.; Cuberas-Borrós, G.; Espinosa, A.; Valero, S.; Hernández, I.; Ruíz, A.; Becker, J.T.; Rosende-Roca, M.; Mauleón, A.; Sotolongo, O.; et al. Cognitive, genetic, and brain perfusion factors associated with four-year incidence of Alzheimer’s disease from mild cognitive impairment. J. Alzheimers Dis. 2014, 41, 739–748. [Google Scholar] [CrossRef] [PubMed]
| Cognitive Training (n = 125) | Comparison Group (n = 34) | Total (n = 159) | Test Statistic | ||
|---|---|---|---|---|---|
| Age, mean (S.D.) | 75.0 (5.8) | 70.4 (4.6) | 74.0 (5.9) | F(1157) = 18.4, p < 0.0011 | |
| Gender, n (%) | Female | 114 (91.2) | 26 (76.5) | 140 (88.1) | = 5.51, p = 0.022 |
| Male | 11 (8.8) | 8 (23.5) | 19 (11.9) | ||
| Higher education, n (%) | No | 43 (34.7) | 8 (25.8) | 51 (32.9) | = 0.88, p = 0.352 |
| Yes | 81 (65.3) | 23 (74.2) | 104 (67.1) | ||
| MMSE baseline total score, mean (S.D.) | 26.7 (1.7) | 27.9 (1.9) | 27.0 (1.8) | F(1157) = 11.78, p < 0.0011 | |
| MMSE total score change from baseline, mean (S.D.) | 1.5 (1.9) | −0.4 (1.5) | 1.1 (2.0) | F(1157) = 28.34, p < 0.0011 | |
| HADS anxiety score, mean (S.D.) | 7.0 (3.4) | 5.9 (4.0) | 6.75 (3.6) | F(1157) = 2.44, p = 0.121 | |
| HADS depression score, mean (S.D.) | 6.3 (3.2) | 5.5 (3.2) | 6.2 (3.2) | F(1157) = 1.97, p = 0.162 | |
| Group | Total (n = 159) | Test Statistics | |||
|---|---|---|---|---|---|
| Cognitive Training (n = 125) | Comparison Group (n = 34) | ||||
| APOE ε4, n (%) | 24 (19.20%) | 5 (14.71%) | 29 (18.24%) | Χ2 = 1.33, df = 2, p = 0.55 | |
| ε4 homozygotes | 3 (2.40%) | 2 (5.88%) | 5 (3.14%) | ||
| Wild type homozygotes | 98 (78.40%) | 27 (79.41%) | 125 (78.62%) | ||
| APOE ε2, n (%) | Heterozygote | 18 (14.40%) | 7 (20.59%) | 25 (15.72%) | Χ2 = 0.77, df = 1, p = 0.38 |
| Wild type homozygotes | 107 (85.60%) | 27 (79.41%) | 134 (84.28%) | ||
| IgG to COVID-19, n (%) | Positive | 37 (29.6%) | 24 (70.59%) | 61 (38.36%) | Χ2 = 18.99, df = 1, p < 0.001 |
| Negative | 88 (70.4%) | 10 (29.41%) | 98 (61.64%) | ||
| IgG to COVID-19, positivity rate, mean (S.D). | 2.54 (4.41) | 5.76 (4.68) | 3.23 (4.65) | F = 13.93, p < 0.001 | |
| Source | Type III Sum of Squares | df | Mean Square | F | p |
|---|---|---|---|---|---|
| Time | 0.653 | 1 | 0.653 | 0.384 | 0.536 |
| Time × Group | 7.272 | 1 | 7.272 | 4.278 | 0.040 |
| Time × IgG against COVID-19 | 0.010 | 1 | 0.010 | 0.006 | 0.939 |
| Time × APOE-ε4 | 3.180 | 2 | 1.590 | 0.935 | 0.395 |
| Time × Group × IgG against COVID-19 | 3.877 | 1 | 3.877 | 2.281 | 0.133 |
| Time × Group × APOE-ε4 | 3.387 | 2 | 1.693 | 0.996 | 0.372 |
| Time × IgG against COVID-19 × APOE-ε4 | 1.144 | 2 | 0.572 | 0.336 | 0.715 |
| Time × Group × IgG against COVID-19 × APOE-ε4 | 2.527 | 1 | 2.527 | 1.487 | 0.225 |
| Error (Time) | 251.597 | 148 | 1.700 |
