Early Myocardial Changes in Patients with Rheumatoid Arthritis without Known Cardiovascular Diseases—A Comprehensive Cardiac Magnetic Resonance Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Group
2.2. Cardiac Magnetic Resonance
2.3. ECG Holter Monitoring
2.4. Control Group
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Silman, A.J.; Pearson, J.E. Epidemiology and genetics of rheumatoid arthritis. Arthritis Res. 2002, 4, 265–272. [Google Scholar] [CrossRef] [Green Version]
- Wolfe, F.; Mitchell, D.M.; Sibley, J.T.; Fries, J.F.; Bloch, D.A.; Williams, C.A.; Spitz, P.W.; Haga, M.; Kleinheksel, S.M.; Cathey, M.A. The mortality of rheumatoid arthritis. Arthritis Rheum. 1994, 37, 481–494. [Google Scholar] [CrossRef] [PubMed]
- Aviña-Zubieta, J.A.; Choi, H.K.; Sadatsafavi, M.; Etminan, M.; Esdaile, J.M.; Lacaille, D. Risk of cardiovascular mortality in patients with rheumatoid arthritis: A meta-analysis of observational studies. Arthritis Rheum. 2008, 59, 1690–1697. [Google Scholar] [CrossRef]
- Avina-Zubieta, J.A.; Thomas, J.; Sadatsafavi, M.; Lehman, A.J.; Lacaille, D. Risk of incident cardiovascular events in patients with rheumatoid arthritis: A meta-analysis of observational studies. Ann. Rheum. Dis. 2012, 71, 1524–1529. [Google Scholar] [CrossRef] [PubMed]
- Maradit-Kremers, H.; Crowson, C.S.; Nicola, P.J.; Ballman, K.V.; Roger, V.L.; Jacobsen, S.J.; Gabriel, S.E. Increased unrecognized coronary heart disease and sudden deaths in rheumatoid arthritis: A population-based cohort study. Arthritis Rheum. 2005, 52, 402–411. [Google Scholar] [CrossRef] [PubMed]
- Mavrogeni, S.; Dimitroulas, T.; Sfikakis, P.P.; Kitas, G.D. Heart involvement in rheumatoid arthritis: Multimodality imaging and the emerging role of cardiac magnetic resonance. Semin. Arthritis Rheum. 2013, 43, 314–324. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Giles, J.T.; Hirano, M.; Yokoe, I.; Nakajima, Y.; Bathon, J.M.; Lima, J.A.; Kobayashi, H. Assessment of myocardial abnormalities in rheumatoid arthritis using a comprehensive cardiac magnetic resonance approach: A pilot study. Arthritis Res. Ther. 2010, 12, R171. [Google Scholar] [CrossRef] [Green Version]
