Ultrasonographic Visualization of the Ovaries to Detect Ovarian Cancer According to Age, Menopausal Status and Body Type
Abstract
1. Introduction
2. Materials and Methods
- (a)
- Aide-to-side movements to achieve sagittal imaging,
- (b)
- 90° rotation to obtain semi-coronal images and angulation of the probe vertically,
- (c)
- Varying the depth of probe insertion to expose different pelvic structures within the field of view.
Statistical Analyses
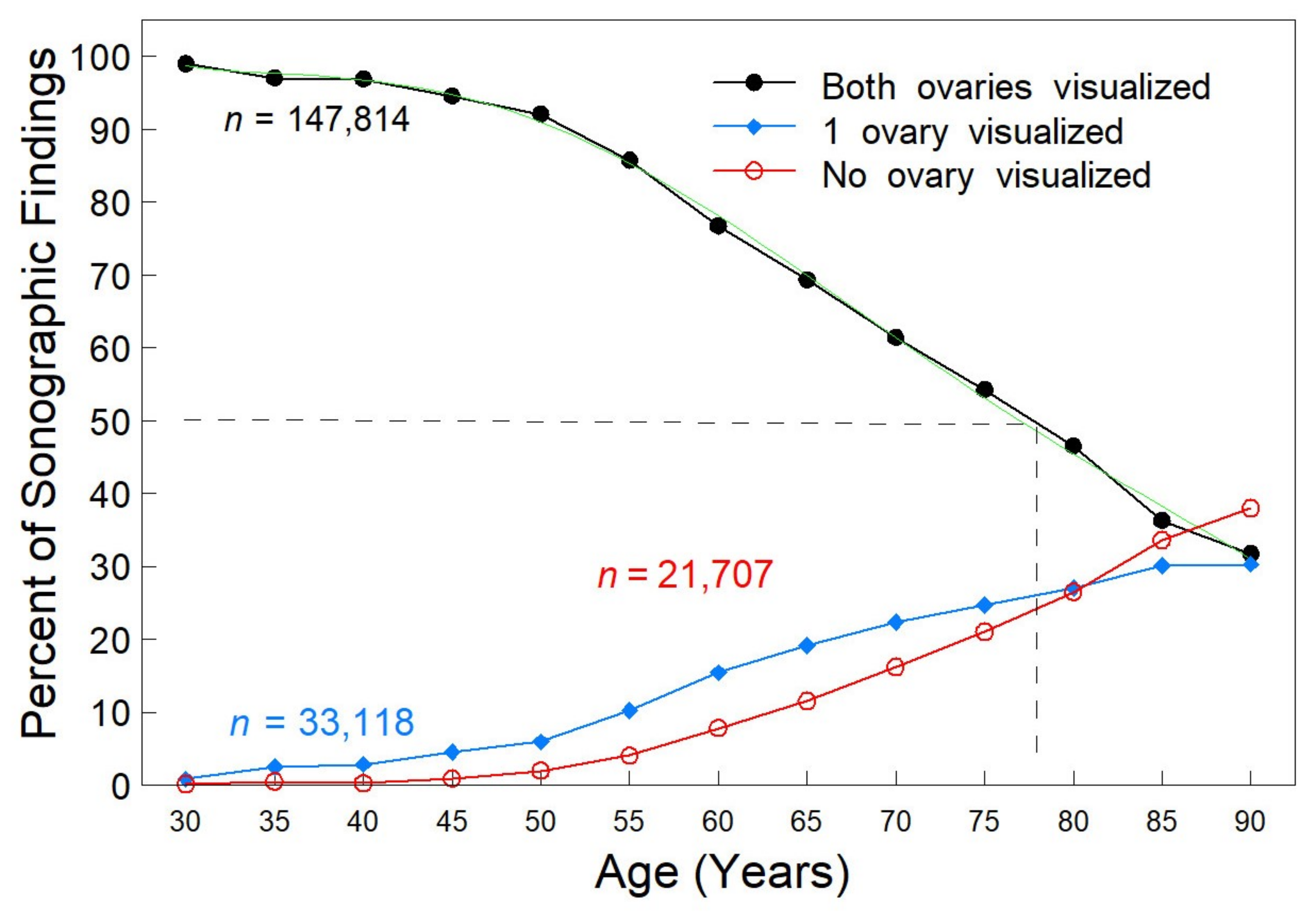
