Natalizumab Induces Changes of Cerebrospinal Fluid Measures in Multiple Sclerosis
Abstract
:1. Introduction
2. Methods
2.1. Study Population
2.2. Observation and Therapy
2.3. MRI Acquisition and Analysis
2.4. Lumbar Puncture and Cerebrospinal Fluid Examination
2.5. Statistical Analysis
3. Results
3.1. Descriptive Characteristics of the Sample
3.2. Correlation between the CSF and Other Clinical and MRI Measures at the Baseline
3.3. Evolution of CSF Measures over the Follow-Up
3.4. Predictors of the Evolution of CSF Measures over the Follow-Up
3.5. Evolution of the Clinical and MRI Measures over the Follow-Up
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Disclosures
Nomenclature
| MS | Multiple sclerosis |
| RRMS | Relapsing-remitting multiple sclerosis |
| NTZ | Natalizumab |
| DMT | Disease modifying therapy |
| CSF | Cerebrospinal fluid |
| OCBs | Oligoclonal bands |
| EDSS | Expanded disability status scale |
| T2LV | T2 lesion volume |
| BPF | Brain parenchymal fraction |
References
- Rudick, R.A.; Stuart, W.H.; Calabresi, P.A.; Confavreux, C.; Galetta, S.L.; Radue, M.S.E.R.S.E.; Lublin, F.D.; Weinstock-Guttman, B.; Wynn, D.R.; Lynn, F.; et al. Natalizumab plus Interferon Beta-1a for Relapsing Multiple Sclerosis. N. Engl. J. Med. 2006, 354, 911–923. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Polman, C.H.; O’Connor, P.W.; Havrdova, E.K.; Hutchinson, M.; Kappos, L.; Miller, D.H.; Phillips, J.T.; Lublin, F.D.; Giovannoni, G.; Wajgt, A.; et al. A Randomized, Placebo-Controlled Trial of Natalizumab for Relapsing Multiple Sclerosis. N. Engl. J. Med. 2006, 354, 899–910. [Google Scholar] [CrossRef] [Green Version]
- Havrdova, E.; Galetta, S.; Hutchinson, M.; Stefoski, D.; Bates, D.; Polman, C.H.; O’Connor, P.W.; Giovannoni, G.; Phillips, J.T.; Lublin, F.D.; et al. Effect of natalizumab on clinical and radiological disease activity in multiple sclerosis: A retrospective analysis of the Natalizumab Safety and Efficacy in Relapsing-Remitting Multiple Sclerosis (AFFIRM) study. Lancet Neurol. 2009, 8, 254–260. [Google Scholar] [CrossRef]
- Horakova, D.; Uher, T.; Krasensky, J.; Seidl, Z.; Ribbens, A.; Van Hecke, W.; Billiet, T.; Koendgen, H.; Freudensprung, U.; Hyde, R.; et al. Long-term effectiveness of natalizumab on MRI outcomes and no evidence of disease activity in relapsing-remitting multiple sclerosis patients treated in a Czech Republic real-world setting: A longitudinal, retrospective study. Mult. Scler. Relat. Disord. 2020, 46, 102543. [Google Scholar] [CrossRef]
- Hannikainen, P.A.; Kosa, P.; Barbour, C.; Bielekova, B. Extensive Healthy Donor Age/Gender Adjustments and Propensity Score Matching Reveal Physiology of Multiple Sclerosis Through Immunophenotyping. Front. Neurol. 2020, 11, 565957. [Google Scholar] [CrossRef]
- Krumbholz, M.; Meinl, I.; Kümpfel, T.; Hohlfeld, R.; Meinl, E. Natalizumab disproportionately increases circulating pre-B and B cells in multiple sclerosis. Neurology 2008, 71, 1350–1354. [Google Scholar] [CrossRef]
