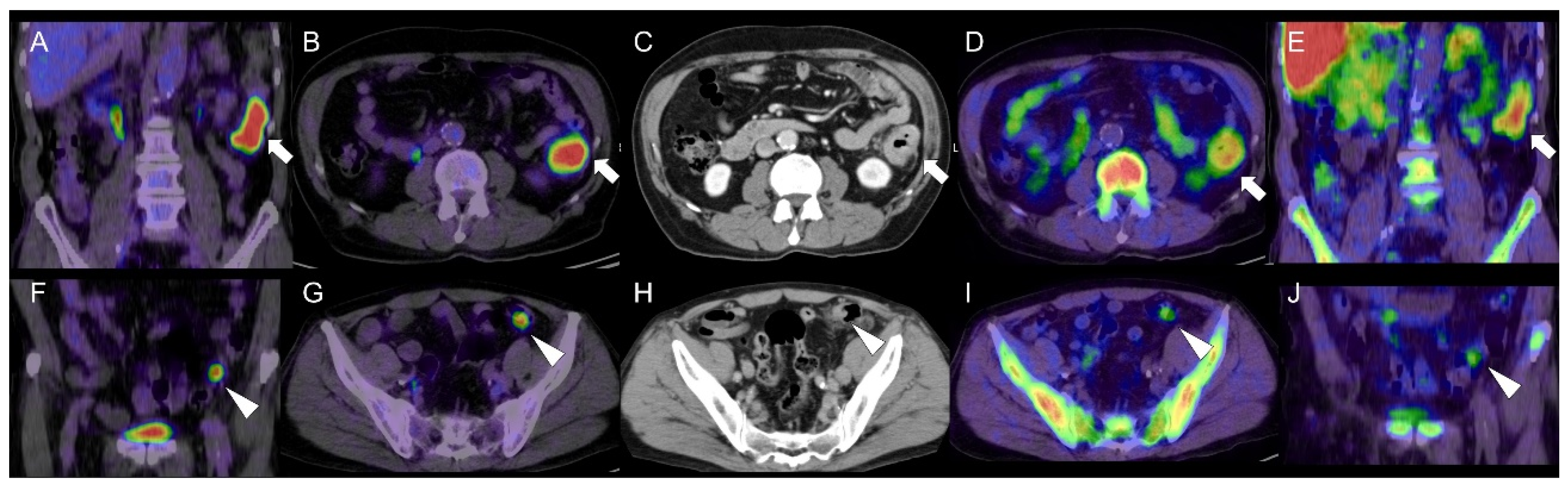
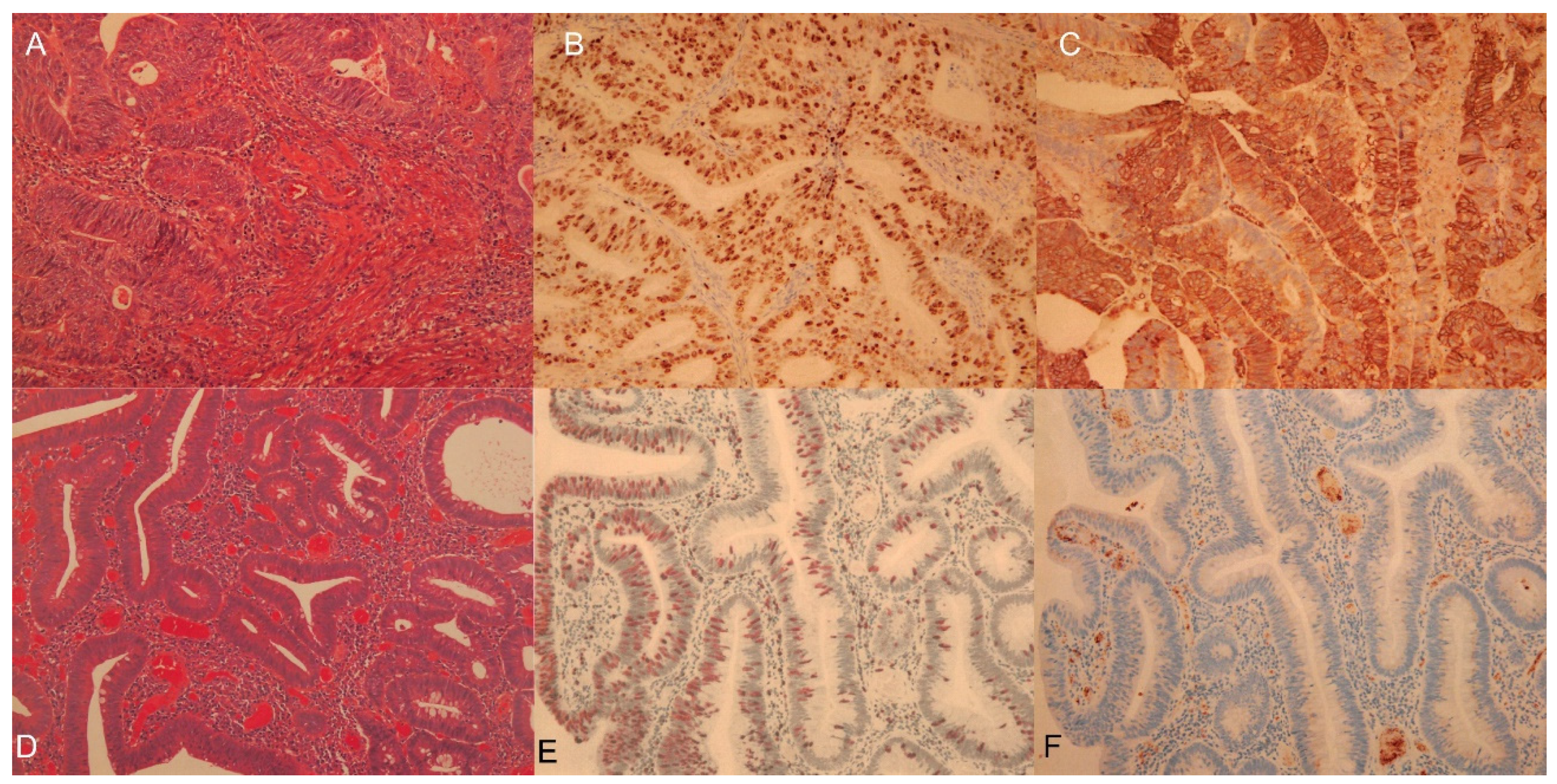
Cell Proliferation PET Imaging with 4DST PET/CT in Colorectal Adenocarcinoma and Adenoma
Abstract
:Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bond, J.H. Polyp guideline: Diagnosis, treatment, and surveillance for patients with colorectal polyps. Practice Parameters Committee of the American College of Gastroenterology. Am. J. Gastroenterol. 2000, 95, 3053–3063. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.D.; Yen, M.F.; Wang, W.M.; Wong, J.M.; Chen, T.H. A case cohort study for the disease natural history of adenoma-carcinoma and de novo carcinoma and surveillance of colon and rectum after polypectomy: Implication for efficacy of colonoscopy. Br. J. Cancer 2003, 88, 1866–1873. [Google Scholar] [CrossRef] [Green Version]
- Winawer, S.J.; Zauber, A.G.; Ho, M.N.; O’Brien, M.J.; Gottlieb, L.S.; Sternberg, S.S.; Waye, J.D.; Schapiro, M.; Bond, J.H.; Panish, J.F.; et al. Prevention of colorectal cancer by colonoscopic polypectomy. The National Polyp Study Workgroup. N. Engl. J. Med. 1993, 329, 1977–1981. [Google Scholar] [CrossRef] [PubMed]
- Drenth, J.P.; Nagengast, F.M.; Oyen, W.J. Evaluation of (pre-)malignant colonic abnormalities: Endoscopic validation of FDG-PET findings. Eur. J. Nucl. Med. Mol. Imaging 2001, 28, 1766–1769. [Google Scholar] [CrossRef]
- Agress, H., Jr.; Cooper, B.Z. Detection of clinically unexpected malignant and premalignant tumors with whole-body FDG PET: Histopathologic comparison. Radiology 2004, 230, 417–422. [Google Scholar] [CrossRef] [PubMed]
- Minamimoto, R.; Senda, M.; Jinnouchi, S.; Terauchi, T.; Yoshida, T.; Inoue, T. Detection of colorectal cancer and adenomas by FDG-PET cancer screening program: Results based on a nationwide Japanese survey. Ann. Nucl. Med. 2013, 28, 212–219. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.R.; Min, J.J.; Song, H.C.; Chong, A.; Kim, G.E.; Choi, C.; Seo, J.H.; Bom, H.S. A stepwise approach using metabolic volume and SUVmax to differentiate malignancy and dysplasia from benign colonic uptakes on 18F-FDG PET/CT. Clin. Nuc. Med. 2012, 37, 134–140. [Google Scholar] [CrossRef] [PubMed]
