Biological Significance of the Protein Changes Occurring in the Cerebrospinal Fluid of Alzheimer’s Disease Patients: Getting Clues from Proteomic Studies
Abstract
1. Introduction
2. Materials and Methods
3. Variability of the Proteomic Approaches
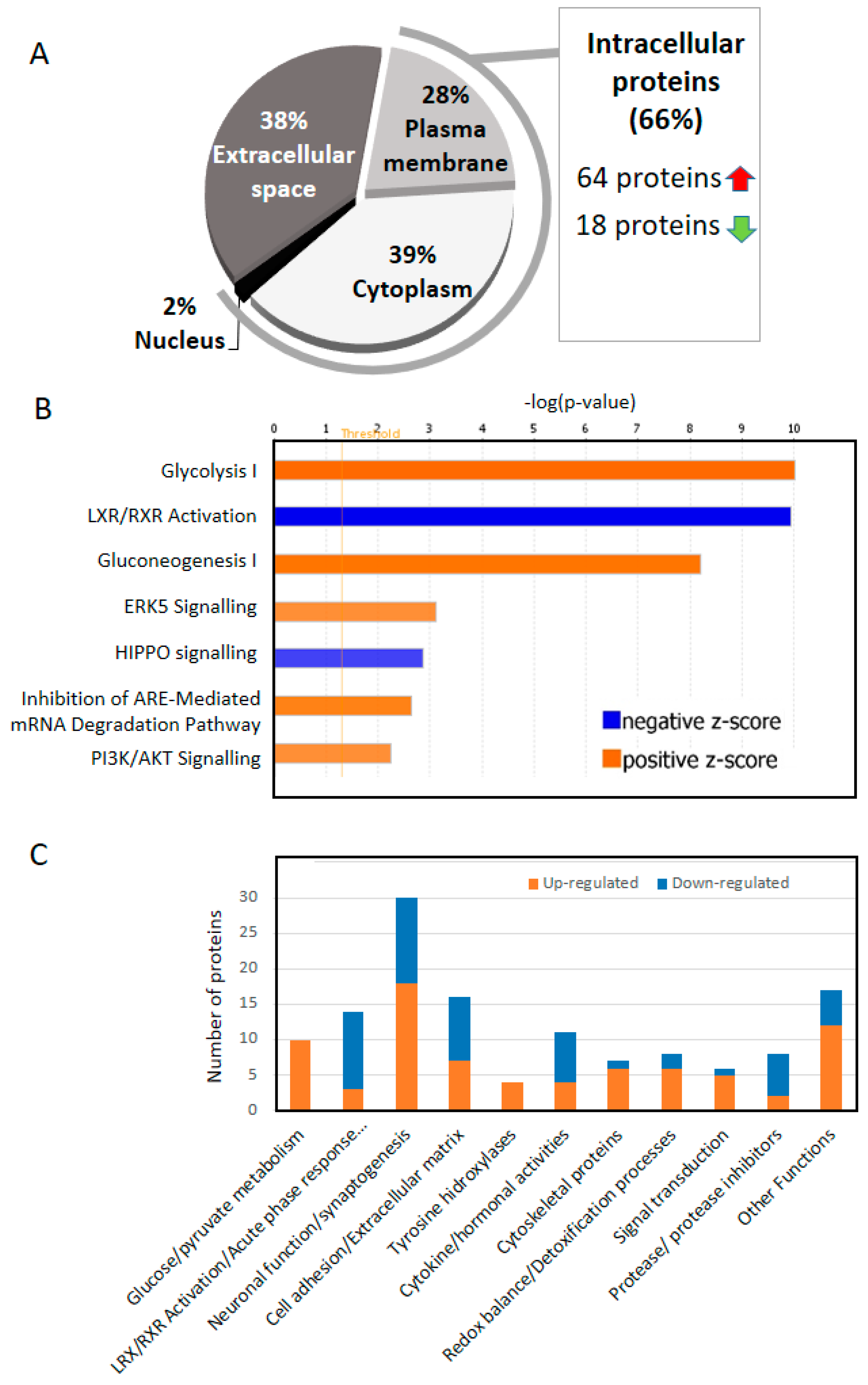
4. Two Thirds of the of the Proteins That Change in the CSF of AD Are Intracellular
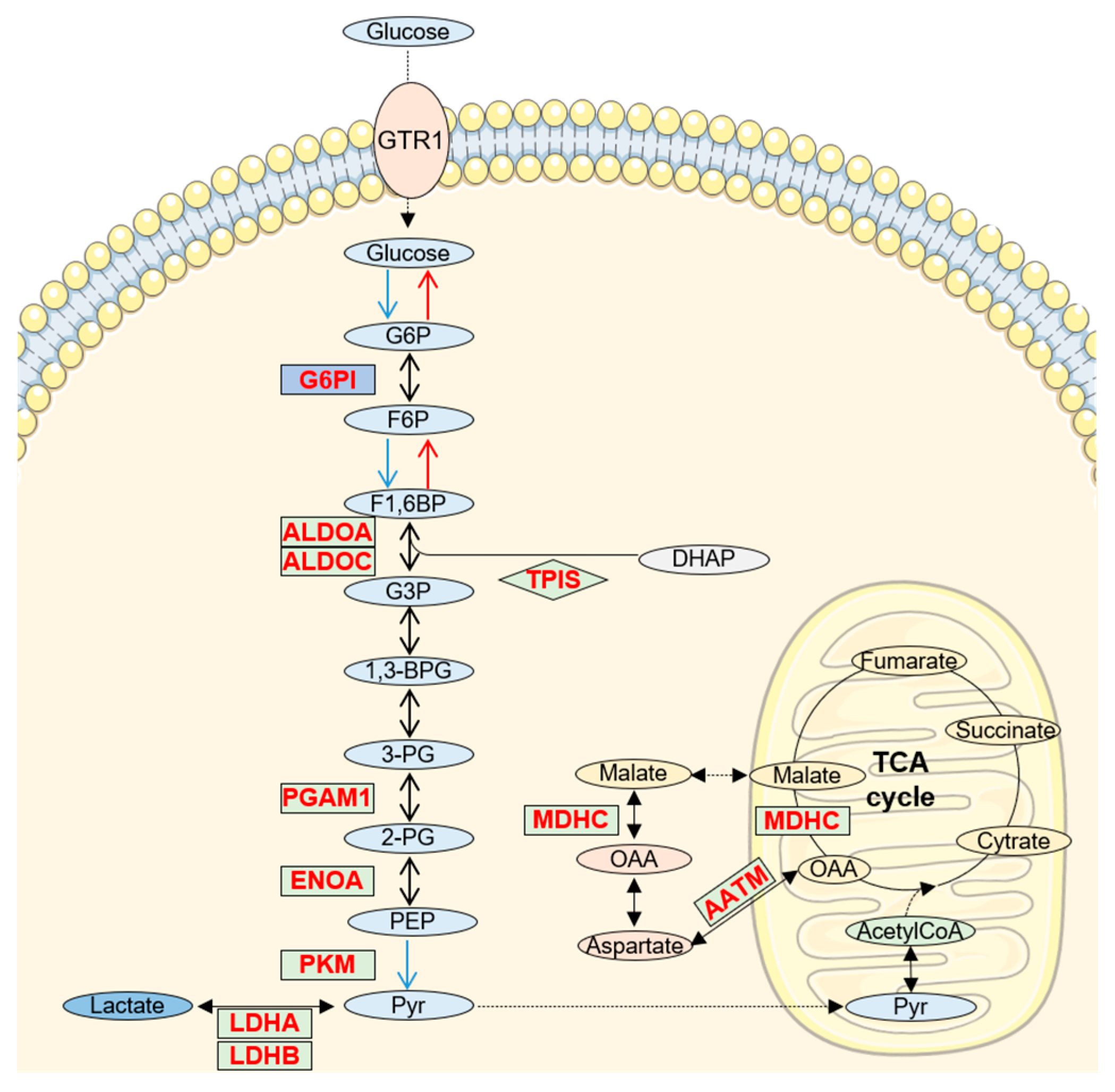
5. Increased Glucose/Pyruvate Metabolism in AD CSF
6. RXR Signaling in CSF (LXR/RXR Activation Pathway)
7. Neuronal Function/Synaptogenesis
8. 14-3-3 Proteins Are up Regulated in CSF
9. Cytokines and Hormones up and down Regulated in CSF
10. Importance of Cofactors in CSF
11. Other amyloid β Interactors
12. New Potential AD Markers
12.1. Ubiquitin C-Terminal Hydrolase L1 (UCHL1)
12.2. C-X-C Motif Chemokine Ligand 16 (CXL16)
12.3. Protein Kinase C and Casein Kinase Substrate in Neurons 1 (PACN1)
12.4. Protein Tyrosine Phosphatase Receptor Type Z1 (PTPRZ)
12.5. Integrin Subunit Alpha M (ITAM)
12.6. Myristoylated Alanine-Rich Protein Kinase C Substrate (MARCS)
13. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Llorens, F.; Schmitz, M.; Ferrer, I.; Zerr, I. CSF biomarkers in neurodegenerative and vascular dementias. Prog. Neurobiol. 2016, 138–140, 36–53. [Google Scholar] [CrossRef]
- Solje, E.; Benussi, A.; Buratti, E.; Remes, A.M.; Haapasalo, A.; Borroni, B. State-of-the-Art Methods and Emerging Fluid Biomarkers in the Diagnostics of Dementia—A Short Review and Diagnostic Algorithm. Diagnostics 2021, 11, 788. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.-Y.; Bawa, K.K.; Ouk, M.; Leung, N.; Yu, D.; Lanctôt, K.L.; Herrmann, N.; Pakosh, M.; Swardfager, W. Neutrophil activation in Alzheimer’s disease and mild cognitive impairment: A systematic review and meta-analysis of protein markers in blood and cerebrospinal fluid. Ageing Res. Rev. 2020, 62, 101130. [Google Scholar] [CrossRef]
- McGrowder, D.A.; Miller, F.; Vaz, K.; Nwokocha, C.; Wilson-Clarke, C.; Anderson-Cross, M.; Brown, J.; Anderson-Jackson, L.; Williams, L.; Latore, L.; et al. Cerebrospinal Fluid Biomarkers of Alzheimer’s Disease: Current Evidence and Future Perspectives. Brain Sci. 2021, 11, 215. [Google Scholar] [CrossRef]
- Baird, A.L.; Westwood, S.; Lovestone, S. Blood-Based Proteomic Biomarkers of Alzheimer’s Disease Pathology. Front. Neurol. 2015, 6, 236. [Google Scholar] [CrossRef] [PubMed]
- Blennow, K. Cerebrospinal fluid protein biomarkers for Alzheimer’s disease. NeuroRX 2004, 1, 213–225. [Google Scholar] [CrossRef]
- Pedrero-Prieto, C.M.; García-Carpintero, S.; Frontiñán-Rubio, J.; Llanos-González, E.; Aguilera García, C.; Alcaín, F.J.; Lindberg, I.; Durán-Prado, M.; Peinado, J.R.; Rabanal-Ruiz, Y. A comprehensive systematic review of CSF proteins and peptides that define Alzheimer’s disease. Clin. Proteom. 2020, 17, 1–24. [Google Scholar] [CrossRef]
- Wesenhagen, K.E.J.; Teunissen, C.E.; Visser, P.J.; Tijms, B.M. Cerebrospinal fluid proteomics and biological heterogeneity in Alzheimer’s disease: A literature review. Crit. Rev. Clin. Lab. Sci. 2019, 57, 86–98. [Google Scholar] [CrossRef]
- Fabregat, A.; Sidiropoulos, K.; Viteri, G.; Forner, O.; Marin-Garcia, P.; Arnau, V.; D’Eustachio, P.; Stein, L.; Hermjakob, H. Reactome pathway analysis: A high-performance in-memory approach. BMC Bioinform. 2017, 18, 142. [Google Scholar] [CrossRef]
- McKetney, J.; Panyard, D.J.; Johnson, S.C.; Carlsson, C.M.; Engelman, C.D.; Coon, J.J. Pilot proteomic analysis of cerebrospinal fluid in Alzheimer’s disease. Proteom. Clin. Appl. 2021, 15, e2000072. [Google Scholar] [CrossRef]
- Zhou, M.; Haque, R.U.; Dammer, E.B.; Duong, D.M.; Ping, L.; Johnson, E.C.B.; Lah, J.J.; Levey, A.I.; Seyfried, N.T. Targeted mass spectrometry to quantify brain-derived cerebrospinal fluid biomarkers in Alzheimer’s disease. Clin. Proteom. 2020, 17, 19. [Google Scholar] [CrossRef]
- Higginbotham, L.; Ping, L.; Dammer, E.B.; Duong, D.M.; Zhou, M.; Gearing, M.; Hurst, C.; Glass, J.D.; Factor, S.A.; Johnson, E.C.B.; et al. Integrated proteomics reveals brain-based cerebrospinal fluid biomarkers in asymptomatic and symptomatic Alzheimer’s disease. Sci. Adv. 2020, 6, aaz9360. [Google Scholar] [CrossRef] [PubMed]
- Bader, J.M.; Geyer, P.E.; Muller, J.B.; Strauss, M.T.; Koch, M.; Leypoldt, F.; Koertvelyessy, P.; Bittner, D.; Schipke, C.G.; Incesoy, E.I.; et al. Proteome profiling in cerebrospinal fluid reveals novel biomarkers of Alzheimer’s disease. Mol. Syst. Biol. 2020, 16, e9356. [Google Scholar] [CrossRef] [PubMed]
- Dayon, L.; Nunez Galindo, A.; Wojcik, J.; Cominetti, O.; Corthesy, J.; Oikonomidi, A.; Henry, H.; Kussmann, M.; Migliavacca, E.; Severin, I.; et al. Alzheimer disease pathology and the cerebrospinal fluid proteome. Alzheimers Res. Ther. 2018, 10, 66. [Google Scholar] [CrossRef] [PubMed]
- Ringman, J.M.; Schulman, H.; Becker, C.; Jones, T.; Bai, Y.; Immermann, F.; Cole, G.; Sokolow, S.; Gylys, K.; Geschwind, D.H.; et al. Proteomic changes in cerebrospinal fluid of presymptomatic and affected persons carrying familial Alzheimer disease mutations. Arch. Neurol. 2012, 69, 96–104. [Google Scholar] [CrossRef] [PubMed]
- Johnson, E.C.B.; Dammer, E.B.; Duong, D.M.; Ping, L.; Zhou, M.; Yin, L.; Higginbotham, L.A.; Guajardo, A.; White, B.; Troncoso, J.C.; et al. Large-scale proteomic analysis of Alzheimer’s disease brain and cerebrospinal fluid reveals early changes in energy metabolism associated with microglia and astrocyte activation. Nat. Med. 2020, 26, 769–780. [Google Scholar] [CrossRef]
- Khoonsari, P.E.; Haggmark, A.; Lonnberg, M.; Mikus, M.; Kilander, L.; Lannfelt, L.; Bergquist, J.; Ingelsson, M.; Nilsson, P.; Kultima, K.; et al. Analysis of the Cerebrospinal Fluid Proteome in Alzheimer’s Disease. PLoS ONE 2016, 11, e0150672. [Google Scholar] [CrossRef] [PubMed]
- Paterson, R.W.; Heywood, W.E.; Heslegrave, A.J.; Magdalinou, N.K.; Andreasson, U.; Sirka, E.; Bliss, E.; Slattery, C.F.; Toombs, J.; Svensson, J.; et al. A targeted proteomic multiplex CSF assay identifies increased malate dehydrogenase and other neurodegenerative biomarkers in individuals with Alzheimer’s disease pathology. Transl. Psychiatry 2016, 6, e952. [Google Scholar] [CrossRef]