- Ntusi, N.A.B.; Piechnik, S.K.; Francis, J.M.; Ferreira, V.M.; Matthews, P.M.; Robson, M.D.; Wordsworth, P.B.; Neubauer, S.; Karamitsos, T.D. Diffuse Myocardial Fibrosis and Inflammation in Rheumatoid Arthritis. JACC Cardiovasc. Imaging 2015, 8, 526–536. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holmström, M.; Koivuniemi, R.; Korpi, K.; Kaasalainen, T.; Laine, M.; Kuuliala, A.; Leirisalo-Repo, M.; Kupari, M.; Kivistö, S. Cardiac magnetic resonance imaging reveals frequent myocardial involvement and dysfunction in active rheumatoid arthritis. Clin. Exp. Rheumatol. 2016, 34, 416–423. [Google Scholar] [PubMed]
- Mordi, I.; Carrick, D.; Bezerra, H.; Tzemos, N. T1 and T2 mapping for early diagnosis of dilated non-ischaemic cardiomyopathy in middle-aged patients and differentiation from normal physiological adaptation. Eur. Heart J. Cardiovasc. Imaging 2016, 17, 797–803. [Google Scholar] [CrossRef] [Green Version]
- aus dem Siepen, F.; Buss, S.J.; Messroghli, D.; Andre, F.; Lossnitzer, D.; Seitz, S.; Keller, M.; Schnabel, P.A.; Giannitsis, E.; Korosoglou, G.; et al. T1 mapping in dilated cardiomyopathy with cardiac magnetic resonance: Quantification of diffuse myocardial fibrosis and comparison with endomyocardial biopsy. Eur. Heart J. Cardiovasc. Imaging 2015, 16, 210–216. [Google Scholar] [CrossRef] [Green Version]
- Bradham, W.; Ormseth, M.J.; Elumogo, C.; Palanisamy, S.; Liu, C.Y.; Lawson, M.A.; Soslow, J.H.; Kawel-Boehm, N.; Bluemke, D.A.; Stein, C.M. Absence of Fibrosis and Inflammation by Cardiac Magnetic Resonance Imaging in Rheumatoid Arthritis Patients with Low to Moderate Disease Activity. J. Rheumatol. 2018, 45, 1078–1084. [Google Scholar] [CrossRef]
- Aletaha, D.; Neogi, T.; Silman, A.J.; Funovits, J.; Felson, D.T.; Bingham III, C.O.; Birnbaum, N.S.; Burmester, G.R.; Bykerk, V.P.; Cohen, M.D.; et al. 2010 Rheumatoid arthritis classification criteria: An American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum. 2010, 62, 2569–2581. [Google Scholar] [CrossRef]
- Prevoo, M.L.; van’T Hof, M.A.; Kuper, H.H.; van Leeuwen, M.A.; van de Putte, L.B.; van Riel, P.L. Modified disease activity scores that include twenty-eight-joint counts. Development and validation in a prospective longitudinal study of patients with rheumatoid arthritis. Arthritis Rheum. 1995, 38, 44–48. [Google Scholar] [CrossRef] [Green Version]
