Clinical Predictors of Neurogenic Lower Urinary Tract Dysfunction in Persons with Multiple Sclerosis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients and Assessment
| Voided volume (VV) | ≤250 mL or ≥500 mL |
| Urinary tract infections (UTI) rate | >0/6 month |
| 24 h standardized voiding frequency (SVF) | ≤4 or ≥13 |
| Post-void residual (PVR) | >70 mL and >100 mL |
| Uroflowmetry [9] | abnormal curve or PVR > 100 mL ormax flow rate < 10 mL/s |
- -
- Correlation between SVF ≥ 13 + VV ≤ 250 mL and compliance < 20 mL/cm H2O.
- -
- Correlation between SVF ≥ 13 + PVR > 100 mL and DSD and DO.
- -
- Correlation between UTI > 0/6 months + PVR > 100 mL and DSD and DO.
2.2. Statistical Analysis
3. Results
3.1. Patient Characteristics, Clinical Parameters, and UDS Findings
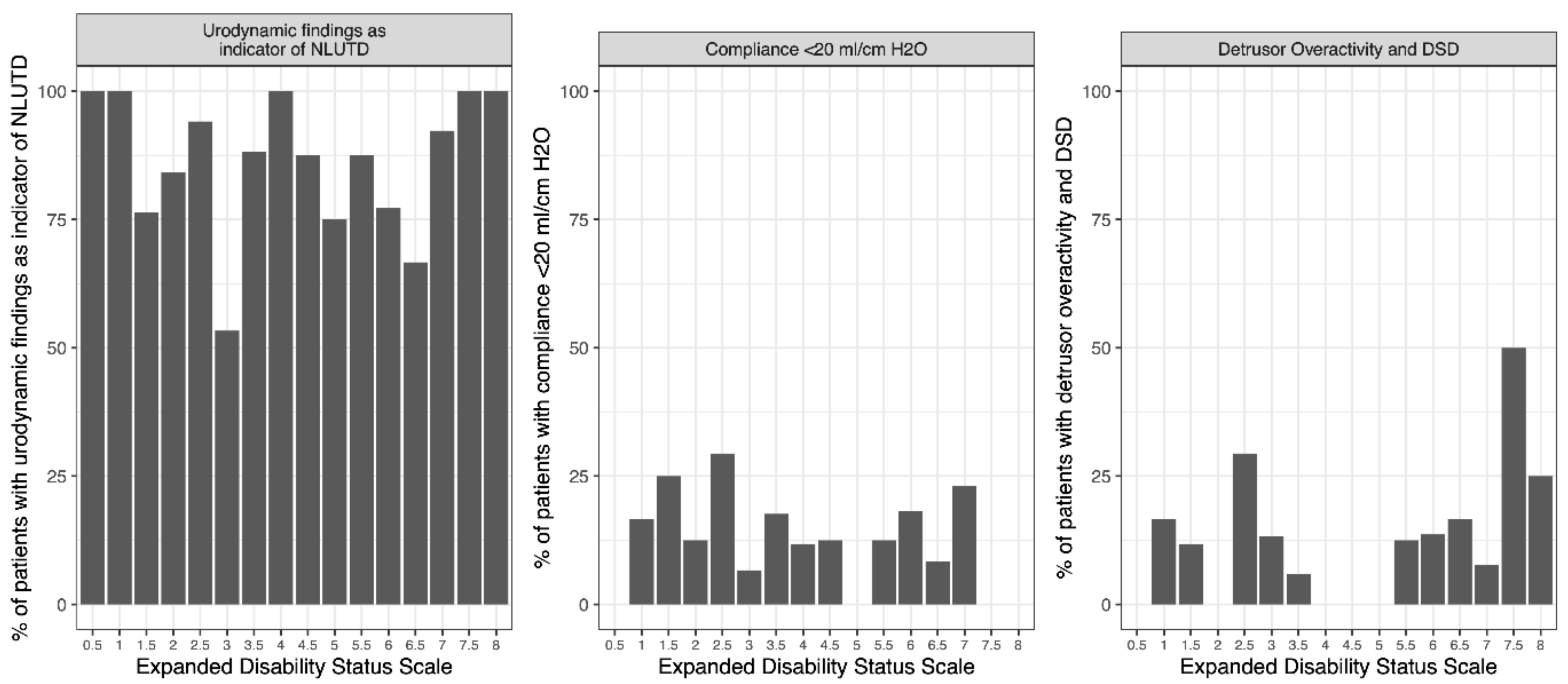
3.2. EDSS Threshold
3.3. Correlations between Clinical Parameters and UDS Findings Indicative of NLUTD or Potential UUTD
3.4. Influence of Combined Clinical Parameters on UDS Findings Indicative of Potential UUTD
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chancellor, M.B.; Blaivas, J.G. Urological and sexual problems in multiple sclerosis. Clin. Neurosci. 1994, 2, 189–195. [Google Scholar]
- Aharony, S.M.; Lam, O.; Corcos, J. Evaluation of lower urinary tract symptoms in multiple sclerosis patients: Review of the literature and current guidelines. Can. Urol. Assoc. J. 2017, 11, 61–64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Litwiller, S.; Frohman, E.; Zimmern, P. Multiple sclerosis and the urologist. J. Urol. 1999, 161, 743–757. [Google Scholar] [CrossRef]
- Kurtzke, J.F. Rating neurologic impairment in multiple sclerosis: An expanded disability status scale (EDSS). Neurology 1983, 33, 1444. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wiedemann, A.; Kaeder, M.; Greulich, W.; Lax, H.; Priebel, J.; Kirschner-Hermanns, R.; Füsgen, I. Which clinical risk factors determine a pathological urodynamic evaluation in patients with multiple sclerosis? An analysis of 100 prospective cases. World J. Urol. 2013, 31, 229–233. [Google Scholar] [CrossRef]
- Giannantoni, A.; Scivoletto, G.; Di Stasi, S.M.; Grasso, M.G.; Agrò, E.F.; Collura, G.; Vespasiani, G. Lower urinary tract dysfunction and disability status in patients with multiple sclerosis. Arch. Phys. Med. Rehabil. 1999, 80, 437–441. [Google Scholar] [CrossRef]
- Koldewijn, E.L.; Hommes, O.R.; Lemmens, W.A.; Debruyne, F.M.; van Kerrebroeck, P.E. Relationship between lower urinary tract abnormalities and disease-related parameters in multiple sclerosis. J. Urol. 1995, 154, 169–173. [Google Scholar] [CrossRef] [Green Version]