- Heywood, W.E.; Galimberti, D.; Bliss, E.; Sirka, E.; Paterson, R.W.; Magdalinou, N.K.; Carecchio, M.; Reid, E.; Heslegrave, A.; Fenoglio, C.; et al. Identification of novel CSF biomarkers for neurodegeneration and their validation by a high-throughput multiplexed targeted proteomic assay. Mol. Neurodegener. 2015, 10, 64. [Google Scholar] [CrossRef] [PubMed]
- Sathe, G.; Na, C.H.; Renuse, S.; Madugundu, A.K.; Albert, M.; Moghekar, A.; Pandey, A. Quantitative Proteomic Profiling of Cerebrospinal Fluid to Identify Candidate Biomarkers for Alzheimer’s Disease. Proteom. Clin. Appl. 2019, 13, e1800105. [Google Scholar] [CrossRef]
- Holtta, M.; Minthon, L.; Hansson, O.; Holmen-Larsson, J.; Pike, I.; Ward, M.; Kuhn, K.; Ruetschi, U.; Zetterberg, H.; Blennow, K.; et al. An integrated workflow for multiplex CSF proteomics and peptidomics-identification of candidate cerebrospinal fluid biomarkers of Alzheimer’s disease. J. Proteome Res. 2015, 14, 654–663. [Google Scholar] [CrossRef]
- Alzate, O.; Osorio, C.; DeKroon, R.M.; Corcimaru, A.; Gunawardena, H.P. Differentially charged isoforms of apolipoprotein E from human blood are potential biomarkers of Alzheimer’s disease. Alzheimers Res. Ther. 2014, 6, 43. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Chakrabarti, A.; Chatterjee, A.; Sengupta, M.B.; Chattopadhyay, P.; Mukhopadhyay, D. Altered levels of amyloid precursor protein intracellular domain-interacting proteins in Alzheimer disease. Alzheimer Dis. Assoc. Disord. 2014, 28, 283–290. [Google Scholar] [CrossRef]
- Khoonsari, P.E.; Shevchenko, G.; Herman, S.; Remnestal, J.; Giedraitis, V.; Brundin, R.; Degerman Gunnarsson, M.; Kilander, L.; Zetterberg, H.; Nilsson, P.; et al. Improved Differential Diagnosis of Alzheimer’s Disease by Integrating ELISA and Mass Spectrometry-Based Cerebrospinal Fluid Biomarkers. J. Alzheimer’s Dis. 2019, 67, 639–651. [Google Scholar] [CrossRef] [PubMed]
- Spellman, D.S.; Wildsmith, K.R.; Honigberg, L.A.; Tuefferd, M.; Baker, D.; Raghavan, N.; Nairn, A.C.; Croteau, P.; Schirm, M.; Allard, R.; et al. Development and evaluation of a multiplexed mass spectrometry based assay for measuring candidate peptide biomarkers in Alzheimer’s Disease Neuroimaging Initiative (ADNI) CSF. Proteom. Clin. Appl. 2015, 9, 715–731. [Google Scholar] [CrossRef]
- Skillback, T.; Mattsson, N.; Hansson, K.; Mirgorodskaya, E.; Dahlen, R.; van der Flier, W.; Scheltens, P.; Duits, F.; Hansson, O.; Teunissen, C.; et al. A novel quantification-driven proteomic strategy identifies an endogenous peptide of pleiotrophin as a new biomarker of Alzheimer’s disease. Sci. Rep. 2017, 7, 13333. [Google Scholar] [CrossRef]
- Manral, P.; Sharma, P.; Hariprasad, G.; Chandralekha; Tripathi, M.; Srinivasan, A. Can apolipoproteins and complement factors be biomarkers of Alzheimer’s disease? Curr. Alzheimer Res. 2012, 9, 935–943. [Google Scholar] [CrossRef]
- Whelan, C.D.; Mattsson, N.; Nagle, M.W.; Vijayaraghavan, S.; Hyde, C.; Janelidze, S.; Stomrud, E.; Lee, J.; Fitz, L.; Samad, T.A.; et al. Multiplex proteomics identifies novel CSF and plasma biomarkers of early Alzheimer’s disease. Acta Neuropathol. Commun. 2019, 7, 169. [Google Scholar] [CrossRef] [PubMed]
- Vafadar-Isfahani, B.; Ball, G.; Coveney, C.; Lemetre, C.; Boocock, D.; Minthon, L.; Hansson, O.; Miles, A.K.; Janciauskiene, S.M.; Warden, D.; et al. Identification of SPARC-like 1 protein as part of a biomarker panel for Alzheimer’s disease in cerebrospinal fluid. J. Alzheimer’s Dis. JAD 2012, 28, 625–636. [Google Scholar] [CrossRef] [PubMed]
- Remnestal, J.; Just, D.; Mitsios, N.; Fredolini, C.; Mulder, J.; Schwenk, J.M.; Uhlen, M.; Kultima, K.; Ingelsson, M.; Kilander, L.; et al. CSF profiling of the human brain enriched proteome reveals associations of neuromodulin and neurogranin to Alzheimer’s disease. Proteom. Clin. Appl. 2016, 10, 1242–1253. [Google Scholar] [CrossRef]
- Brinkmalm, G.; Sjodin, S.; Simonsen, A.H.; Hasselbalch, S.G.; Zetterberg, H.; Brinkmalm, A.; Blennow, K. A Parallel Reaction Monitoring Mass Spectrometric Method for Analysis of Potential CSF Biomarkers for Alzheimer’s Disease. Proteom. Clin. Appl. 2018, 12. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Cunningham, R.; Zetterberg, H.; Asthana, S.; Carlsson, C.; Okonkwo, O.; Li, L. Label-free quantitative comparison of cerebrospinal fluid glycoproteins and endogenous peptides in subjects with Alzheimer’s disease, mild cognitive impairment, and healthy individuals. Proteom. Clin. Appl. 2016, 10, 1225–1241. [Google Scholar] [CrossRef]
- Nilsson, J.; Gobom, J.; Sjodin, S.; Brinkmalm, G.; Ashton, N.J.; Svensson, J.; Johansson, P.; Portelius, E.; Zetterberg, H.; Blennow, K.; et al. Cerebrospinal fluid biomarker panel for synaptic dysfunction in Alzheimer’s disease. Alzheimers Dement. (Amst.) 2021, 13, e12179. [Google Scholar] [CrossRef] [PubMed]
- Hendrickson, R.C.; Lee, A.Y.; Song, Q.; Liaw, A.; Wiener, M.; Paweletz, C.P.; Seeburger, J.L.; Li, J.; Meng, F.; Deyanova, E.G.; et al. High Resolution Discovery Proteomics Reveals Candidate Disease Progression Markers of Alzheimer’s Disease in Human Cerebrospinal Fluid. PLoS ONE 2015, 10, e0135365. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Dey, K.K.; Chen, P.C.; Li, Y.; Niu, M.; Cho, J.H.; Wang, X.; Bai, B.; Jiao, Y.; Chepyala, S.R.; et al. Integrated analysis of ultra-deep proteomes in cortex, cerebrospinal fluid and serum reveals a mitochondrial signature in Alzheimer’s disease. Mol. Neurodegener. 2020, 15, 43. [Google Scholar] [CrossRef] [PubMed]
- Park, S.A.; Jung, J.M.; Park, J.S.; Lee, J.H.; Park, B.; Kim, H.J.; Park, J.H.; Chae, W.S.; Jeong, J.H.; Choi, S.H.; et al. SWATH-MS analysis of cerebrospinal fluid to generate a robust battery of biomarkers for Alzheimer’s disease. Sci. Rep. 2020, 10, 7423. [Google Scholar] [CrossRef] [PubMed]
- Duits, F.H.; Brinkmalm, G.; Teunissen, C.E.; Brinkmalm, A.; Scheltens, P.; Van der Flier, W.M.; Zetterberg, H.; Blennow, K. Synaptic proteins in CSF as potential novel biomarkers for prognosis in prodromal Alzheimer’s disease. Alzheimer’s Res. Ther. 2018, 10, 5. [Google Scholar] [CrossRef] [PubMed]