- Minamimoto, R.; Terauchi, T.; Jinnouchi, S.; Yoshida, T.; Tsukamoto, E.; Shimbo, T.; Ito, K.; Uno, K.; Ohno, H.; Oguchi, K.; et al. Observer variation study of the assessment and diagnosis of incidental colonic FDG uptake. Ann. Nucl. Med. 2013, 27, 468–477. [Google Scholar] [CrossRef] [PubMed]
- Toyohara, J.; Kumata, K.; Fukushi, K.; Irie, T.; Suzuki, K. Evaluation of 4’-[methyl-14C] thiothymidine for in vivo DNA synthesis imaging. J. Nucl. Med. 2006, 47, 1717–1722. [Google Scholar] [PubMed]
- Minamimoto, R.; Toyohara, J.; Seike, A.; Ito, H.; Endo, H.; Morooka, M.; Nakajima, K.; Mitsumoto, T.; Ito, K.; Okasaki, M.; et al. 4’-[Methyl-11C]-Thiothymidine PET/CT for Proliferation Imaging in Non-Small Cell Lung Cancer. J. Nucl. Med. 2011, 53, 199–206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Minamimoto, R.; Nakaigawa, N.; Nagashima, Y.; Toyohara, J.; Ueno, D.; Namura, K.; Nakajima, K.; Yao, M.; Kubota, K. Comparison of 11C-4DST and 18F-FDG PET/CT imaging for advanced renal cell carcinoma: Preliminary study. Abdom. Radiol. 2016, 41, 521–530. [Google Scholar] [CrossRef]
- Fukuda, Y.; Yamamoto, Y.; Mitamura, K.; Ishikawa, R.; Asano, E.; Toyohara, J.; Norikane, T.; Nishiyama, Y. 4′-[methyl-11C]-thiothymidine as a proliferation imaging tracer for detection of colorectal cancer: Comparison with 18F-FDG. Ann. Nucl. Med. 2019, 33, 822–827. [Google Scholar] [CrossRef]
- Güreşci, S.; Özmen, Ö.; Uzman, M.; Şimşek, G.; Tatci, E.; Gökçek, A.; Yeniova, A.Ö. Correlation of Ki-67 proliferation index and 18-fluorodeoxyglucose uptake in colorectal incidental lesions detected by positron emission tomography-computed tomography. Turk. J. Med. Sci. 2016, 46, 1182–1187. [Google Scholar] [CrossRef] [PubMed]
- Luo, Z.-W.; Zhu, M.-G.; Zhang, Z.-Q.; Ye, F.-J.; Huang, W.-H.; Luo, X.-Z. Increased expression of Ki-67 is a poor prognostic marker for colorectal cancer patients: A meta analysis. BMC Cancer 2019, 19, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meyer, H.-J.; Wienke, A.; Surov, A. Associations between GLUT expression and SUV values derived from FDG-PET in different tumors—A systematic review and meta analysis. PLoS ONE 2019, 14, e0217781. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Radovanovic-Dinic, B.; Nagorni, A.; Katic, V.; Katic, V.; Stamenkovic, I.; Zlatic, A. An immunohistochemical study of Ki-67 in colorectal adenoma. Med. Arh. 2009, 63, 16–18. [Google Scholar] [PubMed]
