Information Processing Speed Assessed with Letter Digit Substitution Test in Croatian Sample of Multiple Sclerosis Patients
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Questionnaires
2.2.1. General Questionnaire
2.2.2. Letter Digit Substitution Test (LDST)
2.2.3. Multiple Sclerosis Impact Scale (MSIS-29)
2.3. Validation Procedure
2.4. Statistical Analysis
3. Results
3.1. Overview Results
3.2. Psychometric Properties of the MSIS-29
3.3. Construct Validity of the LDST
3.4. Convergent and Divergent Validity of the LDST
3.5. Multiple Regression Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Howard, J.; Trevick, S.; Younger, D.S. Epidemiology of Multiple Sclerosis. Neurol. Clin. 2016, 34, 919–939. [Google Scholar] [CrossRef]
- Chiaravalloti, N.D.; DeLuca, J. Cognitive impairment in multiple sclerosis. Lancet Neurol 2008, 7, 1139–1151. [Google Scholar] [CrossRef]
- Guimarães, J.; Sá, M.J. Cognitive dysfunction in multiple sclerosis. Front. Neurol. 2012, 3, 74. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Schependom, J.; D‘hooghe, M.B.; Cleynhens, K.; D’hooge, M.; Haelewyck, M.C.; De Keyser, J.; Nagels, G. Reduced information processing speed as primum movens for cognitive decline in MS. Mult. Scler. 2015, 21, 83–91. [Google Scholar] [CrossRef]
- Smith, A. Symbol Digit Modalities Test: Manual; Western Psychological Services: Los Angeles, CA, USA, 1982. [Google Scholar]
- Van der Elst, W.; Van Boxtel, M.P.; Van Breukelen, G.J.; Jolles, J. The Letter Digit Substitution Test: Normative data for 1858 healthy participants aged 24–81 from the Maastricht Aging Study (MAAS): Influence of age, education, and sex. J. Clin. Exp. Neuropsychol. 2006, 28, 998–1009. [Google Scholar] [CrossRef]
- Jolles, J.; van Boxtel, M.P.; Ponds, R.W.; Metsemakers, J.F.; Houx, P.J. The Maastricht aging study (MAAS). The longitudinal perspective of cognitive aging. Tijdschr. Gerontol. Geriatr. 1998, 29, 120–129. [Google Scholar] [PubMed]
