The Diagnostic and Prognostic Utility of Contemporary Cardiac Magnetic Resonance in Suspected Acute Myocarditis
Abstract
:1. Introduction
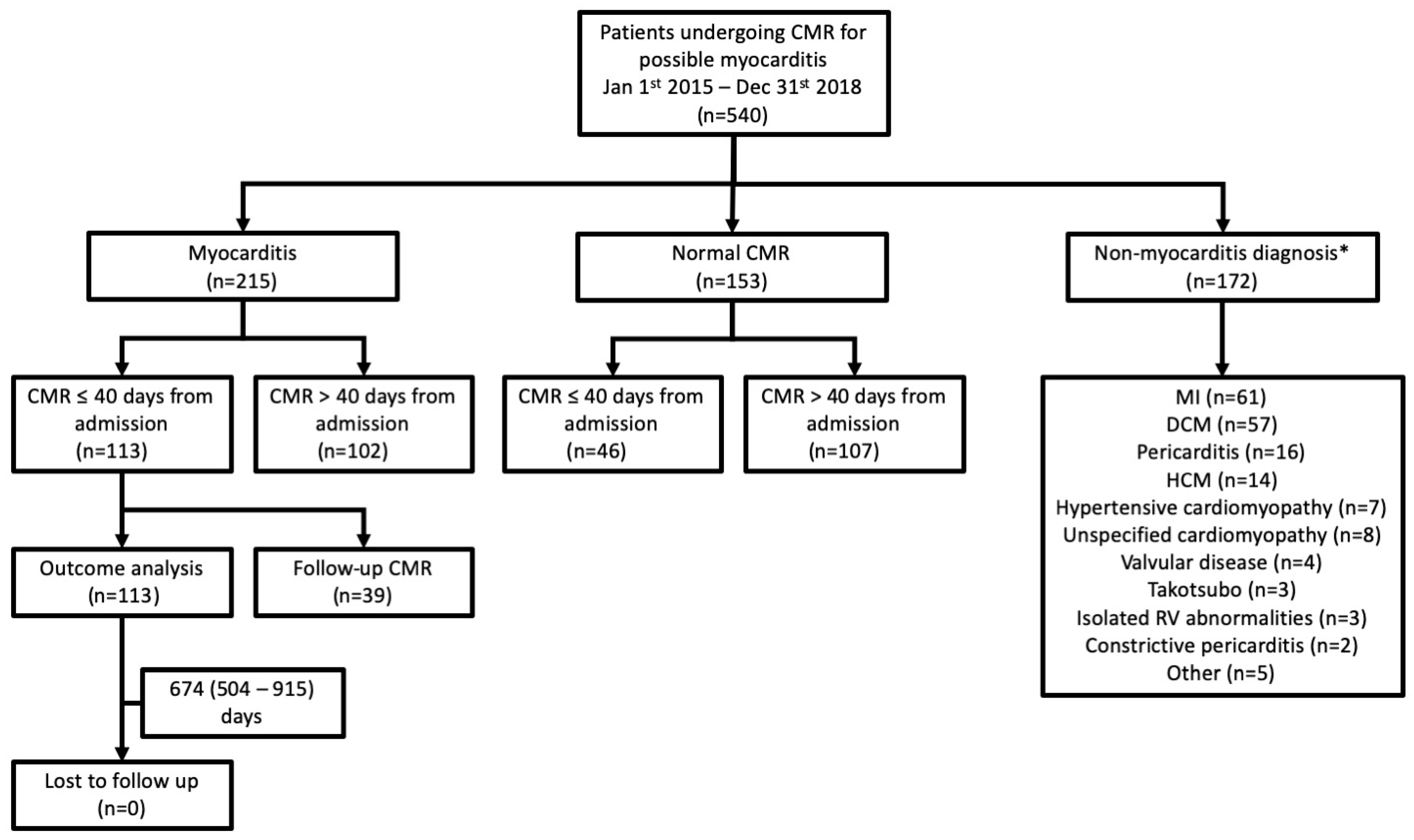
2. Materials and Methods
2.1. Study Design and Participants
2.2. Study Procedures
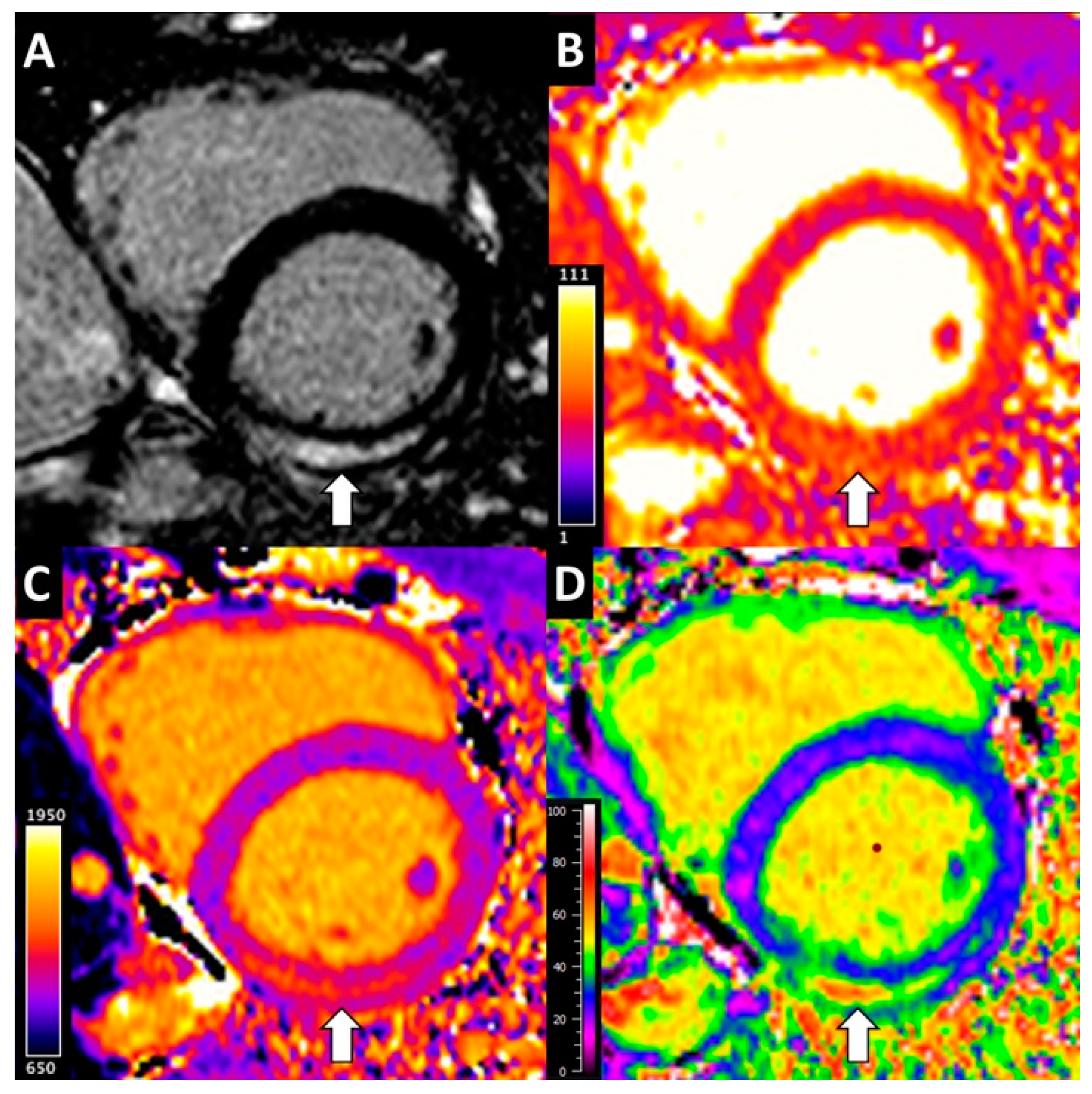
2.3. CMR Analysis
2.4. Study Outcomes
2.5. Statistical Analysis
3. Results
3.1. Diagnostic Yield of CMR
3.2. Relationship between Demographics, Laboratory Findings and Scan Timing, and CMR Diagnosis of Acute Myocarditis
3.3. Relationship between Clinical Presentation and CMR Measurements of Myocardial Injury
3.4. Factors Associated with LV Functional Recovery Following Acute Myocarditis
3.5. Factors Associated with Clinical Outcome Following Acute Myocarditis
4. Discussion
5. Conclusions
6. Limitations
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ferreira, V.M.; Schulz-Menger, J.; Holmvang, G.; Kramer, C.M.; Carbone, I.; Sechtem, U.; Friedrich, M.G. Cardiovascular Magnetic Resonance in Nonischemic Myocardial Inflammation: Expert Recommendations. J. Am. Coll. Cardiol. 2018, 72, 3158–3176. [Google Scholar] [CrossRef]
- Luetkens, J.A.; Homsi, R.; Dabir, D.; Kuetting, D.L.; Marx, C.; Doerner, J.; Schlesinger-Irsch, U.; Andrié, R.; Sprinkart, A.M.; Schmeel, F.C.; et al. Comprehensive Cardiac Magnetic Resonance for Short-Term Follow-Up in Acute Myocarditis. J. Am. Heart Assoc. 2016, 5. [Google Scholar] [CrossRef] [Green Version]
- Bohnen, S.; Radunski, U.; Lund, G.; Ojeda, F.; Looft, Y.; Senel, M.; Radziwolek, L.; Avanesov, M.; Tahir, E.; Stehning, C.; et al. Tissue characterization by T1 and T2 mapping cardiovascular magnetic resonance imaging to monitor myocardial inflammation in healing myocarditis. Eur. Heart J.-Cardiovasc. Imaging 2017, 18, 744–751. [Google Scholar] [CrossRef]
- Baeßler, B.; Schaarschmidt, F.; Dick, A.; Stehning, C.; Schnackenburg, B.; Michels, G.; Maintz, D.; Bunck, A.C. Mapping tissue inhomogeneity in acute myocarditis: A novel analytical approach to quantitative myocardial edema imaging by T2-mapping. J. Cardiovasc. Magn. Reson. 2015, 17, 115. [Google Scholar] [CrossRef] [Green Version]