- Barone, M.; Scavo, M.P.; Papagni, S.; Piscitelli, D.; Guido, R.; Di Lena, M.; Comelli, M.C.; Di Leo, A. ERβ expression in normal, adenomatous and carcinomatous tissues of patients with familial adenomatous polyposis. Scand. J. Gastroenterol. 2010, 45, 1320–1328. [Google Scholar] [CrossRef] [PubMed]
- Haber, R.S.; Rathan, A.; Weiser, K.R.; Pritsker, A.; Itzkowitz, S.H.; Bodian, C.; Slater, G.; Weiss, A.; Burstein, D.E. GLUT1 glucose transporter expression in colorectal carcinoma: A marker for poor prognosis. Cancer 1998, 83, 34–40. [Google Scholar] [CrossRef]
- Greijer, A.E.; Diemen, P.M.D.-V.; Fijneman, R.; Giles, R.H.; Voest, E.E.; Van Hinsbergh, V.W.M.; Meijer, G.A. Presence of HIF-1 and related genes in normal mucosa, adenomas and carcinomas of the colorectum. Virchows Arch. 2008, 452, 535–544. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Wit, M.; Jimenez, C.R.; Carvalho, B.; Belien, J.A.; Delis-van Diemen, P.M.; Mongera, S.; Piersma, S.R.; Vikas, M.; Navani, S.; Pontén, F.; et al. Cell surface proteomics identifies glucose transporter type 1 and prion protein as candidate biomarkers for colorectal adenoma-to-carcinoma progression. Gut 2012, 61, 855–864. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Minamimoto, R.; Endo, H. Cell Proliferation PET Imaging with 4DST PET/CT in Colorectal Adenocarcinoma and Adenoma. Diagnostics 2021, 11, 1658. https://doi.org/10.3390/diagnostics11091658
Minamimoto R, Endo H. Cell Proliferation PET Imaging with 4DST PET/CT in Colorectal Adenocarcinoma and Adenoma. Diagnostics. 2021; 11(9):1658. https://doi.org/10.3390/diagnostics11091658
Chicago/Turabian StyleMinamimoto, Ryogo, and Hisako Endo. 2021. "Cell Proliferation PET Imaging with 4DST PET/CT in Colorectal Adenocarcinoma and Adenoma" Diagnostics 11, no. 9: 1658. https://doi.org/10.3390/diagnostics11091658
APA StyleMinamimoto, R., & Endo, H. (2021). Cell Proliferation PET Imaging with 4DST PET/CT in Colorectal Adenocarcinoma and Adenoma. Diagnostics, 11(9), 1658. https://doi.org/10.3390/diagnostics11091658