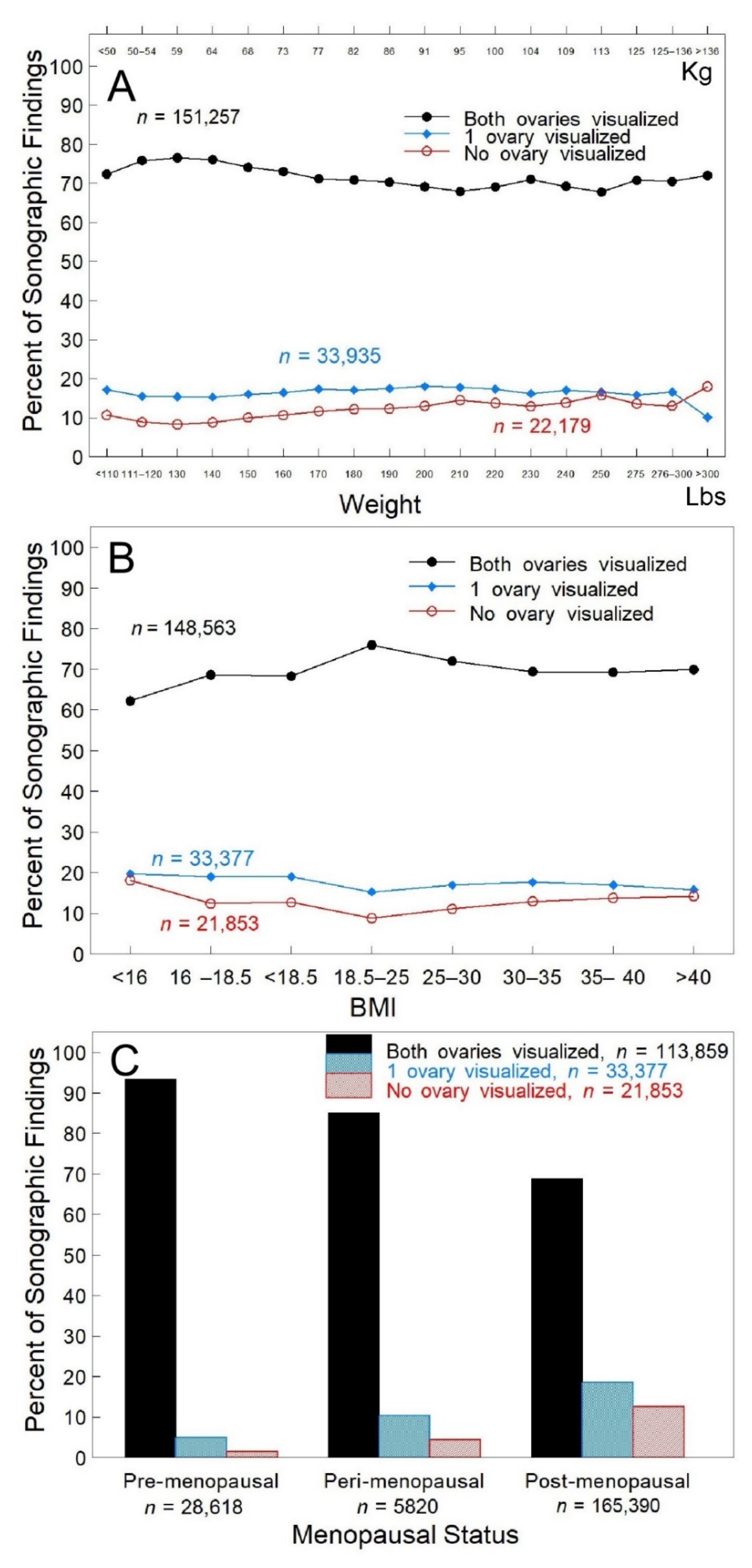
3. Results
4. Discussion
4.1. Clinical Implications
4.2. Research Implications
4.3. Strengths and Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ovarian Cancer Including Fallopian Tube Cancer and Primary Peritoneal Cancer. NCCN Clinical Practice Guidelines in Oncology. Version 3. 9 September 2021. Available online: https://www.nccn.org/professionals/physician_gls/pdf/ovarian.pdf (accessed on 27 December 2020).