- Bohnen, S.; Radunski, U.K.; Lund, G.K.; Kandolf, R.; Stehning, C.; Schnackenburg, B.; Adam, G.; Blankenberg, S.; Muellerleile, K. Performance of T1 and T2 Mapping Cardiovascular Magnetic Resonance to Detect Active Myocarditis in Patients with Recent-Onset Heart Failure. Circ. Cardiovasc. Imaging 2015, 8, e003073. [Google Scholar] [CrossRef] [Green Version]
- Thavendiranathan, P.; Walls, M.; Gir, S.; Verhaert, D.; Rajagopalan, S.; Moore, S.; Raman, S.V. Improved Detection of Myocardial Involvement in Acute Inflammatory Cardiomyopathies Using T2 Mapping. Circ. Cardiovasc. Imaging 2012, 5, 102–110. [Google Scholar] [CrossRef] [Green Version]
- Radunski, U.K.; Lund, G.K.; Stehning, C.; Schnackenburg, B.; Bohnen, S.; Adam, G.; Muellerleile, K. CMR in Patients with Severe Myocarditis Diagnostic Value of Quantitative Tissue Markers ncluding Extracellular Volume Imaging. JACC Cardiovasc. Imaging 2014, 7, 557–575. [Google Scholar]
- Bozkurt, B.; Colvin, M.; Cook, J.; Cooper, L.; Deswal, A.; Fonarow, G.; Francis, G.S.; Lenihan, D.; Lewis, E.F.; McNamara, D.M.; et al. Current Diagnostic and Treatment Strategies for Specific Dilated Cardiomyopathies: A Scientific Statement from the American Heart Association. Circulation 2016, 134, e579–e646. [Google Scholar] [CrossRef]
- Ponikowski, P.; Voors, A.A.; Anker, S.D.; Bueno, H.; Cleland, J.G.F.; Coats, A.J.S.; Falk, V.; Nihoyannopoulos, P.; Parissis, J.T.; Pieske, B.; et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC)Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur. J. Heart Fail. 2016, 37, 2129–2200. [Google Scholar]
- Dastidar, A.G.; Baritussio, A.; De Garate, E.; Drobni, Z.; Biglino, G.; Singhal, P.; Bucciarelli-Ducci, C. Prognostic Role of Cardiac MRI and Conventional Risk Factors in Myocardial Infarction with Nonobstructed Coronary Arteries. JACC Cardiovasc. Imaging 2019, 12, 1973–1982. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harris, P.A.; Taylor, R.; Thielke, R.; Payne, J.; Gonzalez, N.; Conde, J.G. Research electronic data capture (REDCap)—A metadata-driven methodology and workflow process for providing translational research informatics support. J. Biomed. Inform. 2009, 42, 377–381. [Google Scholar] [CrossRef] [Green Version]