- Wijte, D.; McDonnell, L.A.; Balog, C.I.; Bossers, K.; Deelder, A.M.; Swaab, D.F.; Verhaagen, J.; Mayboroda, O.A. A novel peptidomics approach to detect markers of Alzheimer’s disease in cerebrospinal fluid. Methods 2012, 56, 500–507. [Google Scholar] [CrossRef]
- Wildsmith, K.R.; Schauer, S.P.; Smith, A.M.; Arnott, D.; Zhu, Y.; Haznedar, J.; Kaur, S.; Mathews, W.R.; Honigberg, L.A. Identification of longitudinally dynamic biomarkers in Alzheimer’s disease cerebrospinal fluid by targeted proteomics. Mol. Neurodegener. 2014, 9, 22. [Google Scholar] [CrossRef]
- Korecka, M.; Shaw, L.M. Mass spectrometry-based methods for robust measurement of Alzheimer’s Disease biomarkers in biological fluids. J. Neurochem. 2021. [Google Scholar] [CrossRef]
- Rahimi, J.; Woehrer, A. Overview of cerebrospinal fluid cytology. In Neuropathology; Kovacs, G., Alafuzoff, I., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 563–571. [Google Scholar] [CrossRef]
- Guo, T.; Zhang, D.; Zeng, Y.; Huang, T.Y.; Xu, H.; Zhao, Y. Molecular and cellular mechanisms underlying the pathogenesis of Alzheimer’s disease. Mol. Neurodegener. 2020, 15, 40. [Google Scholar] [CrossRef]
- Reiber, H. Dynamics of brain-derived proteins in cerebrospinal fluid. Clin. Chim. Acta 2001, 310, 173–186. [Google Scholar] [CrossRef]
- Stock, A.J.; Kasus-Jacobi, A.; Pereira, H.A. The role of neutrophil granule proteins in neuroinflammation and Alzheimer’s disease. J. Neuroinflamm. 2018, 15, 240. [Google Scholar] [CrossRef] [PubMed]
- Talib, L.L. Platelet biomarkers in Alzheimer’s disease. World J. Psychiatry 2012, 2, 95. [Google Scholar] [CrossRef]
- Arai, K.; Gowert, N.S.; Donner, L.; Chatterjee, M.; Eisele, Y.S.; Towhid, S.T.; Münzer, P.; Walker, B.; Ogorek, I.; Borst, O.; et al. Blood Platelets in the Progression of Alzheimer’s Disease. PLoS ONE 2014, 9, e0090523. [Google Scholar] [CrossRef]
- An, K.; Klyubin, I.; Kim, Y.; Jung, J.; Mably, A.J.; O’Dowd, S.T.; Lynch, T.; Kanmert, D.; Lemere, C.A.; Finan, G.M.; et al. Exosomes neutralize synaptic-plasticity-disrupting activity of Aβ assemblies in vivo. Mol. Brain 2013, 6, 47. [Google Scholar] [CrossRef]
- Li, M.; Huang, L.; Chen, J.; Ni, F.; Zhang, Y.; Liu, F. Isolation of Exosome Nanoparticles from Human Cerebrospinal Fluid for Proteomic Analysis. ACS Appl. Nano Mater. 2021, 4, 3351–3359. [Google Scholar] [CrossRef]
- Zahra, K.; Dey, T.; Ashish; Mishra, S.P.; Pandey, U. Pyruvate Kinase M2 and Cancer: The Role of PKM2 in Promoting Tumorigenesis. Front. Oncol. 2020, 10, 159. [Google Scholar] [CrossRef]
- Bell, S.M.; Burgess, T.; Lee, J.; Blackburn, D.J.; Allen, S.P.; Mortiboys, H. Peripheral Glycolysis in Neurodegenerative Diseases. Int. J. Mol. Sci. 2020, 21, 8924. [Google Scholar] [CrossRef]
- Le Douce, J.; Maugard, M.; Veran, J.; Matos, M.; Jégo, P.; Vigneron, P.-A.; Faivre, E.; Toussay, X.; Vandenberghe, M.; Balbastre, Y.; et al. Impairment of Glycolysis-Derived l-Serine Production in Astrocytes Contributes to Cognitive Deficits in Alzheimer’s Disease. Cell Metab. 2020, 31, 503–517.e508. [Google Scholar] [CrossRef] [PubMed]
- Bigl, M.; Bruckner, M.K.; Arendt, T.; Bigl, V.; Eschrich, K. Activities of key glycolytic enzymes in the brains of patients with Alzheimer’s disease. J. Neural. Transm. 1999, 106, 499–511. [Google Scholar] [CrossRef]
- Goodman, A.B.; Pardee, A.B. Evidence for defective retinoid transport and function in late onset Alzheimer’s disease. Proc. Natl. Acad. Sci. USA 2003, 100, 2901–2905. [Google Scholar] [CrossRef]
- Ray, S.; Das, B.; Dasgupta, S. Potential therapeutic roles of retinoids for prevention of neuroinflammation and neurodegeneration in Alzheimer’s disease. Neural Regen. Res. 2019, 14, 1880. [Google Scholar] [CrossRef]
- Kölsch, H.; Lütjohann, D.; Jessen, F.; Popp, J.; Hentschel, F.; Kelemen, P.; Friedrichs, S.; Maier, T.A.W.; Heun, R. RXRA gene variations influence Alzheimer’s disease risk and cholesterol metabolism. J. Cell. Mol. Med. 2009, 13, 589–598. [Google Scholar] [CrossRef]
- Xiong, H.; Callaghan, D.; Jones, A.; Walker, D.G.; Lue, L.-F.; Beach, T.G.; Sue, L.I.; Woulfe, J.; Xu, H.; Stanimirovic, D.B.; et al. Cholesterol retention in Alzheimer’s brain is responsible for high β- and γ-secretase activities and Aβ production. Neurobiol. Dis. 2008, 29, 422–437. [Google Scholar] [CrossRef] [PubMed]
- Ojo, J.O.; Crynen, G.; Reed, J.M.; Ajoy, R.; Vallabhaneni, P.; Algamal, M.; Leary, P.; Rafi, N.G.; Mouzon, B.; Mullan, M.; et al. Unbiased Proteomic Approach Identifies Unique and Coincidental Plasma Biomarkers in Repetitive mTBI and AD Pathogenesis. Front. Aging Neurosci. 2018, 10, 405. [Google Scholar] [CrossRef] [PubMed]
- Sodhi, R.K.; Singh, N. Retinoids as potential targets for Alzheimer’s disease. Pharmacol. Biochem. Behav. 2014, 120, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Obulesu, M.; Dowlathabad, M.R.; Bramhachari, P.V. Carotenoids and Alzheimer’s Disease: An insight into therapeutic role of retinoids in animal models. Neurochem. Int. 2011, 59, 535–541. [Google Scholar] [CrossRef] [PubMed]
- Fitz, N.F.; Nam, K.N.; Koldamova, R.; Lefterov, I. Therapeutic targeting of nuclear receptors, liver X and retinoid X receptors, for Alzheimer’s disease. Br. J. Pharmacol. 2019, 176, 3599–3610. [Google Scholar] [CrossRef] [PubMed]
- Terwel, D.; Steffensen, K.R.; Verghese, P.B.; Kummer, M.P.; Gustafsson, J.A.; Holtzman, D.M.; Heneka, M.T. Critical Role of Astroglial Apolipoprotein E and Liver X Receptor- Expression for Microglial A Phagocytosis. J. Neurosci. 2011, 31, 7049–7059. [Google Scholar] [CrossRef] [PubMed]
- Zelcer, N.; Khanlou, N.; Clare, R.; Jiang, Q.; Reed-Geaghan, E.G.; Landreth, G.E.; Vinters, H.V.; Tontonoz, P. Attenuation of neuroinflammation and Alzheimer’s disease pathology by liver x receptors. Proc. Natl. Acad. Sci. USA 2007, 104, 10601–10606. [Google Scholar] [CrossRef]
- Andersson, S.; Gustafsson, N.; Warner, M.; Gustafsson, J.A. Inactivation of liver X receptor leads to adult-onset motor neuron degeneration in male mice. Proc. Natl. Acad. Sci. USA 2005, 102, 3857–3862. [Google Scholar] [CrossRef]