- Mattoscio, M.; Nicholas, R.; Sormani, M.P.; Malik, O.; Lee, J.S.; Waldman, A.D.; Dazzi, F.; Muraro, P.A. Hematopoietic mobilization: Potential biomarker of response to natalizumab in multiple sclerosis. Neurology 2015, 84, 1473–1482. [Google Scholar] [CrossRef] [Green Version]
- Carotenuto, A.; Scalia, G.; Ausiello, F.; Moccia, M.; Russo, C.V.; Saccà, F.; De Rosa, A.; Criscuolo, C.; Del Vecchio, L.; Morra, V.B.; et al. RCD4/CD8 ratio during natalizumab treatment in multiple sclerosis patients. J. Neuroimmunol. 2017, 309, 47–50. [Google Scholar]
- Signoriello, E.; Lanzillo, R.; Morra, V.B.; Di Iorio, G.; Fratta, M.; Carotenuto, A.; Lus, G. Lymphocytosis as a response biomarker of natalizumab therapeutic efficacy in multiple sclerosis. Mult. Scler. J. 2015, 22, 921–925. [Google Scholar] [CrossRef] [PubMed]
- Comabella, M.; Sastre-Garriga, J.; Montalban, X. Precision medicine in multiple sclerosis: Biomarkers for diagnosis, prognosis, and treatment response. Curr. Opin. Neurol. 2016, 29, 254–262. [Google Scholar] [CrossRef] [PubMed]
- Stã¼Ve, O.; Marra, C.M.; Jerome, K.R.; Cook, L.; Cravens, P.D.; Cepok, S.; Frohman, E.M.; Phillips, J.T.; Arendt, G.; Hemmer, B.; et al. Immune surveillance in multiple sclerosis patients treated with natalizumab. Ann. Neurol. 2006, 59, 743–747. [Google Scholar] [CrossRef]
- Henriksen, A.C.; Ammitzbøll, C.; Petersen, E.R.; McWilliam, O.; Sellebjerg, F.; von Essen, M.R.; Christensen, J.R. Natalizumab differentially affects plasmablasts and B cells in multiple sclerosis. Mult. Scler. Relat. Disord. 2021, 52, 102987. [Google Scholar] [CrossRef] [PubMed]
- Schlüter, M.; Oswald, E.; Winklmeier, S.; Meinl, I.; Havla, J.; Eichhorn, P.; Meinl, E.; Kümpfel, T. Effects of Natalizumab Therapy on Intrathecal Immunoglobulin G Production Indicate Targeting of Plasmablasts. Neurol. Neuroimmunol. Neuroinflamm. 2021, 8, e1030. [Google Scholar] [CrossRef] [PubMed]
- Harrer, A.; Tumani, H.; Niendorf, S.; Lauda, F.; Geis, C.; Weishaupt, A.; Kleinschnitz, C.; Rauer, S.; Kuhle, J.; Stangel, M.; et al. Cerebrospinal fluid parameters of B cell-related activity in patients with active disease during natalizumab therapy. Mult. Scler. 2013, 19, 1209–1212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Largey, F.; Jelcic, I.; Sospedra, M.; Heesen, C.; Martin, R.; Jelcic, I. Effects of natalizumab therapy on intrathecal antiviral antibody responses in MS. Neurol. Neuroimmunol. Neuroinflamm. 2019, 6, e621. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Warnke, C.; Stettner, M.; Lehmensiek, V.; Dehmel, T.; Mausberg, A.K.; Von Geldern, G.; Gold, R.; Kümpfel, T.; Hohlfeld, R.; Mäurer, M.; et al. Natalizumab exerts a suppressive effect on surrogates of B cell function in blood and CSF. Mult. Scler. J. 2014, 21, 1036–1044. [Google Scholar] [CrossRef] [Green Version]
- Mancuso, R.; Franciotta, D.; Rovaris, M.; Caputo, D.; Sala, A.; Hernis, A.; Agostini, S.; Calvo, M.; Clerici, M. Effects of natalizumab on oligoclonal bands in the cerebrospinal fluid of multiple sclerosis patients: A longitudinal study. Mult. Scler. J. 2014, 20, 1900–1903. [Google Scholar] [CrossRef]