- Ineichen, B.V.; Schneider, M.P.; Hlavica, M.; Hagenbuch, N.; Linnebank, M.; Kessler, T.M. High EDSS can predict risk for upper urinary tract damage in patients with multiple sclerosis. Mult. Scler. J. 2018, 24, 529–534. [Google Scholar] [CrossRef]
- Schäfer, W.; Abrams, P.; Liao, L.; Mattiasson, A.; Pesce, F.; Spangberg, A.; Sterling, A.M.; Zinner, N.R.; Kerrebroeck, P.V. Good urodynamic practices: Uroflowmetry, filling cystometry, and pressure-flow studies. Neurour. Urodyn. 2002, 21, 261–274. [Google Scholar] [CrossRef] [PubMed]
- Rosier, P.F.W.; Schaefer, W.; Lose, G.; Goldman, H.B.; Guralnick, M.; Eustice, S.; Dickinson, T.; Hashim, H. International Continence Society Good Urodynamic Practices and Terms 2016: Urodynamics, uroflowmetry, cystometry, and pressure-flow study. Neurourol. Urodyn. 2017, 36, 1243–1260. [Google Scholar] [CrossRef]
- Domurath, B.; Kurze, I.; Kirschner-Hermanns, R.; Kaufmann, A.; Feneberg, W.; Schmidt, P.; Henze, T.; Flachenecker, P.; Brandt, A.; Vance, W.N.; et al. Neurourological assessment in people with multiple sclerosis (MS): A new evaluated algorithm. Mult. Scler. Relat. Disord. 2020, 44, 102248. [Google Scholar] [CrossRef] [PubMed]
- Team, R. Core. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2017. [Google Scholar]
- Amarenco, G.; Chartier-Kastler, E.; Denys, P.; Jean, J.L.; de Sèze, M.; Lubetzski, C. First-line urological evaluation in multiple sclerosis: Validation of a specific decision-making algorithm. Mult. Scler. J. 2013, 19, 1931–1937. [Google Scholar] [CrossRef] [PubMed]
- Medina-Polo, J.; Adot, J.M.; Allué, M.; Arlandis, S.; Blasco, P.; Casanova, B.; Matías-Guiu, J.; Madurga, B.; Meza-Murillo, E.R.; Müller-Arteaga, C.; et al. Consensus document on the multidisciplinary management of neurogenic lower urinary tract dysfunction in patients with multiple sclerosis. Neurourol. Urodyn. 2020, 39, 762–770. [Google Scholar] [CrossRef] [PubMed]
- Ghezzi, A.; Carone, R.; Del Popolo, G.; Amato, M.P.; Bertolotto, A.; Comola, M.; Del Carro, U.; Di Benedetto, P.; Giannantoni, A.; Lopes de Carvalho, M.L.; et al. Recommendations for the management of urinary disorders in multiple sclerosis: A consensus of the Italian Multiple Sclerosis Study Group. Neurol. Sci. 2011, 32, 1223–1231. [Google Scholar] [CrossRef]
- Averbeck, M.A.; Iacovelli, V.; Panicker, J.; Schurch, B.; Finazzi Agrò, E. Urodynamics in patients with multiple sclerosis: A consensus statement from a urodynamic experts working group. Neurourol. Urodyn. 2020, 39, 73–82. [Google Scholar] [CrossRef]