- Zelcer, N. Liver X receptors as integrators of metabolic and inflammatory signaling. J. Clin. Investig. 2006, 116, 607–614. [Google Scholar] [CrossRef] [PubMed]
- Dominiczak, M.H.; Caslake, M.J. Apolipoproteins: Metabolic role and clinical biochemistry applications. Ann. Clin. Biochem. Int. J. Lab. Med. 2011, 48, 498–515. [Google Scholar] [CrossRef]
- Paula-Lima, A.C.; Tricerri, M.A.; Brito-Moreira, J.; Bomfim, T.R.; Oliveira, F.F.; Magdesian, M.H.; Grinberg, L.T.; Panizzutti, R.; Ferreira, S.T. Human apolipoprotein A–I binds amyloid-β and prevents Aβ-induced neurotoxicity. Int. J. Biochem. Cell Biol. 2009, 41, 1361–1370. [Google Scholar] [CrossRef] [PubMed]
- Schellenberg, G.; Bird, T.; Wijsman, E.; Orr, H.; Anderson, L.; Nemens, E.; White, J.; Bonnycastle, L.; Weber, J.; Alonso, M.; et al. Genetic linkage evidence for a familial Alzheimer’s disease locus on chromosome 14. Science 1992, 258, 668–671. [Google Scholar] [CrossRef]
- Friedman, D.J.; Pollak, M.R. APOL1and Kidney Disease: From Genetics to Biology. Annu. Rev. Physiol. 2020, 82, 323–342. [Google Scholar] [CrossRef]
- Cohen-Cory, S. The developing synapse: Construction and modulation of synaptic structures and circuits. Science 2002, 298, 770–776. [Google Scholar] [CrossRef] [PubMed]
- Masliah, E.; Mallory, M.; Alford, M.; DeTeresa, R.; Hansen, L.A.; McKeel, D.W.; Morris, J.C. Altered expression of synaptic proteins occurs early during progression of Alzheimer’s disease. Neurology 2001, 56, 127–129. [Google Scholar] [CrossRef] [PubMed]
- Selkoe, D.J. Alzheimer’s Disease Is a Synaptic Failure. Science 2002, 298, 789–791. [Google Scholar] [CrossRef] [PubMed]
- Boles, N.C.; Hirsch, S.E.; Le, S.; Corneo, B.; Najm, F.; Minotti, A.P.; Wang, Q.; Lotz, S.; Tesar, P.J.; Fasano, C.A. NPTX1 Regulates Neural Lineage Specification from Human Pluripotent Stem Cells. Cell Rep. 2014, 6, 724–736. [Google Scholar] [CrossRef] [PubMed]
- Sia, G.-M.; Béïque, J.-C.; Rumbaugh, G.; Cho, R.; Worley, P.F.; Huganir, R.L. Interaction of the N-Terminal Domain of the AMPA Receptor GluR4 Subunit with the Neuronal Pentraxin NP1 Mediates GluR4 Synaptic Recruitment. Neuron 2007, 55, 87–102. [Google Scholar] [CrossRef]
- Cummings, D.M.; Benway, T.A.; Ho, H.; Tedoldi, A.; Fernandes Freitas, M.M.; Shahab, L.; Murray, C.E.; Richard-Loendt, A.; Brandner, S.; Lashley, T.; et al. Neuronal and Peripheral Pentraxins Modify Glutamate Release and may Interact in Blood–Brain Barrier Failure. Cereb. Cortex 2017, 27, 3437–3448. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-J.; Wei, M.; Zhang, C.; Maxeiner, S.; Pak, C.; Calado Botelho, S.; Trotter, J.; Sterky, F.H.; Südhof, T.C. Presynaptic Neuronal Pentraxin Receptor Organizes Excitatory and Inhibitory Synapses. J. Neurosci. 2017, 37, 1062–1080. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wang, X.; Zou, H.; Dai, Z.; Feng, S.; Zhang, M.; Xiao, G.; Liu, Z.; Cheng, Q. The Basic Characteristics of the Pentraxin Family and Their Functions in Tumor Progression. Front. Immunol. 2020, 11, 1757. [Google Scholar] [CrossRef] [PubMed]
- Yue, W.; Wang, T.; Zachariah, E.; Lin, Y.; Yang, C.S.; Xu, Q.; DiPaola, R.S.; Tan, X.-L. Transcriptomic analysis of pancreatic cancer cells in response to metformin and aspirin: An implication of synergy. Sci. Rep. 2015, 5, 1–11. [Google Scholar] [CrossRef]
- Karagkounis, G.; Thai, L.; DeVecchio, J.; Gantt, G.A.; Duraes, L.; Pai, R.K.; Kalady, M.F. NPTX2 is associated with neoadjuvant therapy response in rectal cancer. J. Surg. Res. 2016, 202, 112–117. [Google Scholar] [CrossRef]
- Lee, G.W.; Lee, T.H.; Vilcek, J. TSG-14, a tumor necrosis factor- and IL-1-inducible protein, is a novel member of the pentaxin family of acute phase proteins. J. Immunol. 1993, 150, 1804–1812. [Google Scholar]
- Xiao, M.-F.; Xu, D.; Craig, M.T.; Pelkey, K.A.; Chien, C.-C.; Shi, Y.; Zhang, J.; Resnick, S.; Pletnikova, O.; Salmon, D.; et al. NPTX2 and cognitive dysfunction in Alzheimer’s Disease. eLife 2017, 6, e23798. [Google Scholar] [CrossRef]
- De Groot, J.; Sontheimer, H. Glutamate and the biology of gliomas. Glia 2011, 59, 1181–1189. [Google Scholar] [CrossRef]
- Radin, D.P.; Patel, P. A current perspective on the oncopreventive and oncolytic properties of selective serotonin reuptake inhibitors. Biomed. Pharmacother. 2017, 87, 636–639. [Google Scholar] [CrossRef]
- Abad, M.A.; Enguita, M.; DeGregorio-Rocasolano, N.; Ferrer, I.; Trullas, R. Neuronal Pentraxin 1 Contributes to the Neuronal Damage Evoked by Amyloid- and Is Overexpressed in Dystrophic Neurites in Alzheimer’s Brain. J. Neurosci. 2006, 26, 12735–12747. [Google Scholar] [CrossRef] [PubMed]
- Brito-Moreira, J.; Lourenco, M.V.; Oliveira, M.M.; Ribeiro, F.C.; Ledo, J.H.; Diniz, L.P.; Vital, J.F.S.; Magdesian, M.H.; Melo, H.M.; Barros-Aragão, F.; et al. Interaction of amyloid-β (Aβ) oligomers with neurexin 2α and neuroligin 1 mediates synapse damage and memory loss in mice. J. Biol. Chem. 2017, 292, 7327–7337. [Google Scholar] [CrossRef] [PubMed]
- Pettem, K.L.; Yokomaku, D.; Luo, L.; Linhoff, M.W.; Prasad, T.; Connor, S.A.; Siddiqui, T.J.; Kawabe, H.; Chen, F.; Zhang, L.; et al. The Specific α-Neurexin Interactor Calsyntenin-3 Promotes Excitatory and Inhibitory Synapse Development. Neuron 2013, 80, 113–128. [Google Scholar] [CrossRef] [PubMed]
- Um, J.W.; Pramanik, G.; Ko, J.S.; Song, M.Y.; Lee, D.; Kim, H.; Park, K.S.; Südhof, T.C.; Tabuchi, K.; Ko, J. Calsyntenins Function as Synaptogenic Adhesion Molecules in Concert with Neurexins. Cell Rep. 2014, 6, 1096–1109. [Google Scholar] [CrossRef] [PubMed]
- Hintsch, G. The Calsyntenins—A Family of Postsynaptic Membrane Proteins with Distinct Neuronal Expression Patterns. Mol. Cell. Neurosci. 2002, 21, 393–409. [Google Scholar] [CrossRef]
- Uchida, Y.; Gomi, F. The role of calsyntenin-3 in dystrophic neurite formation in Alzheimer’s disease brain. Geriatr. Gerontol. Int. 2016, 16, 43–50. [Google Scholar] [CrossRef]