- Huang, J.; Khademi, M.; Fugger, L.; Lindhe, Ö.; Novakova, L.; Axelsson, M.; Malmeström, C.; Constantinescu, C.; Lycke, J.; Piehl, F.; et al. Inflammation-related plasma and CSF biomarkers for multiple sclerosis. Proc. Natl. Acad. Sci. USA 2020, 117, 12952–12960. [Google Scholar] [CrossRef] [PubMed]
- Meinl, E.; Krumbholz, M.; Hohlfeld, R. B lineage cells in the inflammatory central nervous system environment: Migration, maintenance, local antibody production, and therapeutic modulation. Ann. Neurol. 2006, 59, 880–892. [Google Scholar] [CrossRef]
- Alter, A.; Duddy, M.; Hebert, S.; Biernacki, K.; Prat, A.; Antel, J.P.; Yong, V.W.; Nuttall, R.K.; Pennington, C.J.; Edwards, D.R.; et al. Determinants of human B cell migration across brain endothelial cells. J. Immunol. 2003, 170, 4497–4505. [Google Scholar] [CrossRef] [Green Version]
- Silvy, A.; Altevogt, P.; Mondière, P.; Bella, C.; Defrance, T. A role for the VLA-4 integrin in the activation of human memory B cells. Eur. J. Immunol. 1997, 27, 2757–2764. [Google Scholar] [CrossRef]
- Shapiro-Shelef, M.; Calame, K. Regulation of plasma-cell development. Nat. Rev. Immunol. 2005, 5, 230–242. [Google Scholar] [CrossRef]
- Teunissen, C.E.; Petzold, A.; Bennett, J.L.; Berven, F.S.; Brundin, L.; Comabella, M.; Franciotta, D.; Frederiksen, J.L.; Fleming, J.O.; Furlan, R.; et al. A consensus protocol for the standardization of cerebrospinal fluid collection and biobanking. Neurology 2009, 73, 1914–1922. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uher, T.; Krasensky, J.; Vaneckova, M.; Sobisek, L.; Seidl, Z.; Havrdova, E.K.; Bergsland, N.; Dwyer, M.G.; Horakova, D.; Zivadinov, R. A Novel Semiautomated Pipeline to Measure Brain Atrophy and Lesion Burden in Multiple Sclerosis: A Long-Term Comparative Study. J. Neuroimaging 2017, 27, 620–629. [Google Scholar] [CrossRef] [PubMed]
- Uher, T.; Krasensky, J.; Malpas, C.; Bergsland, N.; Dwyer, M.G.; Havrdova, E.K.; Vaneckova, M.; Horakova, D.; Zivadinov, R.; Kalincik, T. Evolution of Brain Volume Loss Rates in Early Stages of Multiple Sclerosis. Neurol. Neuroimmunol. Neuroinflamm. 2021, 8, e979. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.; Zhanga, Y.; Jenkinson, M.; Chenab, J.; Matthews, P.M.; Federico, A.; de Stefano, N. Accurate, Robust, and Automated Longitudinal and Cross-Sectional Brain Change Analysis. NeuroImage 2002, 17, 479–489. [Google Scholar] [CrossRef] [Green Version]
- Gelineau-Morel, R.; Tomassini, V.; Jenkinson, M.; Johansen-Berg, H.; Matthews, P.M.; Palace, J. The effect of hypointense white matter lesions on automated gray matter segmentation in multiple sclerosis. Hum. Brain Mapp. 2011, 33, 2802–2814. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting Linear Mixed-Effects Models Using lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Ringle, C.M.; Wende, S.; Becker, J.-M. SmartPLS 3. Available online: http://www.smartpls.com (accessed on 27 November 2021).