- Wechsler, D. Manual for the Wechsler Adult Intelligence Scale-Revised; Psychological Corporation: New York, NY, USA, 1981. [Google Scholar]
- Langdon, D.W.; Amato, M.P.; Boringa, J.; Brochet, B.; Foley, F.; Fredrikson, S.; Hamalainen, P.; Hartung, H.P.; Krupp, L.; Penner, I.K. Recommendations for a Brief International Cognitive Assessment for Multiple Sclerosis (BICAMS). Mult. Scler. 2012, 18, 891–898. [Google Scholar] [CrossRef] [Green Version]
- Hobart, J.; Lamping, D.; Fitzpatrick, R.; Riazi, A.; Thompson, A. The Multiple Sclerosis Impact Scale (MSIS-29); a new patient-based outcome measure. Brain 2001, 124, 962–973. [Google Scholar] [CrossRef]
- Rogić Vidaković, M.; Šimić, N.; Poljičanin, A.; Ivanišević, M.N.; Ana, J.; Đogaš, Z. Psychometric properties of the Croatian Version of the Depression, Anxiety, and Stress Scale-21 and Multiple Sclerosis Impact Scale-29 in Multiple Sclerosis Patients. Mult. Scler. Relat. Disord. 2021, 50, 102850. [Google Scholar] [CrossRef]
- Lublin, F.D.; Reingold, S.C.; Cohen, J.A.; Cutter, G.R.; Sorensen, P.S.; Thompson, A.J.; Wolinsky, J.S.; Balcer, L.J.; Banwell, B.; Barkhof, F.; et al. Defining the clinical course of multiple sclerosis: The 2013 revisions. Neurology 2014, 83, 278–286. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kurtzke, J.F. Rating neurologic impairment in multiple sclerosis: An expanded disability status scale (EDSS). Neurology 1983, 33, 1444–14452. [Google Scholar] [CrossRef] [Green Version]
- Møller, J.T.; Cluitmans, P.; Rasmussen, L.S.; Houx, P.; Rasmussen, H.; Canet, J.; Rabbitt, P.; Jolles, J.; Larsen, K.; Hanning, C.D.; et al. Long-term postoperative cognitive dysfunction in the elderly: ISPOCD1 study. Lancet 1998, 351, 857–861. [Google Scholar] [CrossRef]