- Pilozzi, A.; Carro, C.; Whalen, M.; Huang, X. Blood-Brain Barrier Degradation and the Implication of SPARC Protein as a Potential Therapeutic Target for Alzheimer’s Disease. In Alzheimer’s Disease: Drug Discovery; Huang, X., Ed.; Exon Publications: Brisbane, Australia, 2020. [Google Scholar] [CrossRef]
- McCurdy, S.; Baicu, C.F.; Heymans, S.; Bradshaw, A.D. Cardiac extracellular matrix remodeling: Fibrillar collagens and Secreted Protein Acidic and Rich in Cysteine (SPARC). J. Mol. Cell. Cardiol. 2010, 48, 544–549. [Google Scholar] [CrossRef]
- Giannoni, P.; Arango-Lievano, M.; Neves, I.D.; Rousset, M.-C.; Baranger, K.; Rivera, S.; Jeanneteau, F.; Claeysen, S.; Marchi, N. Cerebrovascular pathology during the progression of experimental Alzheimer’s disease. Neurobiol. Dis. 2016, 88, 107–117. [Google Scholar] [CrossRef] [PubMed]
- Awwad, K.; Hu, J.; Shi, L.; Mangels, N.; Abdel Malik, R.; Zippel, N.; Fisslthaler, B.; Eble, J.A.; Pfeilschifter, J.; Popp, R.; et al. Role of secreted modular calcium-binding protein 1 (SMOC1) in transforming growth factor β signalling and angiogenesis. Cardiovasc. Res. 2015, 106, 284–294. [Google Scholar] [CrossRef] [PubMed]
- Bai, B.; Wang, X.; Li, Y.; Chen, P.-C.; Yu, K.; Dey, K.K.; Yarbro, J.M.; Han, X.; Lutz, B.M.; Rao, S.; et al. Deep Multilayer Brain Proteomics Identifies Molecular Networks in Alzheimer’s Disease Progression. Neuron 2020, 105, 975–991.e977. [Google Scholar] [CrossRef]
- Sathe, G.; Albert, M.; Darrow, J.; Saito, A.; Troncoso, J.; Pandey, A.; Moghekar, A. Quantitative proteomic analysis of the frontal cortex in Alzheimer’s disease. J. Neurochem. 2020, 156, 988–1002. [Google Scholar] [CrossRef]
- Barrera-Ocampo, A.; Arlt, S.; Matschke, J.; Hartmann, U.; Puig, B.; Ferrer, I.; Zürbig, P.; Glatzel, M.; Sepulveda-Falla, D.; Jahn, H. Amyloid-β Precursor Protein Modulates the Sorting of Testican-1 and Contributes to Its Accumulation in Brain Tissue and Cerebrospinal Fluid from Patients with Alzheimer Disease. J. Neuropathol. Exp. Neurol. 2016, 75, 903–916. [Google Scholar] [CrossRef]
- Schechter, I.; Ziv, E. Cathepsins S, B and L with aminopeptidases display beta-secretase activity associated with the pathogenesis of Alzheimer’s disease. Biol. Chem. 2011, 392, 555–569. [Google Scholar] [CrossRef]
- Merlo, S.; Sortino, M.A. Estrogen activates matrix metalloproteinases-2 and -9 to increase beta amyloid degradation. Mol. Cell. Neurosci. 2012, 49, 423–429. [Google Scholar] [CrossRef]
- Ichimura, T.; Isobe, T.; Okuyama, T.; Yamauchi, T.; Fujisawa, H. Brain 14-3-3 protein is an activator protein that activates tryptophan 5-monooxygenase and tyrosine 3-monooxygenase in the presence of Ca2+,calmodulin-dependent protein kinase II. FEBS Lett. 1987, 219, 79–82. [Google Scholar] [CrossRef]
- Cho, E.; Park, J.-Y. Emerging roles of 14-3-3γ in the brain disorder. BMB Rep. 2020, 53, 500–511. [Google Scholar] [CrossRef]
- Cornell, B.; Toyo-oka, K. 14-3-3 Proteins in Brain Development: Neurogenesis, Neuronal Migration and Neuromorphogenesis. Front. Mol. Neurosci. 2017, 10, 318. [Google Scholar] [CrossRef]
- Foote, M.; Zhou, Y. 14-3-3 proteins in neurological disorders. Int. J. Biochem. Mol. Biol. 2012, 3, 152–164. [Google Scholar]
- Kelly, J.; Moyeed, R.; Carroll, C.; Albani, D.; Li, X. Gene expression meta-analysis of Parkinson’s disease and its relationship with Alzheimer’s disease. Mol. Brain 2019, 12, 1–10. [Google Scholar] [CrossRef]
- Bartolomucci, A.; Pasinetti, G.M.; Salton, S.R.J. Granins as disease-biomarkers: Translational potential for psychiatric and neurological disorders. Neuroscience 2010, 170, 289–297. [Google Scholar] [CrossRef]
- Bartolomucci, A.; Possenti, R.; Mahata, S.K.; Fischer-Colbrie, R.; Loh, Y.P.; Salton, S.R.J. The Extended Granin Family: Structure, Function, and Biomedical Implications. Endocr. Rev. 2011, 32, 755–797. [Google Scholar] [CrossRef] [PubMed]
- Serby, M.; Richardson, S.B.; Twente, S.; Siekierski, J.; Corwin, J.; Rotrosen, J. CSF somatostatin in Alzheimer’s disease. Neurobiol. Aging 1984, 5, 187–189. [Google Scholar] [CrossRef]
- Sagar, S.M.; Flint Beal, M.; Marshall, P.E.; Landis, D.M.D.; Martin, J.B. Implications of neuropeptides in neurological diseases. Peptides 1984, 5, 255–262. [Google Scholar] [CrossRef]
- Paik, S.; Somvanshi, R.K.; Kumar, U. Somatostatin-Mediated Changes in Microtubule-Associated Proteins and Retinoic Acid–Induced Neurite Outgrowth in SH-SY5Y Cells. J. Mol. Neurosci. 2019, 68, 120–134. [Google Scholar] [CrossRef]
- Sodhi, C.P.; Phadke, S.A.; Batlle, D.; Sahai, A. Hypoxia Stimulates Osteopontin Expression and Proliferation of Cultured Vascular Smooth Muscle Cells. Diabetes 2001, 50, 1482–1490. [Google Scholar] [CrossRef][Green Version]
- Rabenstein, M.; Vay, S.U.; Flitsch, L.J.; Fink, G.R.; Schroeter, M.; Rueger, M.A. Osteopontin directly modulates cytokine expression of primary microglia and increases their survival. J. Neuroimmunol. 2016, 299, 130–138. [Google Scholar] [CrossRef]
- Wung, J.; Perry, G.; Kowalski, A.; Harris, P.R.; Bishop, G.; Trivedi, M.; Johnson, S.; Smith, M.; Denhardt, D.; Atwood, C. Increased Expression of the Remodeling- and Tumorigenic-Associated Factor Osteopontin in Pyramidal Neurons of the Alzheimers Disease Brain. Curr. Alzheimer Res. 2007, 4, 67–72. [Google Scholar] [CrossRef] [PubMed]
- Comi, C.; Carecchio, M.; Chiocchetti, A.; Nicola, S.; Galimberti, D.; Fenoglio, C.; Cappellano, G.; Monaco, F.; Scarpini, E.; Dianzani, U. Osteopontin is Increased in the Cerebrospinal Fluid of Patients with Alzheimer’s Disease and Its Levels Correlate with Cognitive Decline. J. Alzheimer’s Dis. 2010, 19, 1143–1148. [Google Scholar] [CrossRef] [PubMed]
- Sensi, S.L.; Granzotto, A.; Siotto, M.; Squitti, R. Copper and Zinc Dysregulation in Alzheimer’s Disease. Trends. Pharmacol. Sci. 2018, 39, 1049–1063. [Google Scholar] [CrossRef]
- Mold, M.; Ouro-Gnao, L.; Wieckowski, B.M.; Exley, C. Copper prevents amyloid-β1–42 from forming amyloid fibrils under near-physiological conditions in vitro. Sci. Rep. 2013, 3, 1256. [Google Scholar] [CrossRef]