- Matell, H.; Lycke, J.; Svenningsson, A.; Holmén, C.; Khademi, M.; Hillert, J.; Olsson, T.; Piehl, F. Age-dependent effects on the treatment response of natalizumab in MS patients. Mult. Scler. J. 2014, 21, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Kalincik, T.; Vivek, V.; Jokubaitis, V.; Lechner-Scott, J.; Trojano, M.; Izquierdo, G.; Lugaresi, A.; Grand’Maison, F.; Hupperts, R.; Oreja-Guevara, C.; et al. Sex as a determinant of relapse incidence and progressive course of multiple sclerosis. Brain 2013, 136, 3609–3617. [Google Scholar] [CrossRef]
- Scalfari, A.; Lederer, C.; Daumer, M.; Nicholas, R.; Ebers, G.C.; Muraro, A.P. The relationship of age with the clinical phenotype in multiple sclerosis. Mult. Scler. J. 2016, 22, 1750–1758. [Google Scholar] [CrossRef] [PubMed]
- Link, H.; Huang, Y.-M. Oligoclonal bands in multiple sclerosis cerebrospinal fluid: An update on methodology and clinical usefulness. J. Neuroimmunol. 2006, 180, 17–28. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, E.; Nair, K.V.; Hoyt, B.D.; Seale, R.A.; Sillau, S.; Miravalle, A.; Engebretson, E.; Schurr, B.; Corboy, J.R.; Vollmer, T.L.; et al. Brain atrophy rates in patients with multiple sclerosis on long term natalizumab resembles healthy controls. Mult. Scler. Relat. Disord. 2021, 55, 103170. [Google Scholar] [CrossRef] [PubMed]