- De Groot, R.H.M.; Hornstra, G.; Rozendaal, N.; Jolles, J. Memory performance, but not information processing speed, may be reduced during early pregnancy. J. Clin. Exp. Neuropsychol. 2003, 25, 482–488. [Google Scholar] [CrossRef]
- Dijkstra, J.B.; Strik, J.J.M.H.; Lousberg, R.; Prickaerts, J.; Riedel, W.J.; Jolles, J.; van Praag, H.M.; Honig, A. Atypical cognitive profile in patients with depression after myocardial infarction. J. Affect. Disord. 2002, 70, 181–190. [Google Scholar] [CrossRef]
- Jelicic, M.; Van Boxtel, M.P.J.; Houx, P.J.; Jolles, J. Does migraine headache affect cognitive function in the elderly? Report from the Maastricht Aging Study (MAAS). Headache 2000, 40, 715–719. [Google Scholar] [CrossRef] [Green Version]
- Van Boxtel, P.J.; Gaillard, C.; Houx, P.J.; Buntinx, F.; de Leeuw, P.W.; Jolles, J. Can blood pressure predict cognitive task performance in a healthy population sample? J. Hypertens. 1997, 15, 1069–1076. [Google Scholar] [CrossRef] [Green Version]
- Knežević, M.; Tarabić, B.N.; Tomac, P.; Vincek, A.; Ivanda, L. Uloga dobi, spola i obrazovanja u brzini procesiranja informacija [Role of age, gender and education in information processing speed]. Psihologijske Teme 2015, 24, 173–185. [Google Scholar]
- Benedict RHb DeLuca, J.; Phillips, G.; LaRocca, N.; Hudson, L.D.; Rudick, R.; Multiple Sclerosis Outcome Assessments Consortium. Validity of the Symbol Digit Modalities Test as a cognition performance outcome measure for multiple sclerosis. Mult. Scler. 2017, 23, 721–733. [Google Scholar] [CrossRef]
- Brochet, B.; Ruet, A. Cognitive Impairment in Multiple Sclerosis with Regards to Disease Duration and Clinical Phenotypes. Front. Neurol. 2019, 10, 261. [Google Scholar] [CrossRef] [Green Version]