- Singh, I.; Sagare, A.P.; Coma, M.; Perlmutter, D.; Gelein, R.; Bell, R.D.; Deane, R.J.; Zhong, E.; Parisi, M.; Ciszewski, J.; et al. Low levels of copper disrupt brain amyloid-β homeostasis by altering its production and clearance. Proc. Natl. Acad. Sci. USA 2013, 110, 14771–14776. [Google Scholar] [CrossRef]
- Bagheri, S.; Squitti, R.; Haertlé, T.; Siotto, M.; Saboury, A.A. Role of Copper in the Onset of Alzheimer’s Disease Compared to Other Metals. Front. Aging Neurosci. 2018, 9, 446. [Google Scholar] [CrossRef] [PubMed]
- Roberts, B.R.; Ryan, T.M.; Bush, A.I.; Masters, C.L.; Duce, J.A. The role of metallobiology and amyloid-beta peptides in Alzheimer’s disease. J. Neurochem. 2012, 120 (Suppl. 1), 149–166. [Google Scholar] [CrossRef] [PubMed]
- Twomey, P.J.; Viljoen, A.; House, I.M.; Reynolds, T.M.; Wierzbicki, A.S. Relationship between serum copper, ceruloplasmin, and non-ceruloplasmin-bound copper in routine clinical practice. Clin. Chem. 2005, 51, 1558–1559. [Google Scholar] [CrossRef]
- Vašák, M.; Meloni, G. Mammalian Metallothionein-3: New Functional and Structural Insights. Int. J. Mol. Sci. 2017, 18, 1117. [Google Scholar] [CrossRef]
- Eipper, B.A.; Mains, R.E. Peptide α-Amidation. Annu. Rev. Physiol. 1988, 50, 333–344. [Google Scholar] [CrossRef]
- Eipper, B.A.; Stoffers, D.A.; Mains, R.E. The biosynthesis of neuropeptides: Peptide alpha-amidation. Annu. Rev. Neurosci. 1992, 15, 57–85. [Google Scholar] [CrossRef]
- Wand, G.S.; May, C.; May, V.; Whitehouse, P.J.; Rapoport, S.I.; Eipper, B.A. Alzheimer’s disease: Low levels of peptide alpha-amidation activity in brain and CSF. Neurology 1987, 37, 1057–1061. [Google Scholar] [CrossRef]
- Miners, J.S.; Clarke, P.; Love, S. Clusterin levels are increased in Alzheimer’s disease and influence the regional distribution of Aβ. Brain Pathol. 2017, 27, 305–313. [Google Scholar] [CrossRef]
- Thambisetty, M.; Simmons, A.; Velayudhan, L.; Hye, A.; Campbell, J.; Zhang, Y.; Wahlund, L.-O.; Westman, E.; Kinsey, A.; Güntert, A.; et al. Association of Plasma Clusterin Concentration With Severity, Pathology, and Progression in Alzheimer Disease. Arch. Gen. Psychiatry 2010, 67, 739–748. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, C.L.; Koike, M.; Spusta, S.C.; Niemi, E.C.; Yenari, M.; Nakamura, M.C.; Seaman, W.E. A role for TREM2 ligands in the phagocytosis of apoptotic neuronal cells by microglia. J. Neurochem. 2009, 109, 1144–1156. [Google Scholar] [CrossRef]
- Zhao, Y.; Wu, X.; Li, X.; Jiang, L.-L.; Gui, X.; Liu, Y.; Sun, Y.; Zhu, B.; Piña-Crespo, J.C.; Zhang, M.; et al. TREM2 Is a Receptor for β-Amyloid that Mediates Microglial Function. Neuron 2018, 97, 1023–1031.e1027. [Google Scholar] [CrossRef]
- Guerreiro, R.; Wojtas, A.; Bras, J.; Carrasquillo, M.; Rogaeva, E.; Majounie, E.; Cruchaga, C.; Sassi, C.; Kauwe, J.S.K.; Younkin, S.; et al. TREM2 Variants in Alzheimer’s Disease. N. Engl. J. Med. 2013, 368, 117–127. [Google Scholar] [CrossRef]
- Jonsson, T.; Stefansson, H.; Steinberg, S.; Jonsdottir, I.; Jonsson, P.V.; Snaedal, J.; Bjornsson, S.; Huttenlocher, J.; Levey, A.I.; Lah, J.J.; et al. Variant of TREM2 Associated with the Risk of Alzheimer’s Disease. N. Engl. J. Med. 2013, 368, 107–116. [Google Scholar] [CrossRef] [PubMed]
- Giraldo, M.; Lopera, F.; Siniard, A.L.; Corneveaux, J.J.; Schrauwen, I.; Carvajal, J.; Muñoz, C.; Ramirez-Restrepo, M.; Gaiteri, C.; Myers, A.J.; et al. Variants in triggering receptor expressed on myeloid cells 2 are associated with both behavioral variant frontotemporal lobar degeneration and Alzheimer’s disease. Neurobiol. Aging 2013, 34, 2077.e2011–2077.e2018. [Google Scholar] [CrossRef]
- Koike, H.; Seki, H.; Kouchi, Z.; Ito, M.; Kinouchi, T.; Tomioka, S.; Sorimachi, H.; Saido, T.C.; Maruyama, K.; Suzuki, K.; et al. Thimet Oligopeptidase Cleaves the Full-Length Alzheimer Amyloid Precursor Protein at a β-Secretase Cleavage Site in COS Cells. J. Biochem. 1999, 126, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Yamin, R.; Malgeri, E.G.; Sloane, J.A.; McGraw, W.T.; Abraham, C.R. Metalloendopeptidase EC 3.4.24.15 Is Necessary for Alzheimer’s Amyloid-β Peptide Degradation. J. Biol. Chem. 1999, 274, 18777–18784. [Google Scholar] [CrossRef]
- Pollio, G.; Hoozemans, J.J.M.; Andersen, C.A.; Roncarati, R.; Rosi, M.C.; van Haastert, E.S.; Seredenina, T.; Diamanti, D.; Gotta, S.; Fiorentini, A.; et al. Increased expression of the oligopeptidase THOP1 is a neuroprotective response to Aβ toxicity. Neurobiol. Dis. 2008, 31, 145–158. [Google Scholar] [CrossRef]
- Choi, J.; Levey, A.I.; Weintraub, S.T.; Rees, H.D.; Gearing, M.; Chin, L.-S.; Li, L. Oxidative Modifications and Down-regulation of Ubiquitin Carboxyl-terminal Hydrolase L1 Associated with Idiopathic Parkinson’s and Alzheimer’s Diseases. J. Biol. Chem. 2004, 279, 13256–13264. [Google Scholar] [CrossRef]
- Sultana, R.; Boyd-Kimball, D.; Cai, J.; Pierce, W.M.; Klein, J.B.; Merchant, M.; Butterfield, D.A.; Boldyrev, A.; Johnson, P. Proteomics Analysis of the Alzheimer’s Disease Hippocampal Proteome. J. Alzheimer’s Dis. 2007, 11, 153–164. [Google Scholar] [CrossRef] [PubMed]
- Öhrfelt, A.; Johansson, P.; Wallin, A.; Andreasson, U.; Zetterberg, H.; Blennow, K.; Svensson, J. Increased Cerebrospinal Fluid Levels of Ubiquitin Carboxyl-Terminal Hydrolase L1 in Patients with Alzheimer’s Disease. Dement. Geriatr. Cogn. Disord. Extra 2016, 6, 283–294. [Google Scholar] [CrossRef] [PubMed]
- Wein, A.N.; McMaster, S.R.; Takamura, S.; Dunbar, P.R.; Cartwright, E.K.; Hayward, S.L.; McManus, D.T.; Shimaoka, T.; Ueha, S.; Tsukui, T.; et al. CXCR6 regulates localization of tissue-resident memory CD8 T cells to the airways. J. Exp. Med. 2019, 216, 2748–2762. [Google Scholar] [CrossRef] [PubMed]
- Veinotte, L.; Gebremeskel, S.; Johnston, B. CXCL16-positive dendritic cells enhance invariant natural killer T cell-dependent IFNγ production and tumor control. OncoImmunology 2016, 5, e1160979. [Google Scholar] [CrossRef]
- Jadidi-Niaragh, F.; Shegarfi, H.; Naddafi, F.; Mirshafiey, A. The Role of Natural Killer Cells in Alzheimer’s Disease. Scand. J. Immunol. 2012, 76, 451–456. [Google Scholar] [CrossRef]