- Mellergård, J.; Tisell, A.; Blystad, I.; Grönqvist, A.; Blennow, K.; Olsson, B.; Dahle, C.; Vrethem, M.; Lundberg, P.; Ernerudh, J. Cerebrospinal fluid levels of neurofilament and tau correlate with brain atrophy in natalizumab-treated multiple sclerosis. Eur. J. Neurol. 2016, 24, 112–121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garriga, J.S.; Tur, C.; Pareto, D.; Vidal-Jordana, A.; Auger, C.; Rio, J.; Huerga, E.; Tintore, M.; Rovira, A.; Montalban, X. Brain atrophy in natalizumab-treated patients: A 3-year follow-up. Mult. Scler. J. 2014, 21, 749–756. [Google Scholar] [CrossRef]
- Eisele, P.; Szabo, K.; Ebert, A.; Platten, M.; Gass, A. Brain Atrophy in Natalizumab-treated Patients with Multiple Sclerosis: A 5-year Retrospective Study. J. Neuroimaging 2018, 29, 190–192. [Google Scholar] [CrossRef]
- Monreal, E.; de la Maza, S.S.; Costa-Frossard, L.; Walo-Delgado, P.; Zamora, J.; Fernández-Velasco, J.I.; Villarrubia, N.; Espiño, M.; Lourido, D.; Lapuente, P.; et al. Predicting Aggressive Multiple Sclerosis With Intrathecal IgM Synthesis Among Patients With a Clinically Isolated Syndrome. Neurol. Neuroimmunol. Neuroinflamm. 2021, 8, e1047. [Google Scholar] [CrossRef]
- Klein, A.; Selter, R.C.; Hapfelmeier, A.; Berthele, A.; Müller-Myhsok, B.; Pongratz, V.; Gasperi, C.; Zimmer, C.; Mühlau, M.; Hemmer, B. CSF parameters associated with early MRI activity in patients with MS. Neurol. Neuroimmunol. Neuroinflamm. 2019, 6, e573. [Google Scholar] [CrossRef] [Green Version]