| DF | Sum of Squares | Mean Square | F-Ratio | p-Value | ηp2 | |
|---|---|---|---|---|---|---|
| Model | 2 | 76.17 | 38.08 | 11.06 | <0.001 | |
| Baseline Depression HADS score | 1 | 2.44 | 2.44 | 0.71 | 0.401 | 0.005 |
| Group | 1 | 75.76 | 75.76 | 22.00 | <0.001 | 0.14 |
| Error | 137 | 471.72 | 3.44 | |||
| Total(Adjusted) | 139 | 547.89 | 3.94 |
| Effect | B | Standard Error | t | p | 95% CI | |
|---|---|---|---|---|---|---|
| Lower | Upper | |||||
| (Intercept) | 13.59 | 3.31 | 4.10 | <0.001 | 7.05 | 20.14 |
| Age | −0.03 | 0.03 | −1.13 | 0.26 | −0.08 | 0.02 |
| Baseline total MMSE score | −0.37 | 0.08 | −4.46 | <0.001 | −0.54 | −0.21 |
| Group (without training) | −1.59 | 0.36 | −4.43 | <0.001 | −2.30 | −0.88 |
| Model | R2 | R2 Change | df1 | df2 | p |
|---|---|---|---|---|---|
| H0 (Group; Age; MMSE total score) | 0.25 | 0.25 | 3 | 155 | <0.001 |
| Adding ε4 allele of APOE to H0 | 0.28 | 0.04 | 2 | 153 | 0.02 |
| Adding ε2 allele of APOE to H0 | 0.26 | 0.01 | 1 | 154 | 0.24 |
| Adding IgG against SARS-CoV-2 to H0 | 0.25 | 0.00 | 1 | 154 | 0.55 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zorkina, Y.; Syunyakov, T.; Abramova, O.; Andryushchenko, A.; Andreuyk, D.; Abbazova, E.; Goncharov, D.; Rakova, A.; Andriushchenko, N.; Gryadunov, D.; et al. Positive Effect of Cognitive Training in Older Adults with Different APOE Genotypes and COVID-19 History: A 1-Year Follow-Up Cohort Study. Diagnostics 2022, 12, 2312. https://doi.org/10.3390/diagnostics12102312
Zorkina Y, Syunyakov T, Abramova O, Andryushchenko A, Andreuyk D, Abbazova E, Goncharov D, Rakova A, Andriushchenko N, Gryadunov D, et al. Positive Effect of Cognitive Training in Older Adults with Different APOE Genotypes and COVID-19 History: A 1-Year Follow-Up Cohort Study. Diagnostics. 2022; 12(10):2312. https://doi.org/10.3390/diagnostics12102312
Chicago/Turabian StyleZorkina, Yana, Timur Syunyakov, Olga Abramova, Alisa Andryushchenko, Denis Andreuyk, Evgeniya Abbazova, Dmitry Goncharov, Alisa Rakova, Nika Andriushchenko, Dmitry Gryadunov, and et al. 2022. "Positive Effect of Cognitive Training in Older Adults with Different APOE Genotypes and COVID-19 History: A 1-Year Follow-Up Cohort Study" Diagnostics 12, no. 10: 2312. https://doi.org/10.3390/diagnostics12102312
APA StyleZorkina, Y., Syunyakov, T., Abramova, O., Andryushchenko, A., Andreuyk, D., Abbazova, E., Goncharov, D., Rakova, A., Andriushchenko, N., Gryadunov, D., Ikonnikova, A., Fedoseeva, E., Emelyanova, M., Soloveva, K., Pavlov, K., Karpenko, O., Savilov, V., Kurmishev, M., Gurina, O., ... Morozova, A. (2022). Positive Effect of Cognitive Training in Older Adults with Different APOE Genotypes and COVID-19 History: A 1-Year Follow-Up Cohort Study. Diagnostics, 12(10), 2312. https://doi.org/10.3390/diagnostics12102312