- Wells, G.; Becker, J.C.; Teng, J.; Dougados, M.; Schiff, M.; Smolen, J.; Aletaha, D.; Van Riel, P.L.C.M. Validation of the 28-joint Disease Activity Score (DAS28) and European League Against Rheumatism response criteria based on C-reactive protein against disease progression in patients with rheumatoid arthritis, and comparison with the DAS28 based on erythrocyte sedimentation rate. Ann. Rheum. Dis. 2008, 68, 954–960. [Google Scholar] [PubMed]
- Steinbrocker, O.; Traeger, C.H.; Batterman, R.C. Therapeutic criteria in rheumatoid arthritis. J. Am. Med. Assoc. 1949, 140, 659–662. [Google Scholar] [CrossRef]
- Bruce, B.; Fries, J.F. The Stanford Health Assessment Questionnaire: Dimensions and Practical Applications. Health Qual. Life Outcomes 2003, 1, 20. [Google Scholar] [CrossRef] [Green Version]
- Flett, A.S.; Hayward, M.P.; Ashworth, M.T.; Hansen, M.S.; Taylor, A.M.; Elliott, P.M.; McGregor, C.; Moon, J.C. Equilibrium contrast cardiovascular magnetic resonance for the measurement of diffuse myocardial fibrosis: Preliminary validation in humans. Circulation 2010, 122, 138–144. [Google Scholar] [CrossRef]
- Friedrich, M.G.; Sechtem, U.; Schulz-Menger, J.; Holmvang, G.; Alakija, P.; Cooper, L.T.; White, J.A.; Abdel-Aty, H.; Gutberlet, M.; Prasad, S.; et al. Cardiovascular magnetic resonance in myocarditis: A JACC White Paper. J. Am. Coll. Cardiol. 2009, 53, 1475–1487. [Google Scholar] [CrossRef] [Green Version]
- Ferreira, V.M.; Schulz-Menger, J.; Holmvang, G.; Kramer, C.M.; Carbone, I.; Sechtem, U.; Kindermann, I.; Gutberlet, M.; Cooper, L.T.; Liu, P.; et al. Cardiovascular Magnetic Resonance in Nonischemic Myocardial Inflammation: Expert Recommendations. J. Am. Coll. Cardiol. 2018, 72, 3158–3176. [Google Scholar] [CrossRef]
- Lee, A.K.Y.; Andrade, J.; Hawkins, N.M.; Alexander, G.; Bennett, M.T.; Chakrabarti, S.; Laksman, Z.W.; Krahn, A.; Yeung-Lai-Wah, J.A.; Deyell, M.W. Outcomes of untreated frequent premature ventricular complexes with normal left ventricular function. Heart 2019, 105, 1408–1413. [Google Scholar] [CrossRef]