- Schwab, N.; Schneider-Hohendorf, T.; Melzer, N.; Cutter, G.; Wiendl, H. Natalizumab-associated PML: Challenges with incidence, resulting risk, and risk stratification. Neurology 2017, 88, 1197–1205. [Google Scholar] [CrossRef]
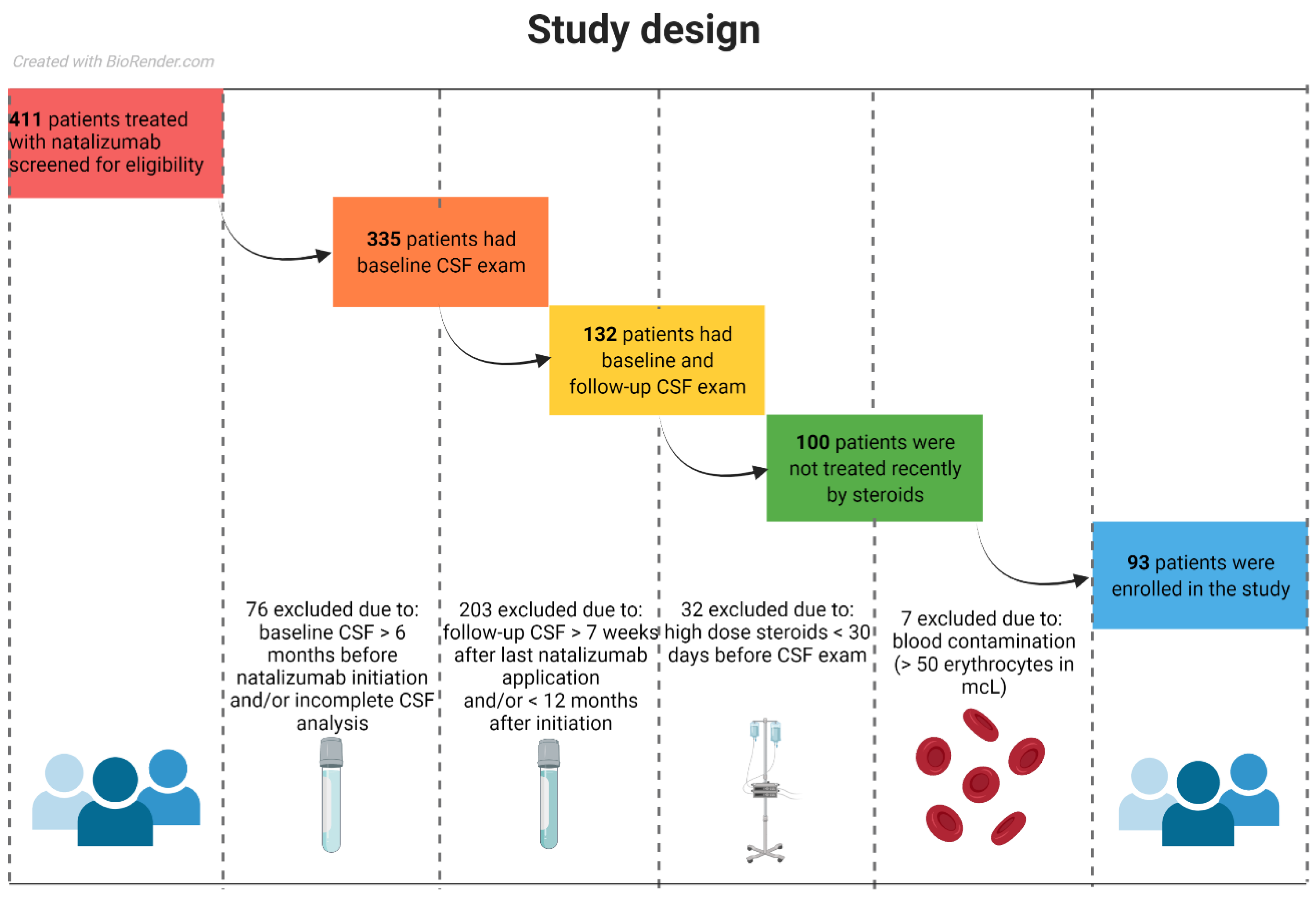
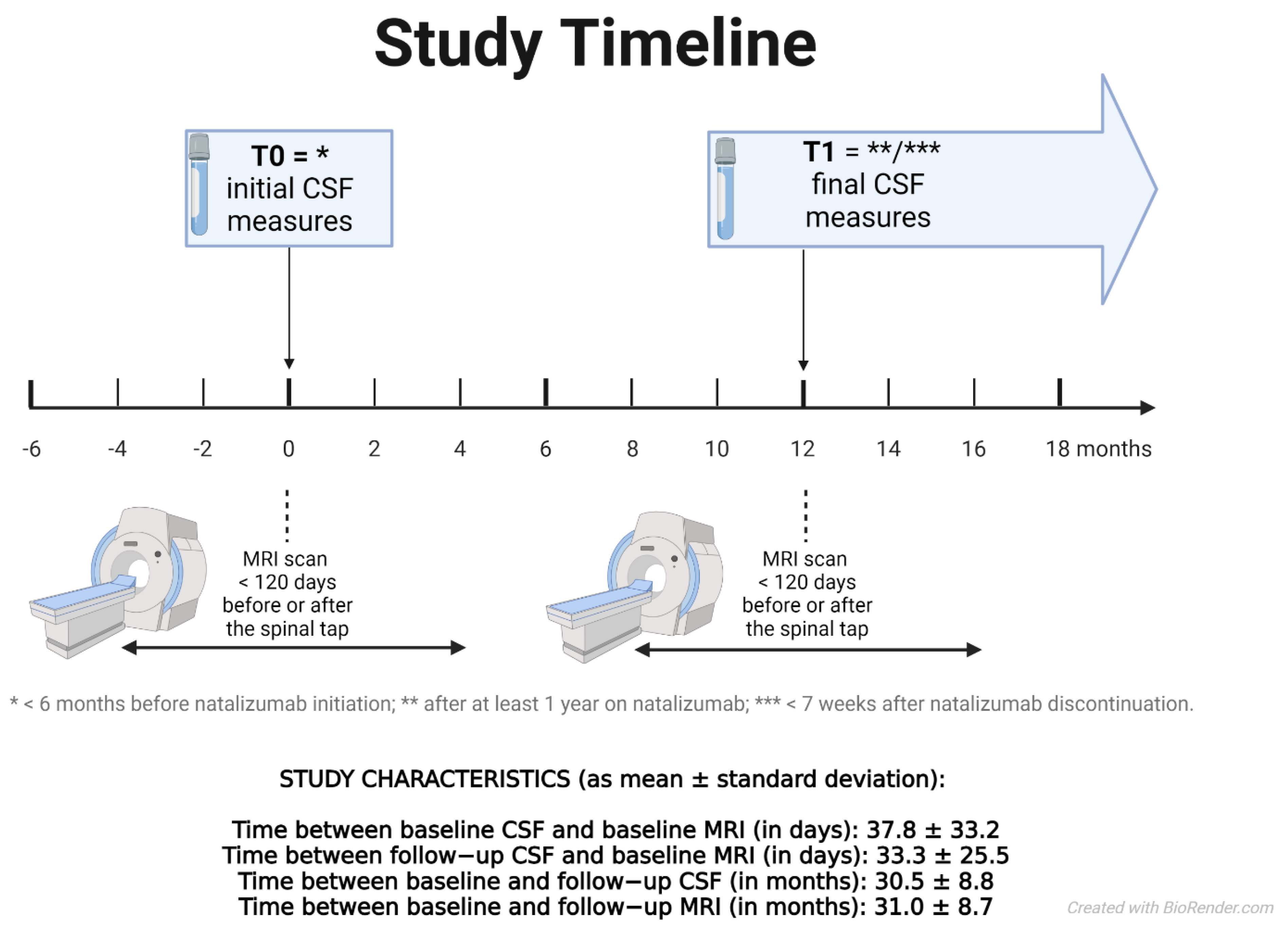

| Patient Characteristics | Baseline | Follow-Up | Statistical Comparison | |
|---|---|---|---|---|
| β (95% CI), p-Value | FDR p-Value **** | |||
| Demographic and clinical: | ||||
| Follow-up duration (years) | -- | 3.1, 2.4 (2.1–3.4) | -- | -- |
| Number of females | 62 (67%) | -- | -- | -- |
| Age (years) | 35.21, 35.30, 12.8 (29.2–42.0) | 38.26, 39.0, 11.6 (32.3–43.9) | -- | -- |
| Disease duration (years) | 9.03, 7.94, 10.4 (3.3–13.7) | 12.08, 11.1, 9.8 (7.2–17.0) | -- | -- |
| EDSS | 3.35, 3.5, 1.5 (2.5–4.0) | 3.31, 3.5, 2.0 (2.0–4.0) | −0.04 (−0.19; 0.11), p = 0.57 | -- |
| Relapses during last 12 months * | 1.67, 2.0, 1 (1–2) | 0.28, 0.0, 0 (0–0) | −4.0 (−5.15; −3.06), p = 6 × 10−15 *** | -- |
| DMT ** | 33.3% no 63.4% low efficacy 3.2% moderate/high efficacy | -- | -- | -- |
| Natalizumab treatment (years) | -- | 5.53, 4.53, 6.7 (2.3–9.0) | -- | -- |
| CSF measures: | ||||
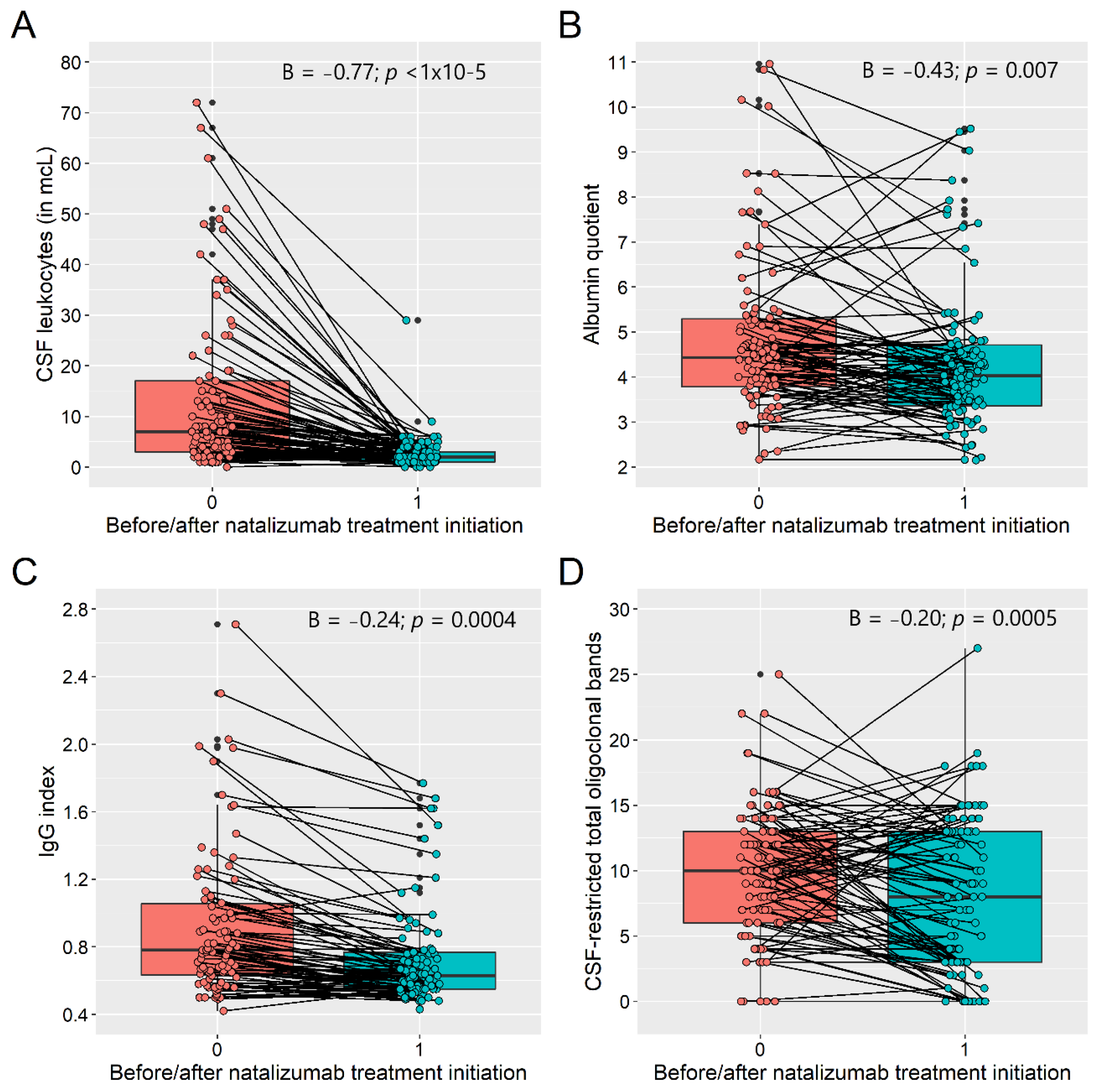
| CSF leukocytes (mcL) † | 4.45, 2.33, 4.7 (1.0–5.7) | 0.93, 0.67, 0.7 (0.3–1.0) | −0.77 (−0.93; −0.60), p < 1 × 10−15 | <0.0001 |