- Rooney, S.; Wood, L.; Moffat, F.; Paul, L. Prevalence of fatigue and its association with clinical features in progressive and non-progressive forms of Multiple Sclerosis. Mult. Scler. Relat. Disord. 2019, 28, 276–282. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Polman, C.H.; Reingold, S.C.; Edan, G.; Filippi, M.; Hartung, H.-P.; Kappos, L.; Lublin, F.D.; Metz, L.M.; McFarland, H.F.; O’Connor, P.W. Diagnostic criteria for multiple sclerosis: 2005 revisions to the “McDonald criteria”. Ann. Neurol. 2005, 58, 840–846. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Katz Sand, I. Classification, diagnosis, and differential diagnosis of multiple sclerosis. Curr. Opin. Neurol. 2015, 28, 193–205. [Google Scholar] [CrossRef]
- Dusankova, J.B.; Kalincik, T.; Havrdova, E.; Benedict, R.H. Cross cultural validation of the Minimal Assessment of Cognitive Function in Multiple Sclerosis (MACFIMS) and the Brief International Cognitive Assessment for Multiple Sclerosis (BICAMS). Clin. Neuropsychol. 2012, 26, 1186–1200. [Google Scholar] [CrossRef]
- Filser, M.; Schreiber, H.; Pöttgen, J.; Ullrich, S.; Lang, M.; Penner, I.K. The Brief International Cognitive Assessment in Multiple Sclerosis (BICAMS): Results from the German Validation Study. J. Neurol. 2018, 265, 2587–2593. [Google Scholar] [CrossRef] [PubMed]
- Goretti, B.; Niccolai, C.; Hakiki, B.; Sturchio, A.; Falautano, M.; Minacapelli, E.; Martinelli, V.; Incerti, C.; Nocentini, U.; Murgia, M.; et al. The Brief International Cognitive Assessment for Multiple Sclerosis (BICAMS): Normative values with gender, age and education corrections in the Italian population. BMC Neurol. 2014, 14, 171. [Google Scholar] [CrossRef] [Green Version]
- Marstrand, L.; Østerberg, O.; Walsted, T.; Skov, A.C.; Schreiber, K.I.; Sellebjerg, F. Brief international cognitive assessment for multiple sclerosis (BICAMS): A danish validation study of sensitivity in early stages of MS. Mult. Scler. Relat. Disord. 2020, 37, 101458. [Google Scholar] [CrossRef]