- Messroghli, D.R.; Moon, J.C.; Ferreira, V.M.; Grosse-Wortmann, L.; He, T.; Kellman, P.; Mascherbauer, J.; Nezafat, R.; Salerno, M.; Schelbert, E.B.; et al. Clinical recommendations for cardiovascular magnetic resonance mapping of T1, T2, T2* and extracellular volume: A consensus statement by the Society for Cardiovascular Magnetic Resonance (SCMR) endorsed by the European Association for Cardiovascular Imaging (EACVI). J. Cardiovasc. Magn. Reson. 2017, 19, 1–24. [Google Scholar] [CrossRef] [Green Version]
- Miller, C.A.; Naish, J.H.; Bishop, P.; Coutts, G.; Clark, D.; Zhao, S.; Schmitt, M. Comprehensive validation of cardiovascular magnetic resonance techniques for the assessment of myocardial extracellular volume. Circ. Cardiovasc. Imaging 2013, 6, 373–683. [Google Scholar] [CrossRef] [Green Version]
- Schelbert, E.B.; Fridman, Y.; Wong, T.C.; Abu Daya, H.; Piehler, K.M.; Kadakkal, A.; Gheorghiade, M. Temporal Relation Between Myocardial Fibrosis and Heart Failure with Preserved Ejection Fraction: Association with Baseline Disease Severity and Subsequent Outcome. JAMA Cardiol. 2017, 2, 995–1006. [Google Scholar] [CrossRef]
- Flett, A.S.; Hasleton, J.; Cook, C.; Hausenloy, D.; Quarta, G.; Ariti, C.; Muthurangu, V.; Moon, J.C. Evaluation of Techniques for the Quantification of Myocardial Scar of Differing Etiology Using Cardiac Magnetic Resonance. JACC Cardiovasc. Imaging 2011, 4, 150–156. [Google Scholar] [CrossRef] [Green Version]
- Biesbroek, P.S.; Hirsch, A.; Zweerink, A.; Van De Ven, P.M.; Beek, A.M.; Groenink, M.; Windhausen, F.; Planken, R.N.; Van Rossum, A.C.; Nijveldt, R. Additional diagnostic value of CMR to the European Society of Cardiology (ESC) position statement criteria in a large clinical population of patients with suspected myocarditis. Eur. Hear. J.-Cardiovasc. Imaging 2017, 19, 1397–1407. [Google Scholar] [CrossRef]