| CSF total protein (mg/L) | 0.34, 0.31, 0.12 (0.27–0.39) | 0.31, 0.3, 0.09 (0.24–0.33) | −0.03 (−0.04; −0.01), p < 0.00007 | 0.0002 |
| CSF total albumin (mg/L) | 0.20, 0.18, 0.07 (0.15–0.22) | 0.19, 0.18, 0.06 (0.15–0.21) | −0.01 (−0.02; 0.00), p = 0.10 | NS |
| QAlb | 4.76, 4.41, 1.6 (3.7–5.3) | 4.34, 4.03, 1.3 (3.4–4.7) | −0.43 (−0.74; −0.11), p = 0.007 | 0.008 |
| Abnormally increased QAlb | 10 from 93 (11%) | 5 from 93 (5%) | ‡ | -- |
| CSF-restricted total OCB | 9.91, 10.0, 7.5 (6.0–13.5) | 7.91, 8.0, 10 (3.0–13.0) | −2.0 (−3.2; −0.9), p = 0.0005 | 0.0007 |
| CSF-restricted OCB positivity | 88 from 93 (95%) | 79 from 93 (85%) | ‡ | -- |
| IgG quotient (n = 90) | 4.45, 3.83, 2.3 (2.6–4.9) | 3.06, 2.67, 1.68 (2.09–3.77) | −1.21 (−1.51; −0.90), p = 6 × 10−15 | <0.0001 |
| IgM quotient † (n = 80) | 1.06, 0.8, 0.78 (0.52–1.3) | 0.81, 0.65, 0.48 (0.42–1.0) | −0.12 (−0.19; −0.06), p = 0.0002 | 0.0004 |
| IgG index (n = 90) | 0.97, 0.78, 0.43 (0.65–1.08) | 0.73, 0.63, 0.22 (0.55–0.77) | −0.24 (−0.38; −0.11), p = 0.0004 | 0.0007 |
| Abnormally increased IgG index | 64 from 92 (69%) | 39 from 87 (45%) | −14.4 (−20.4; −10.8), p = 1 × 10−12 *** | <0.0001 |
| IgM index † (n = 80) | 0.24, 0.18, 0.2 (0.1–0.3) | 0.19, 0.14, 0.13 (0.09–0.22) | −0.04 (−0.06; −0.09), p = 0.003 | 0.004 |
| MRI measures: | ||||