- Estiasari, R.; Fajrina, Y.; Lastri, D.N.; Melani, S.; Maharani, K.; Imran, D.; Pangeran, D.; Sitorus, F. Validity and Reliability of Brief International Cognitive Assessment for Multiple Sclerosis (BICAMS) in Indonesia and the Correlation with Quality of Life. Neurol. Res. Int. 2019, 2019, 4290352. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sandi, D.; Rudisch, T.; Füvesi, J.; Fricska-Nagy, Z.; Huszka, H.; Biernacki, T.; Langdon, D.W.; Langane, E.; Vecsei, L.; Bencsik, K. The Hungarian validation of the Brief International Cognitive Assessment for Multiple Sclerosis (BICAMS) battery and the correlation of cognitive impairment with fatigue and quality of life. Mult. Scler. Relat. Disord. 2015, 4, 499–504. [Google Scholar] [CrossRef] [Green Version]
- Sousa, C.; Rigueiro-Neves, M.; Miranda, T.; Alegria, P.; Vale, J.; Passos, A.M.; Langdon, D.; Sá, M.J. alidation of the brief international cognitive assessment for multiple sclerosis (BICAMS) in the Portuguese population with multiple sclerosis. BMC Neurol. 2018, 18, 172. [Google Scholar] [CrossRef]
- Betscher, E.; Guenter, W.; Langdon, D.W.; Bonek, R. Polish validation of the Brief International Cognitive Assessment for Multiple Sclerosis (BICAMS battery): Correlation of cognitive impairment with mood disorders and fatigue. Neurol. Neurochir. Pol. 2021, 55, 59–66. [Google Scholar] [CrossRef]
- Alarcón, A.N.; Ayala, O.D.; García, J.R.; Montaňés, P. Validation of the Brief International Cognitive Assessment for Multiple Sclerosis (BICAMS) in a Colombian Population. Mult. Scler. Relat. Disord. 2020, 42, 102072. [Google Scholar] [CrossRef]