- Schumm, J.; Greulich, S.; Wagner, A.; Grün, S.; Ong, P.; Bentz, K.; Klingel, K.; Kandolf, R.; Bruder, O.; Schneider, S.; et al. Cardiovascular magnetic resonance risk stratification in patients with clinically suspected myocarditis. J. Cardiovasc. Magn. Reson. 2014, 16, 14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lagan, J.; Schmitt, M.; Miller, C.A. Clinical applications of multi-parametric CMR in myocarditis and systemic inflammatory diseases. Int. J. Cardiovasc. Imaging 2017, 34, 35–54. [Google Scholar] [CrossRef]
- Stensaeth, K.H.; Fossum, E.; Hoffmann, P.; Mangschau, A.; Klow, N.E. Clinical characteristics and role of early cardiac magnetic resonance imaging in patients with suspected ST-elevation myocardial infarction and normal coronary arteries. Int. J. Cardiovasc. Imaging 2010, 27, 355–365. [Google Scholar] [CrossRef] [Green Version]
- Kytö, V.; Sipilä, J.; Rautava, P. The effects of gender and age on occurrence of clinically suspected myocarditis in adulthood. Heart 2013, 99, 1681–1684. [Google Scholar] [CrossRef]
- Frisancho-Kiss, S.; Nyland, J.F.; Davis, S.E.; Frisancho, J.A.; Barrett, M.A.; Rose, N.R.; Fairweather, D. Sex differences in coxsackievirus B3-induced myocarditis: IL-12Rbeta1 signaling and IFN-gamma increase inflammation in males independent from STAT4. Brain Res. 2006, 1126, 139–147. [Google Scholar] [CrossRef]
- Gräni, C.; Eichhorn, C.; Bière, L.; Murthy, V.L.; Agarwal, V.; Kaneko, K.; Cuddy, S.; Aghayev, A.; Steigner, M.; Blankstein, R.; et al. Prognostic Value of Cardiac Magnetic Resonance Tissue Characterization in Risk Stratifying Patients with Suspected Myocarditis. J. Am. Coll. Cardiol. 2017, 70, 1964–1976. [Google Scholar] [CrossRef] [PubMed]
- Monney, P.A.; Sekhri, N.; Burchell, T.; Knight, C.; Davies, C.; Deaner, A.; Sheaf, M.; Baithun, S.; Petersen, S.; Wragg, A.; et al. Acute myocarditis presenting as acute coronary syndrome: Role of early cardiac magnetic resonance in its diagnosis. Heart 2010, 97, 1312–1318. [Google Scholar] [CrossRef]
- Lurz, P.; Eitel, I.; Adam, J.; Steiner, J.; Grothoff, M.; Desch, S.; Fuernau, G.; de Waha, S.; Sareban, M.; Luecke, C.; et al. Diagnostic Performance of CMR Imaging Compared with EMB in Patients with Suspected Myocarditis. JACC Cardiovasc. Imaging 2012, 5, 513–524. [Google Scholar] [CrossRef] [PubMed]