- Nakipoglu, G.F.; Kaya, A.Z.; Orhan, G.; Tezen, O.; Tunc, H.; Ozgirgin, N.; Ak, F. Urinary dysfunction in multiple sclerosis. J. Clin. Neurosci. 2009, 16, 1321–1324. [Google Scholar] [CrossRef]
- Bemelmans, B.L.H.; Hommes, O.R.; Van Kerrebroeck, P.E.V.; Lemmens, W.A.J.G.; Doesburg, W.H.; Debruyne, F.M.J. Evidence for Early Lower Urinary Tract Dysfunction in Clinically Silent Multiple Sclerosis. J. Urol. 1991, 145, 1219–1224. [Google Scholar] [CrossRef]
- De Sèze, M.; Ruffion, A.; Denys, P.; Joseph, P.-A.; Perrouin-Verbe, B. The neurogenic bladder in multiple sclerosis: Review of the literature and proposal of management guidelines. Mult. Scler. J. 2007, 13, 915–928. [Google Scholar] [CrossRef]
- Fowler, C.J.; Panicker, J.N.; Drake, M.; Harris, C.; Harrison, S.C.W.; Kirby, M.; Lucas, M.; Macleod, N.; Mangnall, J.; North, A.; et al. A UK consensus on the management of the bladder in multiple sclerosis. Postgrad. Med. J. 2009, 85, 552–559. [Google Scholar] [CrossRef]
- Çetinel, B.; Tarcan, T.; Demirkesen, O.; Özyurt, C.; Şen, İ.; Erdoğan, S.; Siva, A. Management of lower urinary tract dysfunction in multiple sclerosis: A systematic review and Turkish consensus report. Neurourol. Urodyn. 2013, 32, 1047–1057. [Google Scholar] [CrossRef]
- Colli, E.; Parazzini, F.; Olivieri, L.; Cipriani, S.; Bertozzi, R.; Meschia, M.; Montorsi, F. Number of Daytime Micturitions and Volume Voided per Micturition in the Evaluation of Efficacy of Drugs for Overactive Bladder: Findings from Randomized Clinical Trials. Eur. Urol. 2007, 52, 525–530. [Google Scholar] [CrossRef] [PubMed]
- Van Brummen, H.J.; Heintz, A.P.M.; van der Vaart, C.H. The association between overactive bladder symptoms and objective parameters from bladder diary and filling cystometry. Neurourol. Urodyn. 2004, 23, 38–42. [Google Scholar] [CrossRef] [PubMed]
- Musco, S.; Padilla-Fernández, B.; Del Popolo, G.; Bonifazi, M.; Blok, B.F.M.; Groen, J.; ‘t Hoen, L.; Pannek, J.; Bonzon, J.; Kessler, T.M.; et al. Value of urodynamic findings in predicting upper urinary tract damage in neuro-urological patients: A systematic review. Neurourol. Urodyn. 2018, 37, 1522–1540. [Google Scholar] [CrossRef] [PubMed]
- Wyndaele, J.; Wachter, S.D. Cystometrical Sensory Data from a Normal Population: Comparison of Two Groups of Young Healthy Volunteers Examined with 5 Years Interval. Eur. Urol. 2002, 42, 34–38. [Google Scholar] [CrossRef]
| Mean (SD) | Median (25–75%) | Min–Max | Missing % (n) | |
|---|---|---|---|---|
| Age of patients in years | 49.2 (10.7) | 49 (41–55) | 19–75 | 1.4% (3) |