- Steinberg, J.S.; Varma, N.; Cygankiewicz, I.; Aziz, P.; Balsam, P.; Baranchuk, A.; Cantillon, D.J.; Dilaveris, P.; Dubner, S.J.; El-Sherif, N.; et al. 2017 ISHNE-HRS expert consensus statement on ambulatory ECG and external cardiac monitoring/telemetry. Ann. Noninvasive Electrocardiol. 2017, 22, e12447. [Google Scholar] [CrossRef]
- Caforio, A.L.P.; Pankuweit, S.; Arbustini, E.; Basso, C.; Gimeno-Blanes, J.; Felix, S.B.; Fu, M.; Heliö, T.; Heymans, S.; Jahns, R.; et al. Current state of knowledge on aetiology, diagnosis, management, and therapy of myocarditis: A position statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur. Heart J. 2013, 34, 2636–2648. [Google Scholar] [CrossRef]
- Bozkurt, B.; Colvin, M.; Cook, J.; Cooper, L.T.; Deswal, A.; Fonarow, G.C.; Francis, G.S.; Lenihan, D.; Lewis, E.F.; McNamara, D.M.; et al. Current Diagnostic and Treatment Strategies for Specific Dilated Cardiomyopathies: A Scientific Statement From the American Heart Association. Circulation 2016, 134, e579–e646. [Google Scholar] [CrossRef]
- Ponikowski, P.; Voors, A.A.; Anker, S.D. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC)Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur. Heart J. 2016, 37, 2129–2200. [Google Scholar] [PubMed]
- Yilmaz, A.; Ferreira, V.; Klingel, K.; Kandolf, R.; Neubauer, S.; Sechtem, U. Role of cardiovascular magnetic resonance imaging (CMR) in the diagnosis of acute and chronic myocarditis. Heart Fail. Rev. 2013, 18, 747–760. [Google Scholar] [CrossRef]
- Banka, P.; Robinson, J.D.; Uppu, S.C.; Harris, M.A.; Hasbani, K.; Lai, W.W.; Richmond, M.E.; Fratz, S.; Jain, S.; Johnson, T.R.; et al. Cardiovascular magnetic resonance techniques and findings in children with myocarditis: A multicenter retrospective study. J. Cardiovasc. Magn. Reson. 2015, 17, 96. [Google Scholar] [CrossRef] [Green Version]
- Ricci, F.; Aung, N.; Gallina, S.; Zemrak, F.; Fung, K.; Bisaccia, G.; Paiva, J.M.; Khanji, M.Y.; Mantini, C.; Palermi, S.; et al. Cardiovascular magnetic resonance reference values of mitral and tricuspid annular dimensions: The UK Biobank cohort. J. Cardiovasc. Magn. Reson. 2020, 23, 5. [Google Scholar] [CrossRef]