- Ingkanisorn, W.; Rhoads, K.L.; Aletras, A.; Kellman, P.; Arai, A.E. Gadolinium delayed enhancement cardiovascular magnetic resonance correlates with clinical measures of myocardial infarction. J. Am. Coll. Cardiol. 2004, 43, 2253–2259. [Google Scholar] [CrossRef]
- Mahrholdt, H.; Wagner, A.; Deluigi, C.C.; Kispert, E.; Hager, S.; Meinhardt, G.; Vogelsberg, H.; Fritz, P.; Dippon, J.; Bock, C.-T.; et al. Presentation, Patterns of Myocardial Damage, and Clinical Course of Viral Myocarditis. Circulation 2006, 114, 1581–1590. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caforio, A.L.; Calabrese, F.; Angelini, A.; Tona, F.; Vinci, A.; Bottaro, S.; Ramondo, A.; Carturan, E.; Iliceto, S.; Thiene, G.; et al. A prospective study of biopsy-proven myocarditis: Prognostic relevance of clinical and aetiopathogenetic features at diagnosis. Eur. Heart J. 2007, 28, 1326–1333. [Google Scholar] [CrossRef] [Green Version]
- Grün, S.; Schumm, J.; Greulich, S.; Wagner, A.; Schneider, S.; Bruder, O.; Kispert, E.-M.; Hill, S.; Ong, P.; Klingel, K.; et al. Long-Term Follow-Up of Biopsy-Proven Viral Myocarditis: Predictors of Mortality and Incomplete Recovery. J. Am. Coll. Cardiol. 2012, 59, 1604–1615. [Google Scholar] [CrossRef]
- McCarthy, R.E.; Boehmer, J.; Hruban, R.H.; Hutchins, G.M.; Kasper, E.K.; Hare, J.M.; Baughman, K.L. Long-Term Outcome of Fulminant Myocarditis as Compared with Acute (Nonfulminant) Myocarditis. N. Engl. J. Med. 2000, 342, 690–695. [Google Scholar] [CrossRef] [Green Version]
- Ammirati, E.; Cipriani, M.; Moro, C.; Raineri, C.; Pini, D.; Sormani, P.; Mantovani, R.; Varrenti, M.; Pedrotti, P.; Conca, C.; et al. Clinical Presentation and Outcome in a Contemporary Cohort of Patients with Acute Myocarditis. Circulation 2018, 138, 1088–1099. [Google Scholar] [CrossRef]
| Parameter | All Patients (n = 540) | Females (n = 209) | Males (n = 331) | p Value ∆ |
|---|---|---|---|---|
| Demographics | ||||
| Age (Years) | 47 (33–60) | 52 (41–64) | 44 (29–56) | <0.001 |
| Gender (Female) | 209 (39%) | |||
| Presenting symptoms * | ||||
| Chest pain (n;%) | 354 (66%) | 147 (70%) | 207 (63%) | 0.077 |