- Anggono, V.; Koç-Schmitz, Y.; Widagdo, J.; Kormann, J.; Quan, A.; Chen, C.-M.; Robinson, P.J.; Choi, S.-Y.; Linden, D.J.; Plomann, M.; et al. PICK1 interacts with PACSIN to regulate AMPA receptor internalization and cerebellar long-term depression. Proc. Natl. Acad. Sci. USA 2013, 110, 13976–13981. [Google Scholar] [CrossRef] [PubMed]
- Clayton, E.L.; Anggono, V.; Smillie, K.J.; Chau, N.; Robinson, P.J.; Cousin, M.A. The Phospho-Dependent Dynamin-Syndapin Interaction Triggers Activity-Dependent Bulk Endocytosis of Synaptic Vesicles. J. Neurosci. 2009, 29, 7706–7717. [Google Scholar] [CrossRef]
- Modregger, J. PACSIN 1 interacts with huntingtin and is absent from synaptic varicosities in presymptomatic Huntington’s disease brains. Hum. Mol. Genet. 2002, 11, 2547–2558. [Google Scholar] [CrossRef]
- Takano, M.; Yamashita, T.; Nagano, K.; Otani, M.; Maekura, K.; Kamada, H.; Tsunoda, S.-i.; Tsutsumi, Y.; Tomiyama, T.; Mori, H.; et al. Proteomic analysis of the hippocampus in Alzheimer’s disease model mice by using two-dimensional fluorescence difference in gel electrophoresis. Neurosci. Lett. 2013, 534, 85–89. [Google Scholar] [CrossRef]
- Zhao, X.; Yao, H.; Li, X. Unearthing of Key Genes Driving the Pathogenesis of Alzheimer’s Disease via Bioinformatics. Front. Genet. 2021, 12, 641100. [Google Scholar] [CrossRef] [PubMed]
- Salih, D.A.; Bayram, S.; Guelfi, S.; Reynolds, R.H.; Shoai, M.; Ryten, M.; Brenton, J.W.; Zhang, D.; Matarin, M.; Botia, J.A.; et al. Genetic variability in response to amyloid beta deposition influences Alzheimer’s disease risk. Brain Commun. 2019, 1, fcz022. [Google Scholar] [CrossRef] [PubMed]
- Trovò, L.; Ahmed, T.; Callaerts-Vegh, Z.; Buzzi, A.; Bagni, C.; Chuah, M.; VandenDriessche, T.; D’Hooge, R.; Balschun, D.; Dotti, C.G. Low hippocampal PI(4,5)P2 contributes to reduced cognition in old mice as a result of loss of MARCKS. Nat. Neurosci. 2013, 16, 449–455. [Google Scholar] [CrossRef] [PubMed]


| Uniprot (Human) | Description | Identified in N Articles | References |
|---|---|---|---|
| GLUCOSE/PYRUVATE METABOLISM | |||
| ALDOA | aldolase, fructose-bisphosphate A | 6 (up) * | [10,11,12,13,14,15] |
| ALDOC | aldolase, fructose-bisphosphate C | 2 (up) | [12,13] |
| ENOA | enolase 1 | 2 (up) | [12,13] |
| G6PI | glucose-6-phosphate isomerase | 2 (up) | [12,13] |
| LDHA | lactate dehydrogenase A | 3 (up) | [10,12,13] |
| LDHB | lactate dehydrogenase B | 2 (up) | [16,17] |
| MDHC | malate dehydrogenase 1 | 5 (up) * | [10,12,14,18,19] |
| PGAM1 | phosphoglycerate mutase 1 | 2 (up) | [10,12] |
| KPYM | pyruvate kinase M1/2 | 7 (up) | [11,12,13,14,15,16,20] |
| TPIS | triosephosphate isomerase 1 | 2 (up) | [12,17] |
| RXR SIGNALING (LXR/RXR ACTIVATION PATHWAY) | |||
| FETUA | alpha 2-HS glycoprotein | 2 (down) | [12,21] |
| ALBU # | albumin | 3 (down) * | [17,21,22] |
| AMBP | alpha-1-microglobulin/bikunin precursor | 3 (down) | [12,17,23] |
| APOA1 $ | apolipoprotein A1 | 4 (down) | [17,22,23,24] |
| APOC2 | apolipoprotein C2 | 2 (down) | [17,20] |
| APOL1 | apolipoprotein L1 | 2 (down) | [11,12] |
| C1QB | complement C1q B chain | 3 (down) | [10,17,25] |
| CLUS $ | clusterin | 4 (up) | [19,22,26,27] |
| CERU # | ceruloplasmin | 4 (down) * | [11,12,13,17] |
| KNG1 Δ | kininogen 1 | 3 (down) | [12,13,17] |
| LDLR | low density lipoprotein receptor | 3 (down) | [12,20,28] |
| RET4 | retinol binding protein 4 | 2 (down) | [12,17] |
| SODM Δ | superoxide dismutase 2 | 2 (up) | [12,13] |
| TR11B | TNF receptor superfamily member 11b | 2 (up) | [12,28] |
| NEURONAL FUNCTION/SYNAPTOGENESIS | |||
| ACES | acetylcholinesterase (Cartwright blood group) | 2 (up) | [10,12] |
| APLP1 $ | amyloid beta precursor like protein 1 | 3 (down) * | [17,21,26] |
| APLP2 $ | amyloid beta precursor like protein 2 | 3 (up) | [15,17,26] |
| A4 $ | amyloid beta precursor protein | 3 (down) * | [17,26,29] |
| KCC2G | calcium/calmodulin dependent protein kinase II gamma | 2 (up) | [12,13] |
| CSTN3 ε | calsyntenin 3 | 3 (down) * | [15,17] |
| CPLX2 | complexin 2 | 2 (up) | [10,12] |
| EPHA7 | EPH receptor A7 | 2 (down) | [10,12] |
| NEUM | growth associated protein 43 | 4 (up) | [11,12,14,30] |
| GDIA | GDP dissociation inhibitor 1 | 2 (up) | [10,12] |
| MANF | mesencephalic astrocyte derived neurotrophic factor | 2 (up) | [12,20] |
| MARCS | myristoylated alanine rich protein kinase C substrate | 3 (up) | [10,12,14] |
| MT3 Δ | metallothionein 3 | 2 (down) | [20,21] |
| NFL | neurofilament light | 2 (up) | [12,20] |
| NFM | neurofilament medium | 3 (up) | [11,12,26] |
| NPTX1 | neuronal pentraxin 1 | 3 (down) | [25,31,32] |
| NPTX2 | neuronal pentraxin 2 | 4 (down) | [12,20,25,33] |
| NPTXR | neuronal pentraxin receptor | 5 (down) | [11,12,25,33,34] |
| NEUG | neurogranin | 4 (up) | [12,20,30,33] |
| NRX1A | neurexin 1 | 4 (down) * | [12,21,25,31] |
| NRX2A | neurexin 2 | 3 (down) | [12,20,25] |
| NRX3B | neurexin 3 | 2 (down) | [20,31] |
| PACN1 | protein kinase C and casein k. substrate in neurons 1 | 2 (up) | [11,12] |
| PCLO | piccolo presynaptic cytomatrix protein | 2 (up) | [12,26] |
| RP3A | rabphilin 3A | 2 (up) | [12,20] |
| RTN4 | reticulon 4 | 2 (up) | [12,26] |
| SYUG | synuclein gamma | 2 (up) | [10,12] |
| SYN1 | synapsin I | 2 (up) | [11,12] |
| TKNK | tachykinin precursor 3 | 2 (down) | [21,26] |
| TREM2 $,ε | triggering receptor expressed on myeloid cells 2 | 2 (up) | [12,19] |
| CELL ADHESION/EXTRACELLULAR MATRIX | |||
| C1QT1 | C1q and TNF related 1 | 2 (down) | [11,12] |
| C1QT5 | C1q and TNF related 5 | 2 (up) | [12,35] |
| CD99 ε | CD99 molecule (Xg blood group) | 2 (up) | [15,26] |
| NCHL1 | cell adhesion molecule L1 like | 2 (down) | [17,32] |
| FBLN3 | EGF containing fibulin extracellular matrix protein 1 | 2 (down) | [17,20] |
| FBLN1 | fibulin 1 | 3 (down) | [10,13,17] |
| ITAM | integrin subunit alpha M | 3 (up) | [11,12,20] |
| MUC18 | melanoma cell adhesion molecule | 4 (down) | [15,17,25,32] |
| MMP2 Δ | matrix metallopeptidase 2 | 2 (down) | [12,28] |
| NID2 | nidogen 2 | 2 (down) | [12,13] |
| PGRP2 | peptidoglycan recognition protein 2 | 2 (down) | [11,12,17] |
| SMOC1 | SPARC related modular calcium binding 1 | 7 (up) * | [10,11,12,13,14,15,35] |