- Hämäläinen, P.; Leo, V.; Therman, S.; Ruutiainen, J. Validation of the Finnish version of the Brief International Cognitive Assessment for Multiple Sclerosis (BICAMS) and evaluation of the applicability of the Multiple Sclerosis Neuropsychological Questionnaire (MSNQ) and the Fatigue Scale for Motor and Cognitive Functions (FSMC). Brain Behav. 2021, 11, e02087. [Google Scholar] [PubMed]
- Polychroniadou, E.; Bakirtzis, C.; Langdon, D.; Lagoudaki, R.; Kesidou, E.; Theotokis, P.; Tsalikakis, D.; Poulatsidou, K.; Kyriazis, O.; Boziki, M.; et al. Validation of the Brief International Cognitive Assessment for Multiple Sclerosis (BICAMS) in Greek population with multiple sclerosis. Mult. Scler. Relat. Disord. 2016, 9, 68–72. [Google Scholar] [CrossRef] [Green Version]
- Niino, M.; Fukazawa, T.; Kira, J.I.; Okuno, T.; Mori, M.; Sanjo, N.; Ohashi, T.; Fukaura, H.; Fujimori, J.; Shimizu, Y.; et al. Validation of the Brief International Cognitive Assessment for Multiple Sclerosis in Japan. Mult. Scler. J. Exp. Transl. Clin. 2017, 3, 2055217317748972. [Google Scholar] [CrossRef] [PubMed]
- Maubeuge, N.; Deloire, M.S.A.; Brochet, B.; Erhlé, N.; Charré-Morin, J.; Saubusse, A.; Ruet, A.; BICAFMS Study Investigators. French validation of the Brief International Cognitive Assessment for Multiple Sclerosis. Rev. Neurol. 2021, 177, 73–79. [Google Scholar] [CrossRef] [PubMed]
| Control Subjects (N = 196) | People with MS (N = 87) | Test | p-Value | |
|---|---|---|---|---|
| Age in years (mean ± SD) Female (mean ± SD) Male (mean ± SD) | 44.2 ± 14.2 43.8 ± 13.3 45.0 ± 16.2 | 42.8 ± 12.2 42.7 ± 12.5 42.1 ± 10.9 | t = 0.9 | p = 0.37 |
| Age (range) | 18–81 | 19–73 | ||
| Sex | χ2 = 3.5 | p = 0.06 | ||
| Female | 70% | 81% | ||
| Male | 30% | 18% | ||
| EDSS (median ± IQR, range) | 1.0 ± 2.5, 0–80–9 | |||
| EDSS † | 1.0 ± 2.0 | |||
| EDSS †† | 6 ± 1.0 | |||
| LDST and self-report scale (mean ± SD) | ||||
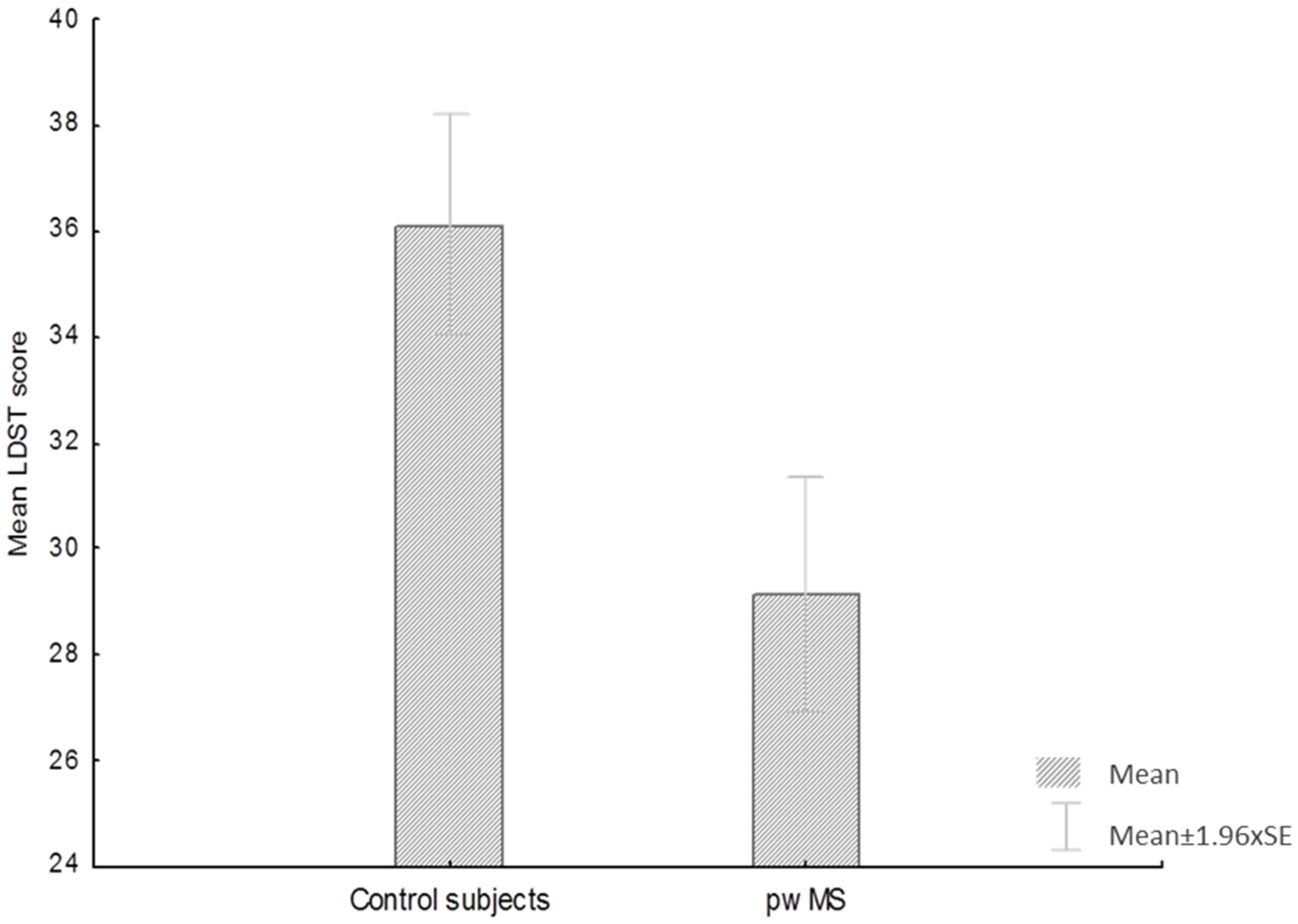