| Palpitations/arrhythmia (n;%) | 68 (13%) | 25 (12%) | 43 (13%) | 0.791 |
| Heart failure (n;%) | 66 (12%) | 26 (12%) | 40 (12%) | 0.894 |
| Viral prodrome (n;%) | 50 (9%) | 13 (6%) | 37 (11%) | 0.067 |
| Systemic infection (n;%) | 33 (6%) | 9 (4%) | 24 (7%) | 0.198 |
| Pre-/syncope (n;%) | 22 (4%) | 8 (4%) | 14 (4%) | 1.000 |
| Generally unwell (n;%) | 18 (3%) | 6 (3%) | 12 (4%) | 0.807 |
| GI symptoms (n;%) | 17 (3%) | 6 (3%) | 11 (3%) | 1.000 |
| Cardiac arrest (n;%) | 6 (1%) | 2 (1%) | 4 (1%) | 1.000 |
| Hypotension (n;%) | 4 (1%) | 1 (<1%) | 3 (1%) | 1.000 |
| CMR diagnosis † | ||||
| Myocarditis (n;%) | 215 (40%) | 55 (26%) | 160 (48%) | < 0.001 |
| Normal scan (n;%) | 153 (28%) | 90 (43%) | 63 (19%) | <0.001 |
| Myocardial infarction (n;%) | 61 (11%) | 26 (12%) | 35 (11%) | 0.577 |
| Reversible Ischaemia (n;%) | 2 (<1%) | 1 (<1%) | 1 (<1%) | 1.000 |
| HCM (n;%) | 14 (3%) | 8 (4%) | 6 (2%) | 0.171 |
| DCM (n;%) | 57 (11%) | 13 (6%) | 44 (13%) | 0.009 |
| Hypertensive cardiomyopathy (n;%) | 7 (1%) | 1 (<1%) | 6 (2%) | 0.257 |
| Unspecified cardiomyopathy (n;%) | 8 (1%) | 4 (2%) | 4 (1%) | 0.717 |
| Pericarditis (n;%) | 16 (3%) | 6 (3%) | 10 (3%) | 1.000 |
| Constrictive pericarditis (n;%) | 2 (<1%) | 0 (0%) | 2 (<1%) | 0.525 |
| Valvular disease (n;%) | 4 (1%) | 1 (<1%) | 3 (1%) | 1.000 |
| Takotsubo (n;%) | 3 (1%) | 3 (1%) | 0 (0%) | 0.057 |
| Isolated RV abnormalities (n;%) | 3 (1%) | 1 (<1%) | 2 (<1%) | 1.000 |
| Vasculitis (n;%) | 1 (<1%) | 1 (<1%) | 0 (0%) | 1.000 |
| Amyloid (n;%) | 1 (<1%) | 0 (0%) | 1 (<1%) | 1.000 |
| Sarcoid (n;%) | 1 (<1%) | 0 (0%) | 1 (<1%) | 1.000 |
| Parameter | Myocarditis (n = 113) | Normal (n = 46) | p Value |
|---|---|---|---|
| Demographics | |||
| Age (years) | 40 (24–52) | 44 (30–58) | 0.074 |
| Gender (female) | 27 (24%) | 31 (67%) | <0.001 |
| Presentation to scan interval (days) | 15 (5–26) | 26 (18–31) | <0.001 |
| Laboratory findings | |||
| WBC (×109/L) * | 9.3 (7.2–12.2) | 8.1 (6.5–10.4) | 0.044 |
| CRP (mg/L) † | 21 (4–67) | 3 (1–21) | <0.001 |
| Abnormal troponin (n;%) ∆ | 103 (96%) | 35 (76%) | <0.001 |
| CMR findings | |||
| LV EDV/BSA (mL/m2) | 84 ± 16 | 79 ± 15 | 0.058 |