- Mahrholdt, H.; Goedecke, C.; Wagner, A.; Meinhardt, G.; Athanasiadis, A.; Vogelsberg, H.; Fritz, P.; Klingel, K.; Kandolf, R.; Sechtem, U. Cardiovascular magnetic resonance assessment of human myocarditis: A comparison to histology and molecular pathology. Circulation 2004, 109, 1250–1258. [Google Scholar] [CrossRef] [Green Version]
- Greulich, S.; Mayr, A.; Kitterer, D.; Latus, J.; Henes, J.; Vecchio, F.; Kaesemann, P.; Patrascu, A.; Greiser, A.; Groeninger, S.; et al. Advanced myocardial tissue characterisation by a multi-component CMR protocol in patients with rheumatoid arthritis. Eur. Radiol. 2017, 27, 4639–4649. [Google Scholar] [CrossRef] [Green Version]
- Hua, C.; Daien, C.I.; Combe, B.; Landewe, R. Diagnosis, prognosis and classification of early arthritis: Results of a systematic review informing the 2016 update of the EULAR recommendations for the management of early arthritis. RMD Open 2017, 3, e000406. [Google Scholar] [CrossRef] [PubMed]
- Lehmonen, L.; Vuorinen, A.M.; Koivuniemi, R.; Leirisalo-Repo, M.; Holmström, M.; Kivistö, S.; Kaasalainen, T. One-Year Follow-up Study Detects Myocardial Changes with Cardiovascular Magnetic Resonance Tagging in Active Rheumatoid Arthritis. Acad. Radiol. 2018, 25, 476–485. [Google Scholar] [CrossRef]
- Tański, W.; Gać, P.; Chachaj, A.; Sobieszczańska, M.; Poręba, R.; Szuba, A. Selected clinical parameters and changes in cardiac morphology and function assessed by magnetic resonance imaging in patients with rheumatoid arthritis and ankylosing spondylitis without clinically apparent heart disease. Clin. Rheumatol. 2021, 40, 4701–4711. [Google Scholar] [CrossRef]
- Ntusi, N.A.B.; Francis, J.M.; Gumedze, F.; Karvounis, H.; Matthews, P.M.; Wordsworth, P.B.; Neubauer, S.; Karamitsos, T.D. Cardiovascular magnetic resonance characterization of myocardial and vascular function in rheumatoid arthritis patients. Hell. J. Cardiol. 2019, 60, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.A.; Lee, Y.J.; Salerno, M. Diagnostic Performance of Extracellular Volume, Native T1, and T2 Mapping Versus Lake Louise Criteria by Cardiac Magnetic Resonance for Detection of Acute Myocarditis: A Meta-Analysis. Circ. Cardiovasc. Imaging 2018, 11, e007598. [Google Scholar] [CrossRef] [Green Version]