- Moorthy, R.S. Transvaginal Sonography. Med. J. Armed Forces India 2000, 56, 181–183. [Google Scholar] [CrossRef]
- Jacobs, I.; Gentry-Maharaj, A.; Burnell, M.; Manchanda, R.; Singh, N.; Sharma, A.; Ryan, A.; Seif, M.W.; Amso, N.N.; Turner, G.; et al. Sensitivity of transvaginal ultrasound screening for endometrial cancer in postmenopausal women: A case-control study within the UKCTOCS cohort. Lancet Oncol. 2011, 12, 38–48. [Google Scholar] [CrossRef]
- Van Nagell, J.; Burgess, B.; Miller, R.; Baldwin, L.; DeSimone, C.; Ueland, F.; Huang, B.; Chen, Q.; Kryscio, R.; Pavlik, E. Survival of Women with Type I and II Epithelial Ovarian Cancer Detected by Ultrasound Screening. Obstet. Gynecol. 2018, 132, 1091–1100. [Google Scholar] [CrossRef] [PubMed]
- Suh-Burgmann, E.; Brasic, N.; Jha, P.; Hung, Y.Y.; Goldstein, R.B. Ultrasound characteristics of early-stage high-grade serous ovarian cancer. Am. J. Obstet. Gynecol. 2021, 225, 409.e1–409.e8. [Google Scholar] [CrossRef] [PubMed]
- Cohen Ben-Meir, L.; Mashiach, R.; Eisenberg, V.H. External Validation of the IOTA Classification in Women with Ovarian Masses Suspected to Be Endometrioma. J. Clin. Med. 2021, 10, 2971. [Google Scholar] [CrossRef] [PubMed]
- Tomasińska, A.; Stukan, M.; Badocha, M.; Myszewska, A. Accuracy of Pretreatment Ultrasonography Assessment of Intra-Abdominal Spread in Epithelial Ovarian Cancer: A Prospective Study. Diagnostics 2021, 11, 1600. [Google Scholar] [CrossRef] [PubMed]
- Carter, J.; Fowler, J.; Carson, L.; Carlson, J.; Twiggs, L.B. How accurate is the pelvic examination as compared to transvaginal sonography? A prospective, comparative study. J. Reprod. Med. 1994, 39, 32–34. [Google Scholar] [PubMed]
- Ueland, F.R.; Depriest, P.D.; Desimone, C.P.; Pavlik, E.J.; Lele, S.M.; Kryscio, R.J.; van Nagell, J.R. The accuracy of examination under anesthesia and transvaginal sonography in evaluating ovarian size. Gynecol. Oncol. 2005, 99, 400–403. [Google Scholar] [CrossRef] [PubMed]
- Pavlik, E.J.; DePriest, P.D.; Gallion, H.H.; Ueland, F.R.; Reedy, M.B.; Kryscio, R.J.; van Nagell, J.R. Ovarian Volume Related to Age. Gynecol. Oncol. 2000, 77, 410–412. [Google Scholar] [CrossRef] [PubMed]
- Pavlik, E.J.; Liu, C.; DePriest, P.D.; Gallion, H.H.; Ueland, F.R.; Kryscio, R.J.; van Nagell, J.R. Relating Ovarian Size to Age, Menopausal Status, and Use of Hormones. Gynecol. Oncol. 2001, 80, 333–334. [Google Scholar] [CrossRef] [PubMed]
- David, G.; Weismiller, D.G. Menopause. Prim. Care 2009, 36, 199–226. [Google Scholar] [CrossRef]
- National Institute of Aging. Available online: https://www.nia.nih.gov/health/what-menopause (accessed on 27 December 2020).
- Mayo Clinic–Perimenopause. Available online: https://www.mayoclinic.org/diseases-conditions/perimenopause/symptoms-causes/syc-20354666 (accessed on 27 December 2020).
- Higgins, R.V.; van Nagell, J.R., Jr.; Woods, C.H.; Thompson, E.A.; Kryscio, R.J. Interobserver variation in ovarian measurements using transvaginal sonography. Gynecol. Oncol. 1990, 39, 69–71. [Google Scholar] [CrossRef]
- Raine-Fenning, N.J.; Campbell, B.K.; Clewes, J.S.; Johnson, I.R. The interobserver reliability of ovarian volume measurement is improved with three-dimensional ultrasound, but dependent upon technique. Ultrasound Med. Biol. 2003, 12, 1685–1690. [Google Scholar] [CrossRef]
- VassarStats: Website for Statistical Computation. Available online: http://vassarstats.net/index.html (accessed on 25 October 2020).
- National Institute of Diabetes and Digestive and Kidney Diseases: Overweight & Obesity Statistics. Available online: https://www.niddk.nih.gov/health-information/health-statistics/overweight-obesity#estimation (accessed on 25 October 2020).
- Glanc, P.; O’Hayon, B.E.; Singh, D.K.; Bokhari, S.A.J.; Maxwell, C.V. Challenges of Pelvic Imaging in Obese Women. Radio Graph. 2012, 32, 1839–1862. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Your Guide to Menopause. Available online: https://www.webmd.com/menopause/guide/menopause-information#1-2 (accessed on 27 December 2020).
- Age at Menopause. Available online: https://www.cdc.gov/nchs/data/series/sr_11/sr11_019.pdf (accessed on 27 December 2020).