| Age of MS onset in years | 35.5 (11) | 34 (28–42) | 14–71 | 2.9% (6) |
| Disease duration in years | 13.7 (9.5) | 13 (6–20) | 0–46 | 2.9% (6) |
| MS Type | % (n) | |||
| PPMS | 10.9% (22) | 2.4% (5) | ||
| RRMS | 46.5% (94) | |||
| SPMS | 42.6% (86) |
| % (n) | Mean (SD) | Median (25–75%) | Min–Max | Missing % (n) | ||
|---|---|---|---|---|---|---|
| BD | Daily fluid intake [mL] | 1908.7 (619.1) | 1850 (1500–2200) | 500–5000 | 2.9% (6) | |
| Daily urine outtake [mL] | 1705.3 (689.7) | 1600 (1200–2158) | 555–3950 | 19.3% (40) | ||
| Average VV [mL] | 239 (122.6) | 215 (150–300) | 50–925 | 18.4% (38) | ||
| Average VF at day | 8.2 (3.8) | 7 (6–10) | 2–30 | 8.7% (18) | ||
| Average VF at night | 1.8 (1.9) | 1 (1–2) | 0–14 | 7.7% (16) | ||
| UTI | Per 6 month | |||||
| 0 | 74.2% (147) | 4.3% (9) | ||||
| 1 | 9.6% (19) | |||||
| 2 | 5.6% (11) | |||||
| 3 | 6.6% (13) | |||||
| >3 | 4% (8) | |||||
| EDSS | 4.1 (2) | 4 (2.5–6) | 0.5–8 | 8.7% (18) | ||
| UF | Qmax [mL/s] | 1 (12) | 16.7 (11–23) | 0–68.1 | 19.3% (40) | |
| VV [mL] | 260.8 (212.3) | 209 (120–336.5) | 0–1300 | 14% (29) | ||
| PVR [mL] | 80.5 (101.7) | 42.5 (12.8–113.2) | 0–580 | 11.1% (23) | ||
| Abnormal curve | 55.7% (98) | 15% (31) | ||||
| UDS | First desire to void [mL] | 207.5 (126.9) | 177 (112–286) | 8–710 | 6.8% (14) | |
| Strong desire to void [mL] | 302.3 (141.5) | 286 (202–385) | 33–828 | 14.5% (30) | ||
| Compliance [mL/cm H2O] | 63.4 (60.1) | 52 (25–83) | 0–453.4 | 3.4% (7) | ||
| DO | 40.1% (81) | 2.4% (5) | ||||
| DSD | 31.2% (59) | 8.7% (18) | ||||
| Max. BC [mL] | 405.8 (158) | 401 (299–495) | 80–1000 | 3.4% (7) | ||
| Symptomatology with Regard to NLUTD | |||
|---|---|---|---|
| Abnormal UDS | |||
| n | % | n | |
| Symptomatic | 101 | 87 | 88 |
| Asymptomatic | 106 | 79 | 84 |
| Symptomatology with Regard to Risk of UUTD | |||
| Abnormal UDS | |||
| n | % | n | |
| Symptomatic | 101 | 26 | 26 |
| Asymptomatic | 99 | 13 | 13 |
| EDSS | Compliance < 20 mL/cm H2O | DO and DSD | ||
|---|---|---|---|---|
| No | Yes | No | Yes | |
| <5 | 83.3% (95) | 16.7% (19) | 90.7% (107) | 9.3% (11) |
| ≥5 | 87.3% (62) | 12.7% (9) | 85.9% (61) | 14.1% (10) |
| Odds Ratio | p -Value | Sens | Spec | PPV | NPV | |
|---|---|---|---|---|---|---|
| Compliance < 20 mL/cm H2O | 0.73 (0.27–1.82) | 0.53 | 0.32 | 0.61 | 0.13 | 0.83 |
| DO and DSD | 1.59 (0.57–4.39) | 0.34 | 0.48 | 0.64 | 0.14 | 0.91 |
| Urodynamic Findings Indicative of NLUTD | ||||||
|---|---|---|---|---|---|---|
| Odds Ratio | p-Value | Sens | Spec | PPV | NPV | |
| SVF ≤ 4/24 h | 0 (0–1.06) | 0.03 | 0.00 | 0.94 | 0.00 | 0.16 |
| SVF ≥ 13/24 h | 7.4 (2.15–39.66) | 0.00 | 0.44 | 0.90 | 0.96 | 0.25 |