- Kobayashi, H.; Kobayashi, Y.; Yokoe, I.; Akashi, Y.; Takei, M.; Giles, J.T. Magnetic Resonance Imaging–Detected Myocardial Inflammation and Fibrosis in Rheumatoid Arthritis: Associations with Disease Characteristics and N-Terminal Pro–Brain Natriuretic Peptide Levels. Arthritis Care Res. 2017, 69, 1304–1311. [Google Scholar] [CrossRef] [PubMed]
- Mavrogeni, S.; Karabela, G.; Stavropoulos, E.; Gialafos, E.; Sfendouraki, E.; Kyrou, L.; Kolovou, G. Imaging patterns of heart failure in rheumatoid arthritis evaluated by cardiovascular magnetic resonance. Int. J. Cardiol. 2013, 168, 4333–4335. [Google Scholar] [CrossRef]
- Giles, J.T.; Fernandes, V.; Lima, J.A.; Bathon, J.M. Myocardial dysfunction in rheumatoid arthritis: Epidemiology and pathogenesis. Arthritis Res. Ther. 2005, 7, 195–207. [Google Scholar] [CrossRef] [Green Version]
- Lévy, L.; Fautrel, B.; Barnetche, T.; Schaeverbeke, T. Incidence and risk of fatal myocardial infarction and stroke events in rheumatoid arthritis patients. A systematic review of the literature. Clin. Exp. Rheumatol. 2008, 26, 673–679. [Google Scholar]
- Lindhardsen, J.; Ahlehoff, O.; Gislason, G.H.; Madsen, O.R.; Olesen, J.B.; Torp-Pedersen, C.; Hansen, P.R. The risk of myocardial infarction in rheumatoid arthritis and diabetes mellitus: A Danish nationwide cohort study. Ann. Rheum. Dis. 2011, 70, 929–934. [Google Scholar] [CrossRef] [Green Version]
- Voyles, W.F.; Searles, R.P.; Bankhurst, A.D. Myocardial infarction caused by rheumatoid vasculitis. Arthritis Rheum. 1980, 23, 860–863. [Google Scholar] [CrossRef] [PubMed]
- Tłustochowicz, W.; Piotrowicz, R.; Cwetsch, A.; Raczka, A.; Kramarz, E.; Nowak, J. 24-h ECG monitoring in patients with rheumatoid arthritis. Eur. Heart J. 1995, 16, 848–851. [Google Scholar] [CrossRef] [PubMed]
- Gräni, C.; Eichhorn, C.; Bière, L.; Murthy, V.L.; Agarwal, V.; Kaneko, K.; Cuddy, S.; Aghayev, A. Prognostic Value of Cardiac Magnetic Resonance Tissue Characterization in Risk Stratifying Patients With Suspected Myocarditis. J. Am. Coll. Cardiol. 2017, 70, 1964–1976. [Google Scholar] [CrossRef] [PubMed]