- Gold, E.B. The Timing of the Age at Which Natural Menopause Occurs. Obstet. Gynecol. Clin. N. Am. 2011, 38, 425–440. [Google Scholar] [CrossRef] [PubMed]
| All Subjects (n = 29,877) | All Encounters (n = 202,639) | |
|---|---|---|
| Age (y) | 55.0, 55 (20–91) | 60.1, 60 (20–95) |
| Weight (kg) | 73, 70.3 (38–204) | 72.3, 69.4 (36–205) |
| Height (cm) | 163.5, 162.6 (119–198) | 163.6, 162.6 (119–198) |
| BMI | 27.3, 26 (13–80) | 27, 26 (13–80) |
| Pre-menopausal Peri-menopausal Post-menopausal | 5966 (20.9%) 1262 (4.4%) 21,251 (74.6%) | 28,618 (14.3%) 5820 (2.9%) 165,390 (82.8%) |
| Age (yrs) | Total n | Both Ovaries Visualized | PR [95% CI] | OR [95% CI] |
|---|---|---|---|---|
| 20–30 | 1182 | 1170 | 1 | 1 |
| 31–35 | 2439 | 2366 | 0.98 (0.9712–0.9889) | 0.3324 (0.1798–0.6146) |
| 36–40 | 5103 | 4943 | 0.9786 (0.9712–0.9860) | 0.3169 (0.1756–0.5717) |
| 41–45 | 8483 | 8019 | 0.955 (0.9477–0.9624) | 0.1773 (0.0996–0.3154) |
| 46–50 | 13,096 | 12,056 | 0.93 (0.9229–0.9372) | 0.1189 (0.0671–0.2107) |
| 51–55 | 33,202 | 28,463 | 0.8661 (0.8598–0.8724) | 0.0616 (0.0349–0.1089] |
| 56–60 | 39,278 | 30,132 | 0.775 (0.7689–0.7812) | 0.0338 (0.0191–0.0597) |
| 61–65 | 38,077 | 26,396 | 0.7003 (0.6942–0.7065) | 0.0232 (0.0131–0.0409) |
| 66–70 | 30,164 | 18,522 | 0.6203 (0.6138–0.6270) | 0.0163 (0.0092–0.0288) |
| 71–75 | 18,823 | 10,210 | 0.548 (0.5402–0.5559) | 0.0122 (0.0069–0.0215) |
| 76–80 | 9077 | 4221 | 0.4698 (0.4592–0.4806) | 0.0089 (0.0050–0.0158) |
| 81–85 | 3009 | 1092 | 0.3666 (0.3496–0.3845) | 0.0058 (0.0033–0.0104) |
| >85 | 706 | 224 | 0.3205 (0.2876–0.3572) | 0.0048 (0.0026–0.0086) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pavlik, E.J.; Brekke, E.; Gorski, J.; Baldwin-Branch, L.; Miller, R.; DeSimone, C.P.; Dietrich, C.S.; Gallion, H.S.; Ueland, F.R.; van Nagell, J.R., Jr. Ultrasonographic Visualization of the Ovaries to Detect Ovarian Cancer According to Age, Menopausal Status and Body Type. Diagnostics 2022, 12, 128. https://doi.org/10.3390/diagnostics12010128
Pavlik EJ, Brekke E, Gorski J, Baldwin-Branch L, Miller R, DeSimone CP, Dietrich CS, Gallion HS, Ueland FR, van Nagell JR Jr. Ultrasonographic Visualization of the Ovaries to Detect Ovarian Cancer According to Age, Menopausal Status and Body Type. Diagnostics. 2022; 12(1):128. https://doi.org/10.3390/diagnostics12010128
Chicago/Turabian StylePavlik, Edward J., Emily Brekke, Justin Gorski, Lauren Baldwin-Branch, Rachel Miller, Christopher P. DeSimone, Charles S. Dietrich, Holly S. Gallion, Frederick Rand Ueland, and John R. van Nagell, Jr. 2022. "Ultrasonographic Visualization of the Ovaries to Detect Ovarian Cancer According to Age, Menopausal Status and Body Type" Diagnostics 12, no. 1: 128. https://doi.org/10.3390/diagnostics12010128
APA StylePavlik, E. J., Brekke, E., Gorski, J., Baldwin-Branch, L., Miller, R., DeSimone, C. P., Dietrich, C. S., Gallion, H. S., Ueland, F. R., & van Nagell, J. R., Jr. (2022). Ultrasonographic Visualization of the Ovaries to Detect Ovarian Cancer According to Age, Menopausal Status and Body Type. Diagnostics, 12(1), 128. https://doi.org/10.3390/diagnostics12010128