| LDST | 36.6 ± 10.2 | 29.2 ± 10.4 | t = 5.2 | p < 0.01 |
| MSIS-29 PHYS | 45.1 ± 18.0 | |||
| MSIS-29 PSY | 22.1 ± 9.7 |
| % | LDST (Mean ± SD) | Test | p-Value | ||
|---|---|---|---|---|---|
| Sex | Women | 82.0 | 29.3 (10.4) | p = 0.22 | >0.05 |
| Men | 18.0 | 28.6 (10.6) | |||
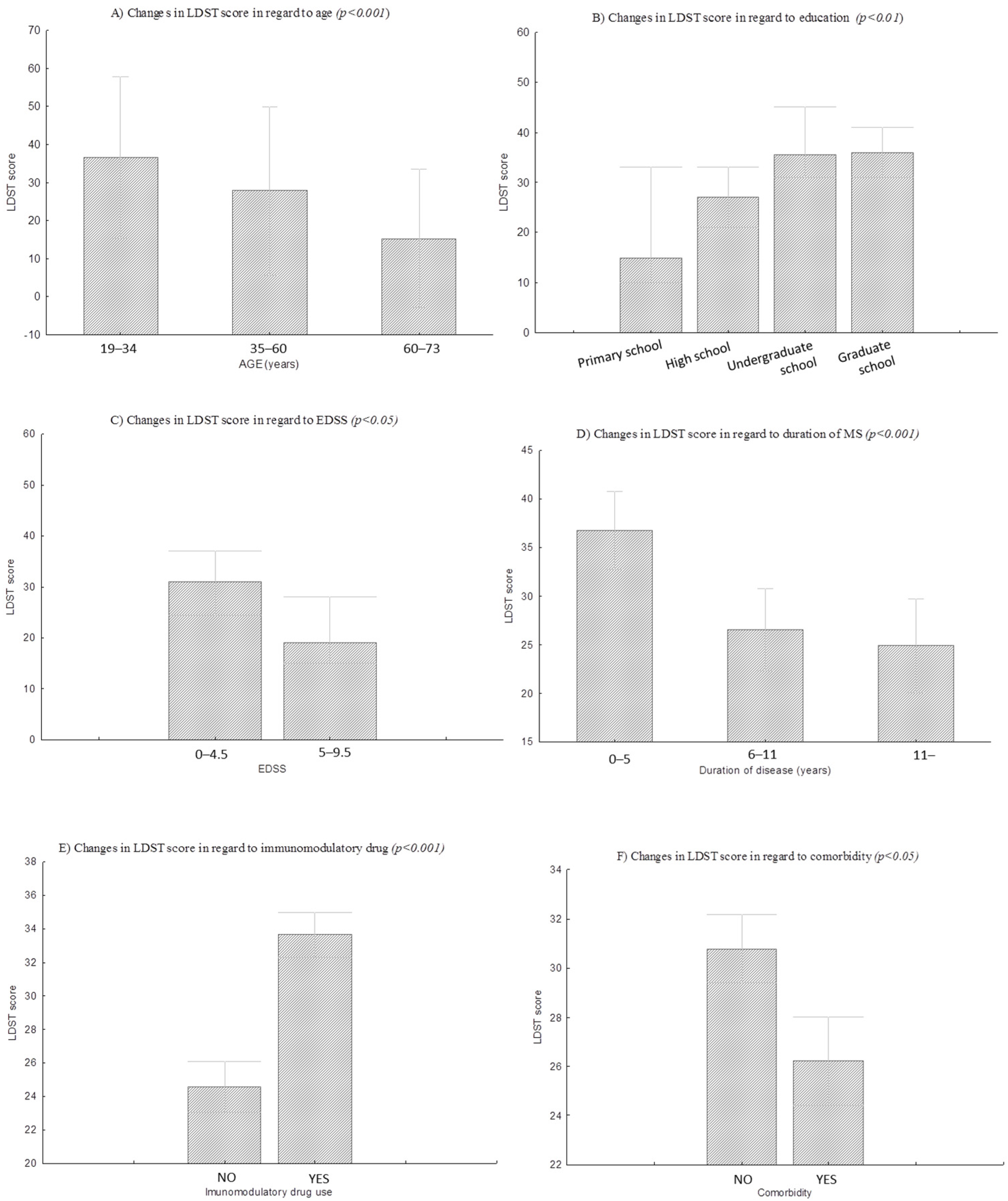
| Age (years) | 19–34 years | 28.7 | 36.5 (8.5) | H = 26.2 | p < 0.001 |
| 35–60 years | 62.0 | 27.8 (8.9) | |||
| 60–73 years | 9.3 | 15.3 (7.3) | |||
| Education | Primary school | 4.3 | 19.3 (12.1) | H = 15.3 | p < 0.01 |
| High school | 65.5 | 26.8 (9.5) | |||
| Undergraduate studies | 9.5 | 37.4 (7.6) | |||
| Graduate study | 20.7 | 34.2 (10.2) | |||
| EDSS | EDSS † | 85 | 29.8 (10.3) | Z = 1.96 | <0.05 |
| EDSS †† | 15 | 22 (9.1) | |||
| Comorbidity | YES | 64.4 | 26.2 (10.0) | t = 1.99 | <0.05 |
| NO | 35.6 | 30.8 (10.3) | |||
| Duration of MS disease | 0 to 5 years | 32.2 | 35.6 (8.5) | F = 10.8 | <0.001 |
| 6 to 11 years | 43.5 | 26.8 (9.3) | |||
| over 11 years | 24.3 | 24.9 (10.8) | |||
| MS type | RRMS | 79.0 | 30.2 (10.2) | H = 6.5 | >0.05 |
| SPMS | 2.5 | 28.5 (4.9) | |||
| PPMS | 9.2 | 20.3 (9.7) | |||
| Not known | 6.8 | 26.6 (11.8) | |||
| Immunomodulatory drug | YES | 51.0 | 24.6 (9.9) | t = −4.5 | <0.001 |
| NO | 49.0 | 33.6 (8.9) |
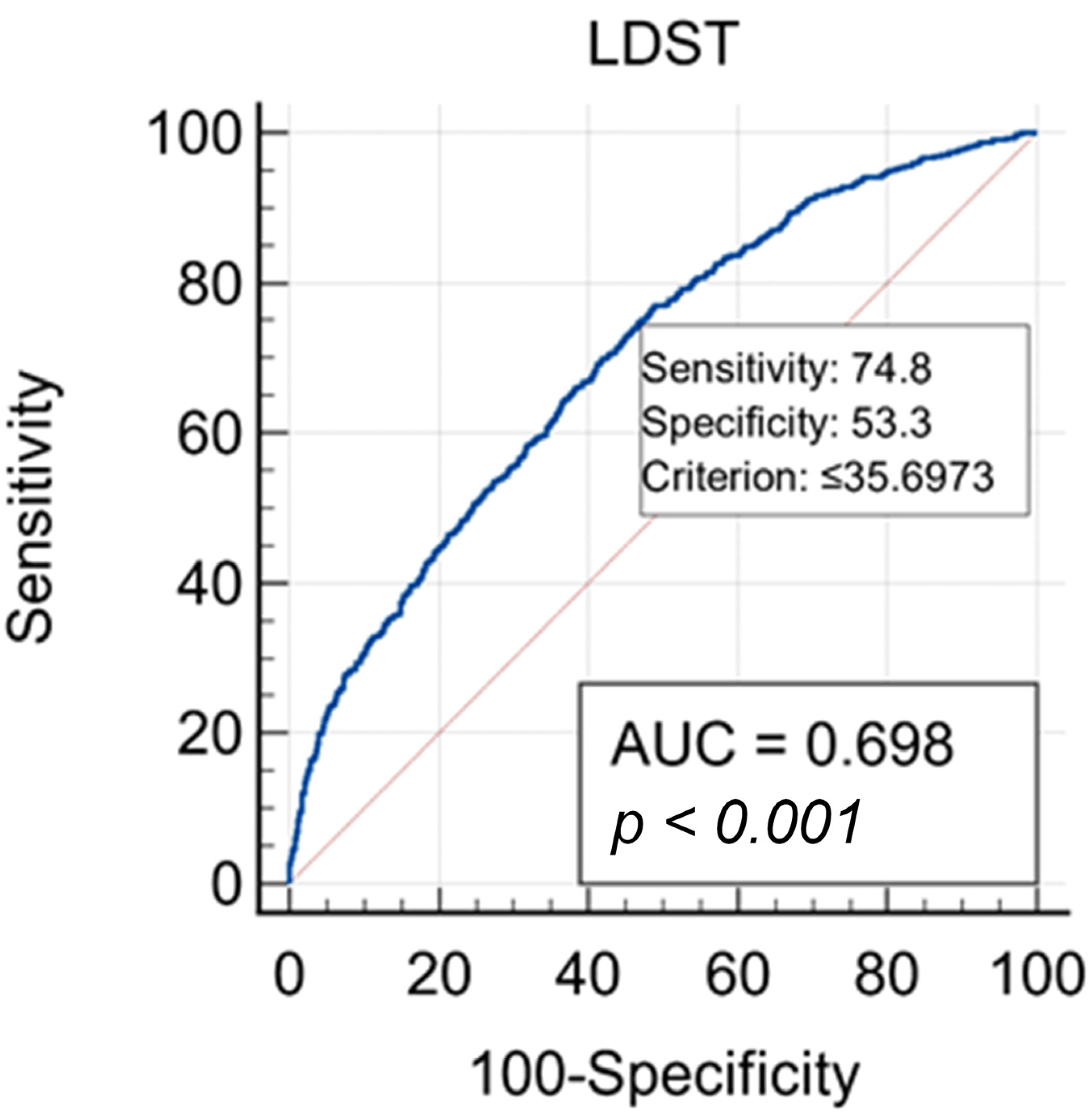
| LDST Scores | Sensitivity | Specificity | +LR | −LR |
|---|---|---|---|---|
| ≤15 | 9.40 | 98.60 | 6.71 | 0.92 |
| ≤20 | 20.90 | 95.40 | 4.54 | 0.83 |
| ≤25 | 35.10 | 86.70 | 2.64 | 0.75 |
| ≤30 | 53.90 | 71.90 | 1.92 | 0.64 |
| ≤35 | 71.50 | 55.60 | 1.61 | 0.51 |
| ≤40 | 86.10 | 36.20 | 1.35 | 0.38 |
| ≤45 | 94.20 | 22.30 | 1.21 | 0.26 |
| ≤50 | 97.90 | 9.60 | 1.08 | 0.22 |
| LDST | MSIS-29 PHYS | MSIS-29 PSY | EDSS | |
|---|---|---|---|---|
| LDST | - | −0.64 ** | −0.40 ** | −0.36 * |