| VV ≤ 250 mL | 4.53 (1.85–11.99) | 0.00 | 0.64 | 0.72 | 0.91 | 0.31 |
| VV ≥ 500 mL | 0.3 (0.11–0.87) | 0.02 | 0.10 | 0.72 | 0.62 | 0.15 |
| Uroflowmetry | 1.91 (0.75–5.02) | 0.20 | 0.54 | 0.62 | 0.81 | 0.31 |
| PVR > 70 mL | 6.43 (1.87–34.4) | 0.00 | 0.40 | 0.91 | 0.95 | 0.24 |
| PVR > 100 mL | 4.17 (1.20–22.46) | 0.02 | 0.30 | 0.91 | 0.94 | 0.21 |
| UTI > 0/6 months | 3.91 (1.13–21.00) | 0.03 | 0.29 | 0.91 | 0.94 | 0.20 |
| Compliance < 20 mL/cm H2O | ||||||
| Odds Ratio | p-Value | Sens | Spec | PPV | NPV | |
| SVF ≤ 4/24 h | 0 (0–28.03) | 1.00 | 0.00 | 0.99 | 0.00 | 0.84 |
| SVF ≥ 13/24 h | 3.7 (1.51–9.61) | 0.00 | 0.66 | 0.66 | 0.27 | 0.91 |
| VV ≤ 250 mL | 3.91 (1.22–16.56) | 0.01 | 0.83 | 0.45 | 0.19 | 0.94 |
| VV ≥ 500 mL | 0.59 (0.06–2.71) | 0.74 | 0.09 | 0.86 | 0.09 | 0.86 |
| Uroflowmetry | 0.25 (0.02–1.39) | 0.09 | 0.22 | 0.46 | 0.03 | 0.88 |
| PVR > 70 mL | 0.75 (0.27–1.94) | 0.66 | 0.30 | 0.64 | 0.13 | 0.84 |
| PVR > 100 mL | 0.76 (0.23–2.11) | 0.64 | 0.22 | 0.73 | 0.12 | 0.84 |
| UTI > 0/6 months | 2.52 (1.03–6.10) | 0.04 | 0.43 | 0.77 | 0.25 | 0.88 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Beck, J.; Jaekel, A.K.; Zeller, F.L.; Kowollik, M.; Kurze, I.; Kaufmann, A.; Feneberg, W.; Brandt, A.; Flachenecker, P.; Henze, T.; et al. Clinical Predictors of Neurogenic Lower Urinary Tract Dysfunction in Persons with Multiple Sclerosis. Diagnostics 2022, 12, 191. https://doi.org/10.3390/diagnostics12010191
Beck J, Jaekel AK, Zeller FL, Kowollik M, Kurze I, Kaufmann A, Feneberg W, Brandt A, Flachenecker P, Henze T, et al. Clinical Predictors of Neurogenic Lower Urinary Tract Dysfunction in Persons with Multiple Sclerosis. Diagnostics. 2022; 12(1):191. https://doi.org/10.3390/diagnostics12010191
Chicago/Turabian StyleBeck, Janina, Anke Kirsten Jaekel, Federico Leopoldo Zeller, Michael Kowollik, Ines Kurze, Albert Kaufmann, Wolfgang Feneberg, Anna Brandt, Peter Flachenecker, Thomas Henze, and et al. 2022. "Clinical Predictors of Neurogenic Lower Urinary Tract Dysfunction in Persons with Multiple Sclerosis" Diagnostics 12, no. 1: 191. https://doi.org/10.3390/diagnostics12010191
APA StyleBeck, J., Jaekel, A. K., Zeller, F. L., Kowollik, M., Kurze, I., Kaufmann, A., Feneberg, W., Brandt, A., Flachenecker, P., Henze, T., Domurath, B., Schmidt, P., Vance, W. N., Goldschmidt, F., Kirschner-Hermanns, R. K. M., & Knüpfer, S. C. (2022). Clinical Predictors of Neurogenic Lower Urinary Tract Dysfunction in Persons with Multiple Sclerosis. Diagnostics, 12(1), 191. https://doi.org/10.3390/diagnostics12010191