- Grigoratos, C.; Di Bella, G.; Aquaro, G.D. Diagnostic and prognostic role of cardiac magnetic resonance in acute myocarditis. Heart Fail. Rev. 2019, 24, 81–90. [Google Scholar] [CrossRef]

| Study Group (n = 18) | |
|---|---|
| Age, median (IQR), y | 41 (38–45) |
| Female sex, n (%) | 15 (83) |
| BMI, median (IQR), kg/m2 | 23.7 (20.3–26.6) |
| Time from RA diagnosis, median (IQR), y | 8.5 (5–11) |
| Time from symptom onset until diagnosis (treatment start), median (IQR), months | 3 (2–6) |
| ESR, median (IQR), mm/h | 10 (5–18) |
| CRP, median (IQR), mg/dL | 4 (4–5) |
| Positive RF, n (%) | 15 (83) |
| Positive CCP, n (%) | 16 (89) |
| Swelled joints, median (IQR), number | 0.5 (0–3) |
| Painful joints, median (IQR), number | 2 (0–6) |
| VAS, median (IQR) | 32 (11–53) |
| DAS28 ESR, median (IQR) | 3.0 (2.2–4.0) |
| DAS28 CRP, median (IQR) | 2.9 (1.6–4.0) |
| Disease activity, n (%) | |
| − remission | 6 (33) |
| − low | 5 (28) |
| − moderate | 5 (28) |
| − high | 2 (11) |
| X-ray erosion, n (%) | 12 (67) |
| X-ray Steinbrocker scale, median (IQR) | 3 (2–3) |
| HAQ, median (IQR) | 0.63 (0.13–1) |
| Smoking, n (%) | |
| − current | 2 (11) |
| − former | 6 (33) |
| Treatment, n (%) | |
| − steroids | 2 (11) |
| − methotrexate | 13 (72) |
| − leflunomide | 5 (28) |
| − sulfasalazine | 2 (11) |
| − chloroquine | 1 (5) |
| − tocilizumab | 2 (11) |
| − anti-TNF | 1 (5) |
| Study Group (n = 18) | Control Group (n = 10) | p-Value | |
|---|---|---|---|
| Age, median (IQR), y | 41 (38–45) | 37 (36–45) | 0.17 |
| Female sex, n (%) | 15 (83) | 8 (80%) | 1.00 |
| LVEDVI, median (IQR), mL/m2 | 77 (68–85) | 84 (80–85) | 0.19 |
| LVESVI, median (IQR), mL/m2 | 31 (27–34) | 31 (30–33) | 0.77 |
| LVSVI, median (IQR), mL/m2 | 49 (45–51) | 51 (50–53) | 0.19 |
| LVEF, median (IQR), % | 61 (59–62) | 62 (61–63) | 0.36 |
| LVMI, median (IQR), kg/m2 | 60 (51–66) | 60 (56–65) | 0.63 |
| RVEDVI, median (IQR), mL/m2 | 79 (71–88) | 85 (80–86) | 0.10 |
| RVESVI, median (IQR), mL/m2 | 32 (27–36) | 35 (31–37) | 0.26 |
| RVSVI, median (IQR), mL/m2 | 48 (42–53) | 51 (48–54) | 0.46 |
| RVEF, median (IQR), % | 61 (56–66) | 59 (57–61) | 0.53 |
| LAA, median (IQR), cm2 | 23 (20–24) | 24 (23–26) | 0.11 |
| RAA, median (IQR), cm2 | 21 (18–23) | 23 (22–24) | 0.12 |