| SMOC2 | SPARC related modular calcium binding 2 | 2 (up) | [12,28] |
| SPRC | secreted protein acidic and cysteine rich | 3 (up) | [17,24,26] |
| TICN1 | SPARC (osteonectin), cwcv and kazal like domains proteoglycan 1 | 3 (down) * | [10,17,21] |
| SPON1 | spondin 1 | 3 (up) | [12,15,26] |
| 14-3-3 PROTEINS | |||
| 1433B | tyrosine 3-monooxygenase/tryptophan 5-monooxygenase activation protein beta | 3 (up) | [11,12,20] |
| 1433E | tyrosine 3-monooxygenase/tryptophan 5-monooxygenase activation protein epsilon | 5 (up) | [10,11,12,33,36] |
| 1433G | tyrosine 3-monooxygenase/tryptophan 5-monooxygenase activation protein gamma | 4 (up) | [10,12,13,20] |
| 1433Z | tyrosine 3-monooxygenase/tryptophan 5-monooxygenase activation protein zeta | 5 (up) | [10,11,12,13,33] |
| CYTOKINE (C)/HORMONAL (H) ACTIVITIES | |||
| CCKN | cholecystokinin (H) | 2 (up) | [13,26] |
| CMGA | chromogranin A (H) | 4 (down) * | [21,31,34,36] |
| SCG1 | chromogranin B (H) | 3 (down) * | [12,17,21] |
| CXL16 | C-X-C motif chemokine ligand 16 (C) | 3 (up) | [10,20,26] |
| MIF | macrophage migration inhibitory factor (C) | 2 (up) | [12,13] |
| SCG2 | secretogranin II (H) | 6 (down) * | [12,17,20,21,26,31] |
| SCG3 | secretogranin III (H) | 3 (down) * | [17,21,26] |
| OSTP | secreted phosphoprotein 1 (C) | 7 (up) & | [11,12,13,15,18,19,26] |
| SMS | Somatostatin (H) | 3 (down) | [12,20,26] |
| VGF | VGF nerve growth factor inducible (H) | 11 (down) * | [11,12,21,24,26,31,34,36,37,38] |
| VIP | vasoactive intestinal peptide (H) | 2 (down) | [12,20] |
| CYTOSKELETAL PROTEINS | |||
| GELS $ | gelsolin | 3 (down) | [11,12,20] |
| K22E | keratin 2 | 2 (up) | [11,12] |
| K1C9 | keratin 9 | 2 (up) | [20,29] |
| MAP1B | microtubule associated protein 1B | 2 (up) | [11,12] |
| MTAP2 | microtubule associated protein 2 | 3 (up) | [11,12,20] |
| TAU | microtubule associated protein tau | 4 (up) | [11,12,13,20] |
| STMN1 | stathmin 1 | 2 (up) | [11,12] |
| REDOX BALANCE/DETOXIFICATION PROCESSES | |||
| AATM | glutamic-oxaloacetic transaminase 2 | 2 (up) | [12,13] |
| GSHR | glutathione-disulfide reductase | 2 (up) | [12,13] |
| GSTO1 | glutathione S-transferase omega 1 | 2 (up) | [12,13] |
| PARK7 | Parkinsonism associated deglycase | 2 (up) | [12,13] |
| PPIA | peptidylprolyl isomerase A | 2 (up) | [12,13] |
| PPIB | peptidylprolyl isomerase B | 2 (down) | [10,17] |
| SODE #,ε | superoxide dismutase 3 | 2 (down) | [10,15] |
| TRXR2 | thioredoxin reductase 2 | 2 (up) | [12,20] |
| SIGNAL TRANSDUCTION | |||
| IGF1R | insulin like growth factor 1 receptor | 2 (up) | [12,20] |
| IBP6 ε | insulin like growth factor binding protein 6 | 2 (down) | [12,15] |
| IMPA1 | inositol monophosphatase 1 | 2 (up) | [12,13] |
| PEBP1 | phosphatidylethanolamine binding protein 1 | 2 (up) | [10,12] |
| PTPRZ | protein tyrosine phosphatase receptor type Z1 | 3 (up) | [12,17,26] |
| SH3L3 | SH3 domain binding glutamate rich protein like | 2 (up) | [12,13] |
| PROTEASE/PROTEASE INHIBITORS | |||
| CFAD | complement factor D | 2 (down) | [17,20] |
| FETUB Δ | fetuin B | 2 (down) | [11,12] |
| PCS1N | proprotein convertase subtilisin/kexin type 1 inhibitor | 5 (down) * | [10,17,21,25,32] |
| NEC2 | proprotein convertase subtilisin/kexin type 2 | 2 (down) | [12,21] |
| ZPI | serpin family A member 10 | 2 (down) | [12,13] |
| THOP1 | thimet oligopeptidase 1 | 2 (up) | [12,20] |
| UCHL1 | ubiquitin C-terminal hydrolase L1 | 3 (up) | [11,12,19] |
| WFDC1 | WAP four-disulfide core domain 1 | 2 (down) | [12,20] |
| OTHER FUNCTIONS | |||
| ARP21 | cAMP regulated phosphoprotein 21 | 2 (up) | [11,12] |
| VAS1 | ATPase H+ transporting accessory protein 1 | 2 (down) | [12,17] |
| B3GN8 | UDP-GlcNAc:betaGal beta-1,3-N-acetylglucosaminyltransferase 8 | 2 (down) | [11,12] |
| CH3L1 | chitinase 3 like 1 | 7 (up) | [11,12,18,19,24,28,39] |
| CHIT1 | chitotriosidase-1 | 3 (up) | [11,12,28] |
| TETN | C-type lectin domain family 3 member B | 2 (down) | [12,29] |
| DDAH1 Δ | dimethylarginine dimethylaminohydrolase 1 | 2 (up) | [12,20] |
| FABPH | fatty acid binding protein 3 | 4 (up) | [10,11,12,25] |
| FKB1A | FKBP prolyl isomerase 1A | 2 (up) | [12,13] |
| GUAD | guanine deaminase | 2 (up) | [11,12] |
| HBG2 Δ | hemoglobin subunit gamma 2 | 2 (down) | [11,12] |
| HPRT | hypoxanthine phosphoribosyltransferase 1 | 3 (up) | [10,12,13] |
| OLR1 | oxidized low density lipoprotein receptor 1 | 2 (up) | [12,20] |
| AMD #,ε | peptidylglycine alpha-amidating monooxygenase | 2 (up) | [15,20] |
| PBIP1 | PBX homeobox interacting protein 1 | 2 (down) | [17,20] |
| SLIT2 | slit guidance ligand 2 | 2 (up) | [12,35] |
| SYWC | tryptophanyl-tRNA synthetase 1 | 2 (up) | [12,20] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pedrero-Prieto, C.M.; Frontiñán-Rubio, J.; Alcaín, F.J.; Durán-Prado, M.; Peinado, J.R.; Rabanal-Ruiz, Y. Biological Significance of the Protein Changes Occurring in the Cerebrospinal Fluid of Alzheimer’s Disease Patients: Getting Clues from Proteomic Studies. Diagnostics 2021, 11, 1655. https://doi.org/10.3390/diagnostics11091655
Pedrero-Prieto CM, Frontiñán-Rubio J, Alcaín FJ, Durán-Prado M, Peinado JR, Rabanal-Ruiz Y. Biological Significance of the Protein Changes Occurring in the Cerebrospinal Fluid of Alzheimer’s Disease Patients: Getting Clues from Proteomic Studies. Diagnostics. 2021; 11(9):1655. https://doi.org/10.3390/diagnostics11091655
Chicago/Turabian StylePedrero-Prieto, Cristina M., Javier Frontiñán-Rubio, Francisco J. Alcaín, Mario Durán-Prado, Juan R. Peinado, and Yoana Rabanal-Ruiz. 2021. "Biological Significance of the Protein Changes Occurring in the Cerebrospinal Fluid of Alzheimer’s Disease Patients: Getting Clues from Proteomic Studies" Diagnostics 11, no. 9: 1655. https://doi.org/10.3390/diagnostics11091655
APA StylePedrero-Prieto, C. M., Frontiñán-Rubio, J., Alcaín, F. J., Durán-Prado, M., Peinado, J. R., & Rabanal-Ruiz, Y. (2021). Biological Significance of the Protein Changes Occurring in the Cerebrospinal Fluid of Alzheimer’s Disease Patients: Getting Clues from Proteomic Studies. Diagnostics, 11(9), 1655. https://doi.org/10.3390/diagnostics11091655