| MSIS-29 PHYS | - | 0.76 ** | 0.48 ** | |
| MSIS-29 PSY | - | 0.20 | ||
| EDSS | - |
| LDST | |||
|---|---|---|---|
| Step1 | Step2 | ||
| Predictors | β | β | |
| Step 1 | Age | −0.50 ** | −0.49 ** |
| Education | 0.24 ** | 0.18 | |
| R2 | 0.41 ** | ||
| Step 2 | EDSS | −0.19 ** | |
| Comorbidity | −0.03 | ||
| Duration of disease | −0.08 | ||
| R2 | 0.47 ** | ||
| ΔR2 | 0.046 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jerković, A.; Matijaca, M.; Proroković, A.; Šikić, A.; Košta, V.; Ćurković Katić, A.; Dolić, K.; Duka Glavor, K.; Šoda, J.; Đogaš, Z.; et al. Information Processing Speed Assessed with Letter Digit Substitution Test in Croatian Sample of Multiple Sclerosis Patients. Diagnostics 2022, 12, 111. https://doi.org/10.3390/diagnostics12010111
Jerković A, Matijaca M, Proroković A, Šikić A, Košta V, Ćurković Katić A, Dolić K, Duka Glavor K, Šoda J, Đogaš Z, et al. Information Processing Speed Assessed with Letter Digit Substitution Test in Croatian Sample of Multiple Sclerosis Patients. Diagnostics. 2022; 12(1):111. https://doi.org/10.3390/diagnostics12010111
Chicago/Turabian StyleJerković, Ana, Meri Matijaca, Ana Proroković, Anđela Šikić, Vana Košta, Ana Ćurković Katić, Krešimir Dolić, Klaudia Duka Glavor, Joško Šoda, Zoran Đogaš, and et al. 2022. "Information Processing Speed Assessed with Letter Digit Substitution Test in Croatian Sample of Multiple Sclerosis Patients" Diagnostics 12, no. 1: 111. https://doi.org/10.3390/diagnostics12010111
APA StyleJerković, A., Matijaca, M., Proroković, A., Šikić, A., Košta, V., Ćurković Katić, A., Dolić, K., Duka Glavor, K., Šoda, J., Đogaš, Z., & Rogić Vidaković, M. (2022). Information Processing Speed Assessed with Letter Digit Substitution Test in Croatian Sample of Multiple Sclerosis Patients. Diagnostics, 12(1), 111. https://doi.org/10.3390/diagnostics12010111