| IVSd, median (IQR), mm | 9 (7.5–9) | 9 (8–9) | 0.72 |
| PWd, median (IQR), mm | 8 (7–8) | 8 (8–8.5) | 0.29 |
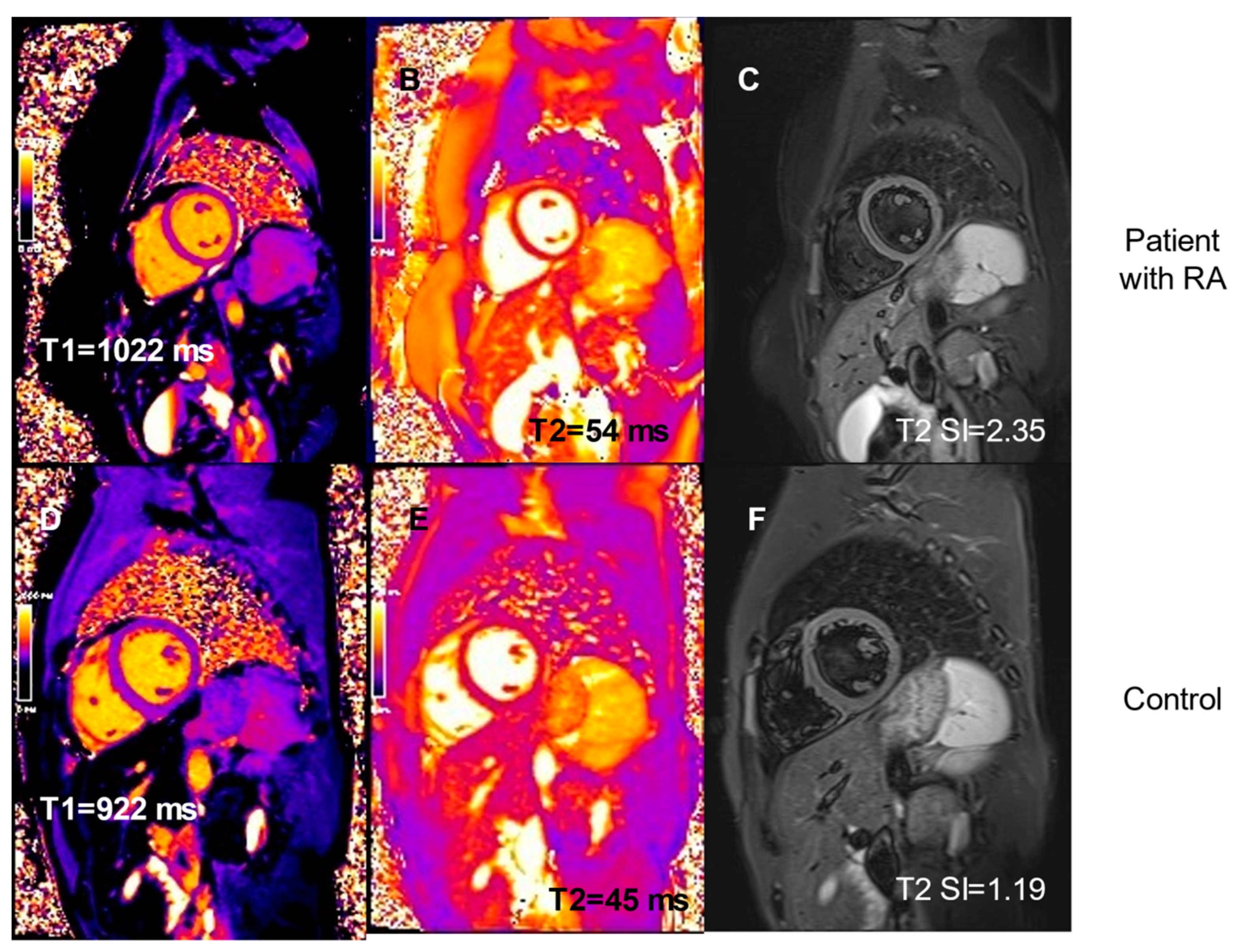
| T1 pre-contrast, median | 1028 (1001–1040) | 992 (984–1012) | 0.04 |
| (IQR), ms | |||
| T1post-contrast, median (IQR), ms | 507 (475–550) | 586 (565–593) | 0.0002 |
| T2, median (IQR), ms | 49 (48–50) | 45 (45–46) | 0.003 |
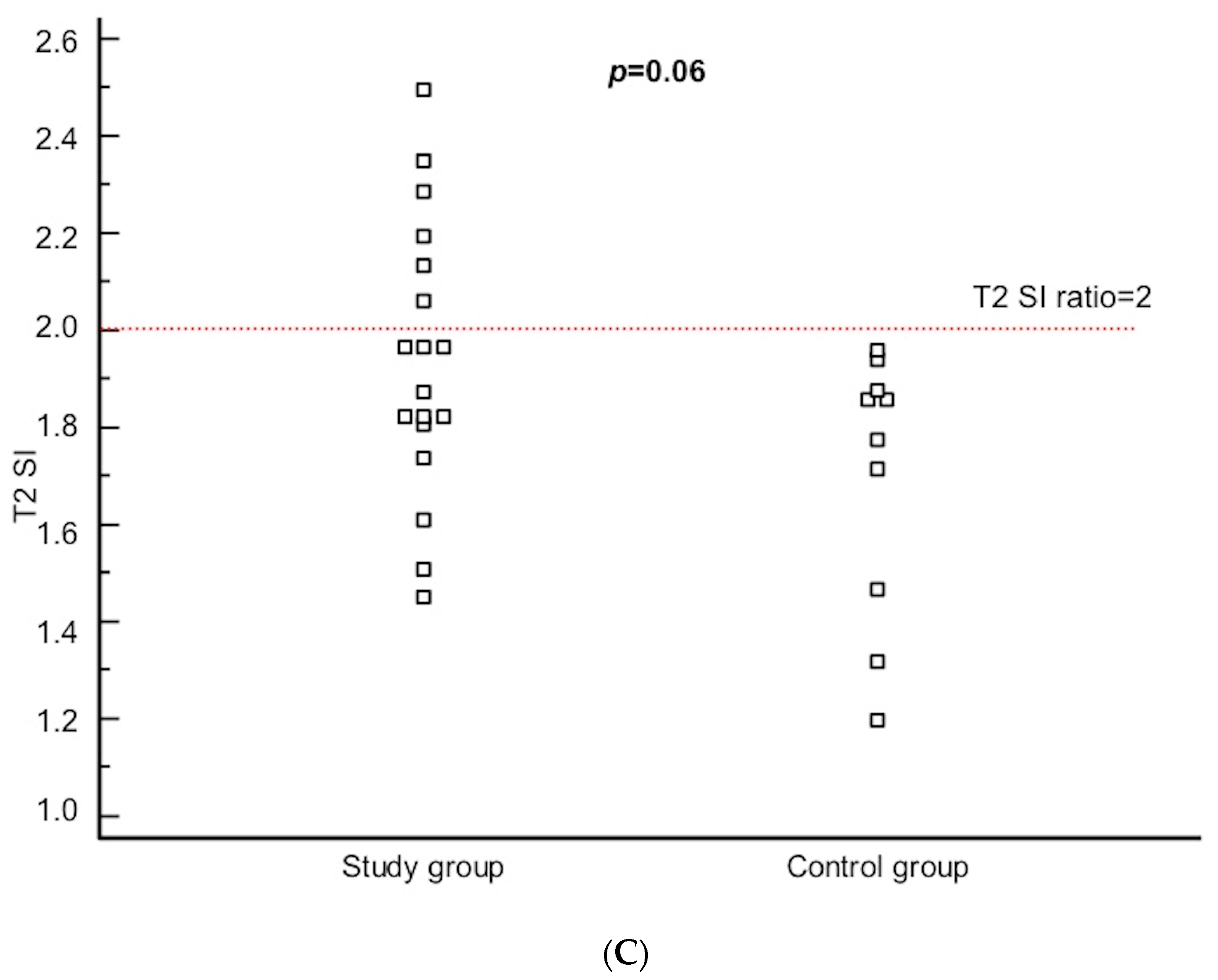
| T2 SI, median (IQR) | 1.92 (1.80–2.13) | 1.82 (1.47–1.88) | 0.06 |
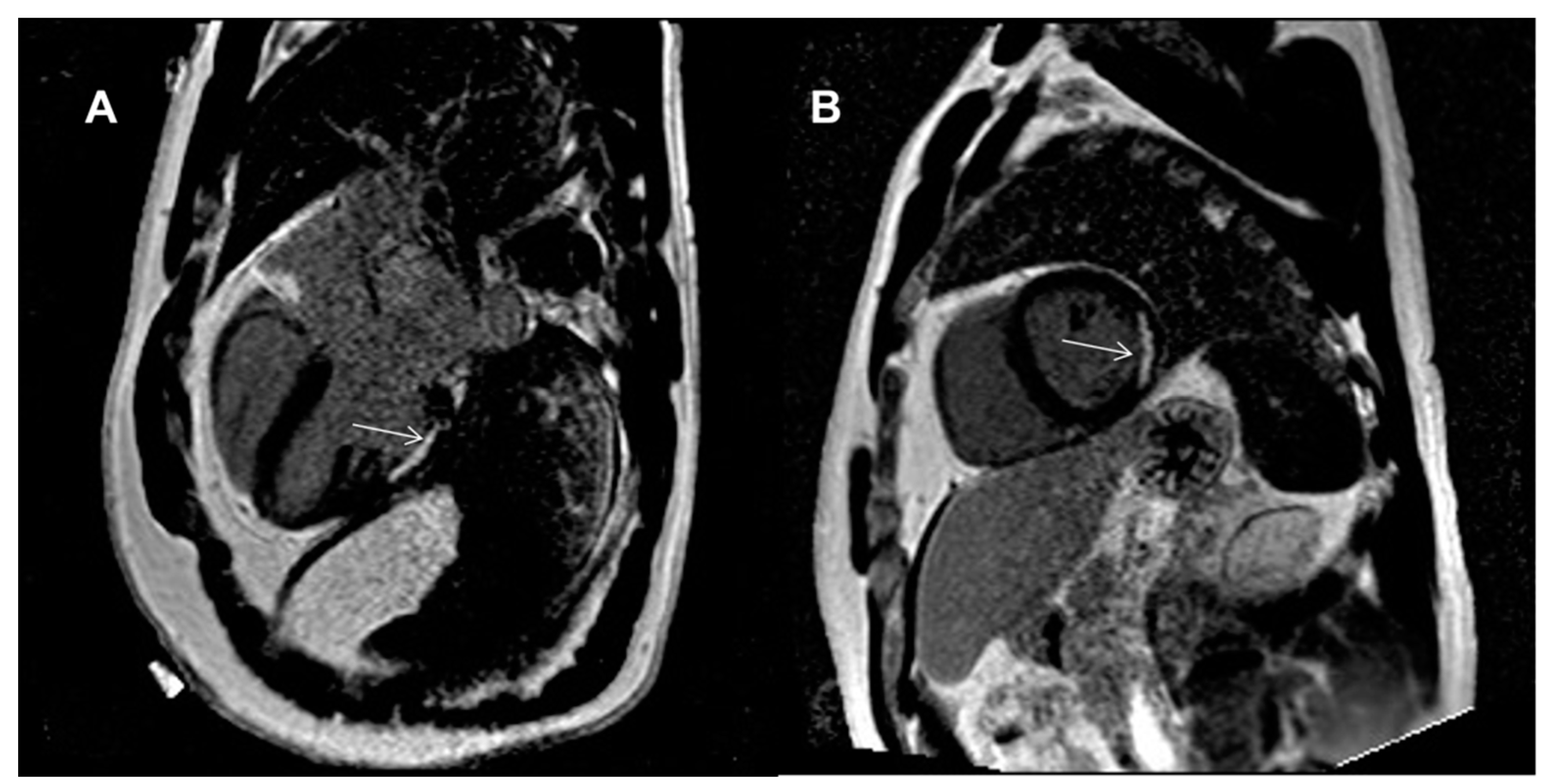
| LGE, n (%) | - | ||
| − subendocardial | 1 (6) | 0 (0) | |
| − mid-wall | 0 (0) | 0 (0) | |
| − subepicardial | 0 (0) | 0 (0) | |
| ECV, median (IQR), % | 27 (25–30) | 27 (25–28) | 0.50 |
| Pericardialeffusion, n (%) | 0 (0) | 0 (0) | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Malczuk, E.; Tłustochowicz, W.; Kramarz, E.; Kisiel, B.; Marczak, M.; Tłustochowicz, M.; Małek, Ł.A. Early Myocardial Changes in Patients with Rheumatoid Arthritis without Known Cardiovascular Diseases—A Comprehensive Cardiac Magnetic Resonance Study. Diagnostics 2021, 11, 2290. https://doi.org/10.3390/diagnostics11122290
Malczuk E, Tłustochowicz W, Kramarz E, Kisiel B, Marczak M, Tłustochowicz M, Małek ŁA. Early Myocardial Changes in Patients with Rheumatoid Arthritis without Known Cardiovascular Diseases—A Comprehensive Cardiac Magnetic Resonance Study. Diagnostics. 2021; 11(12):2290. https://doi.org/10.3390/diagnostics11122290
Chicago/Turabian StyleMalczuk, Ewa, Witold Tłustochowicz, Elżbieta Kramarz, Bartłomiej Kisiel, Magdalena Marczak, Małgorzata Tłustochowicz, and Łukasz A. Małek. 2021. "Early Myocardial Changes in Patients with Rheumatoid Arthritis without Known Cardiovascular Diseases—A Comprehensive Cardiac Magnetic Resonance Study" Diagnostics 11, no. 12: 2290. https://doi.org/10.3390/diagnostics11122290
APA StyleMalczuk, E., Tłustochowicz, W., Kramarz, E., Kisiel, B., Marczak, M., Tłustochowicz, M., & Małek, Ł. A. (2021). Early Myocardial Changes in Patients with Rheumatoid Arthritis without Known Cardiovascular Diseases—A Comprehensive Cardiac Magnetic Resonance Study. Diagnostics, 11(12), 2290. https://doi.org/10.3390/diagnostics11122290