| LV ESV/BSA (mL/m2) | 33 ± 13 | 26 ± 8 | <0.001 |
| LV EF (%) | 61 ± 9 | 67 ± 6 | <0.001 |
| LV mass/BSA (g/m2) | 65 ± 13 | 53 ± 12 | <0.001 |
| RV EDV/BSA (mL/m2) | 90 ± 17 | 82 ± 16 | 0.006 |
| RV ESV/BSA (mL/m2) | 40 ± 11 | 31 ± 9 | <0.001 |
| RV EF (%) | 56 ± 7 | 62 ± 6 | <0.001 |
| LA area/BSA (cm2/m2) | 12 ± 2 | 12 ± 2 | 0.823 |
| RA area/BSA (cm2/m2) | 12 ± 2 | 11 ± 2 | 0.059 |
| LGE (g) | 5.5 (2.9–10.7) | 0.0 (0.0–0.0) | <0.001 |
| LGE present (n;%) | 109 (97%) | 0 (0%) | <0.001 |
| T2 mapping (ms) µ | |||
| 1.5 T | 50 (49–52) | 49 (46–50) | 0.055 |
| 3 T | 41 (39–43) | 40 (38–42) | 0.434 |
| SD | 4.2 (3.5–5.2) | 3.9 (3.1–4.8) | 0.096 |
| T1 mapping (ms) | |||
| 1.5 T | 1044 (1018–1079) | 1019 (1008–1052) | 0.023 |
| 3 T | 1244 (1221–1274) | 1224 (1201–1264) | 0.112 |
| SD | 56.4 (45.6–72.4) | 46.9 (40.5–56.9) | <0.001 |
| ECV (%) ∂ | 27.08 (25.4–30.3) | 26.10 (24.26–27.87) | 0.029 |
| Parameter | Baseline | Follow-Up | p Value |
|---|---|---|---|
| Demographics | |||
| Age (years) | 33.6 (22.2–45.4) | ||
| Presentation to scan interval (days) | 5 (3–13) | 189 (166–209) | |
| Gender (female) | 7 (18%) | ||
| CMR findings | |||
| LV EDV/BSA (mL/m2) | 88 ± 15 | 88 ± 14 | 0.691 |
| LV ESV/BSA (mL/m2) | 35 ± 11 | 33 ± 10 | 0.086 |
| LV EF (%) | 60 ± 8 | 63 ± 7 | 0.04 |
| LV mass/BSA (g/m2) | 68 ± 11 | 63 ± 10 | 0.002 |
| RV EDV/BSA (mL/m2) | 95 ± 13 | 96 ± 14 | 0.367 |
| RV ESV/BSA (mL/m2) | 44 ± 10 | 41 ± 9 | 0.045 |
| RV EF (%) | 55 ± 7 | 57 ± 6 | 0.023 |
| LA area/BSA (cm2/m2) | 12 ± 2 | 12 ± 2 | 0.806 |
| RA area/BSA (cm2/m2) | 12 ± 2 | 12 ± 2 | 0.705 |
| LGE (g) | 6.9 (4.0–16.6) | 3.06 (1.8–6.5) | <0.001 |
| T2 mapping (ms) * | |||
| 1.5 T | 50 (49–52) | 47 (46–49) | <0.001 |
| 3 T | 40 (38–44) | 37 (36–41) | 0.046 |
| SD | 4.7 (3.9–5.7) | 3.9 (3.3–4.6) | <0.001 |
| T1 mapping (ms) | |||
| 1.5 T | 1054 (1026–1089) | 1015 (991–1030) | <0.001 |
| 3 T | 1251 (1227–1423) | 1224 (1167–1312) | 0.028 |
| SD | 62.0 (48.1–77.5) | 47.1 (39.1–56.7) | 0.006 |
| ECV (%) | 27.13 (25.39–30.99) | 25.87 (24.34–28.18) | <0.001 |
| Baseline Variables | B (SE) | t Value | p Value |
|---|---|---|---|