| T2-LV (mL) (n1 = 64; and n2 = 45) † | 7.1, 3.1, 9.0 (1.5–10.5) | 7.9, 4.0, 8.7 (1.8–10.5) | 0.00 (−0.08; 0.08), p = 0.99 | NS |
| BPF (%) (n = 78) | 84.4, 84.6, 3.2 (83.0–86.2) | 84.35, 84.54, 3.4 (82.7–86.1) | −0.08 (−0.29; 0.11), p = 0.40 | NS |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ganapathy Subramanian, R.; Horakova, D.; Vaneckova, M.; Lorincz, B.; Krasensky, J.; Kubala Havrdova, E.; Uher, T. Natalizumab Induces Changes of Cerebrospinal Fluid Measures in Multiple Sclerosis. Diagnostics 2021, 11, 2230. https://doi.org/10.3390/diagnostics11122230
Ganapathy Subramanian R, Horakova D, Vaneckova M, Lorincz B, Krasensky J, Kubala Havrdova E, Uher T. Natalizumab Induces Changes of Cerebrospinal Fluid Measures in Multiple Sclerosis. Diagnostics. 2021; 11(12):2230. https://doi.org/10.3390/diagnostics11122230
Chicago/Turabian StyleGanapathy Subramanian, Ranjani, Dana Horakova, Manuela Vaneckova, Balazs Lorincz, Jan Krasensky, Eva Kubala Havrdova, and Tomas Uher. 2021. "Natalizumab Induces Changes of Cerebrospinal Fluid Measures in Multiple Sclerosis" Diagnostics 11, no. 12: 2230. https://doi.org/10.3390/diagnostics11122230
APA StyleGanapathy Subramanian, R., Horakova, D., Vaneckova, M., Lorincz, B., Krasensky, J., Kubala Havrdova, E., & Uher, T. (2021). Natalizumab Induces Changes of Cerebrospinal Fluid Measures in Multiple Sclerosis. Diagnostics, 11(12), 2230. https://doi.org/10.3390/diagnostics11122230