| Univariate models | |||
| Age | 0.12 ± 0.09 | 1.379 | 0.176 |
| Gender (male) | 1.38 ± 2.95 | 0.468 | 0.643 |
| CRP (per 1 mg/L increase) * | −0.01 ± 0.014 | −0.357 | 0.723 |
| Troponin I (per 100 ng/L increase) † | −0.02 ± 0.01 | −1.234 | 0.226 |
| T2 (per 1 ms increase) µ | −0.50 ± 0.38 | −1.309 | 0.201 |
| T1 (per 1 ms increase) ∂ | −0.01 ± 0.02 | −0.591 | 0.559 |
| ECV (per 1% increase) | −0.41 ± 0.23 | −1.779 | 0.083 |
| LGE (per 1 g increase) | −0.27 ± 0.10 | −2.699 | 0.010 |
| LV EF (per 1% increase) | 0.50 ± 0.13 | 3.858 | <0.001 |
| Multivariable model ∆ | |||
| LV EF (per 1% increase) | 050 ± 0.13 | 2.858 | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lagan, J.; Fortune, C.; Hutchings, D.; Bradley, J.; Naish, J.H.; Timoney, R.; Prescott, D.; Bain, H.D.C.; Bangi, T.; McIntosh, J.; et al. The Diagnostic and Prognostic Utility of Contemporary Cardiac Magnetic Resonance in Suspected Acute Myocarditis. Diagnostics 2022, 12, 156. https://doi.org/10.3390/diagnostics12010156
Lagan J, Fortune C, Hutchings D, Bradley J, Naish JH, Timoney R, Prescott D, Bain HDC, Bangi T, McIntosh J, et al. The Diagnostic and Prognostic Utility of Contemporary Cardiac Magnetic Resonance in Suspected Acute Myocarditis. Diagnostics. 2022; 12(1):156. https://doi.org/10.3390/diagnostics12010156
Chicago/Turabian StyleLagan, Jakub, Christien Fortune, David Hutchings, Joshua Bradley, Josephine H. Naish, Richard Timoney, Daniel Prescott, Hamish D. C. Bain, Tasneem Bangi, Jerome McIntosh, and et al. 2022. "The Diagnostic and Prognostic Utility of Contemporary Cardiac Magnetic Resonance in Suspected Acute Myocarditis" Diagnostics 12, no. 1: 156. https://doi.org/10.3390/diagnostics12010156
APA StyleLagan, J., Fortune, C., Hutchings, D., Bradley, J., Naish, J. H., Timoney, R., Prescott, D., Bain, H. D. C., Bangi, T., McIntosh, J., Egdell, R., Irwin, R. B., Clark, D., Schelbert, E. B., Nucifora, G., Schmitt, M., & Miller, C. A. (2022). The Diagnostic and Prognostic Utility of Contemporary Cardiac Magnetic Resonance in Suspected Acute Myocarditis. Diagnostics, 12(1), 156. https://doi.org/10.3390/diagnostics12010156







