The Role of EUS-Guided FNA and FNB in Autoimmune Pancreatitis
Abstract
:1. Introduction
2. Fine-Needle Aspiration (FNA) and Fine-Needle Biopsy (FNB)
2.1. FNA in AIP
2.1.1. FNA for the Differential Diagnosis between AIP and Pancreatic Cancer
2.1.2. FNA for the Diagnosis AIP
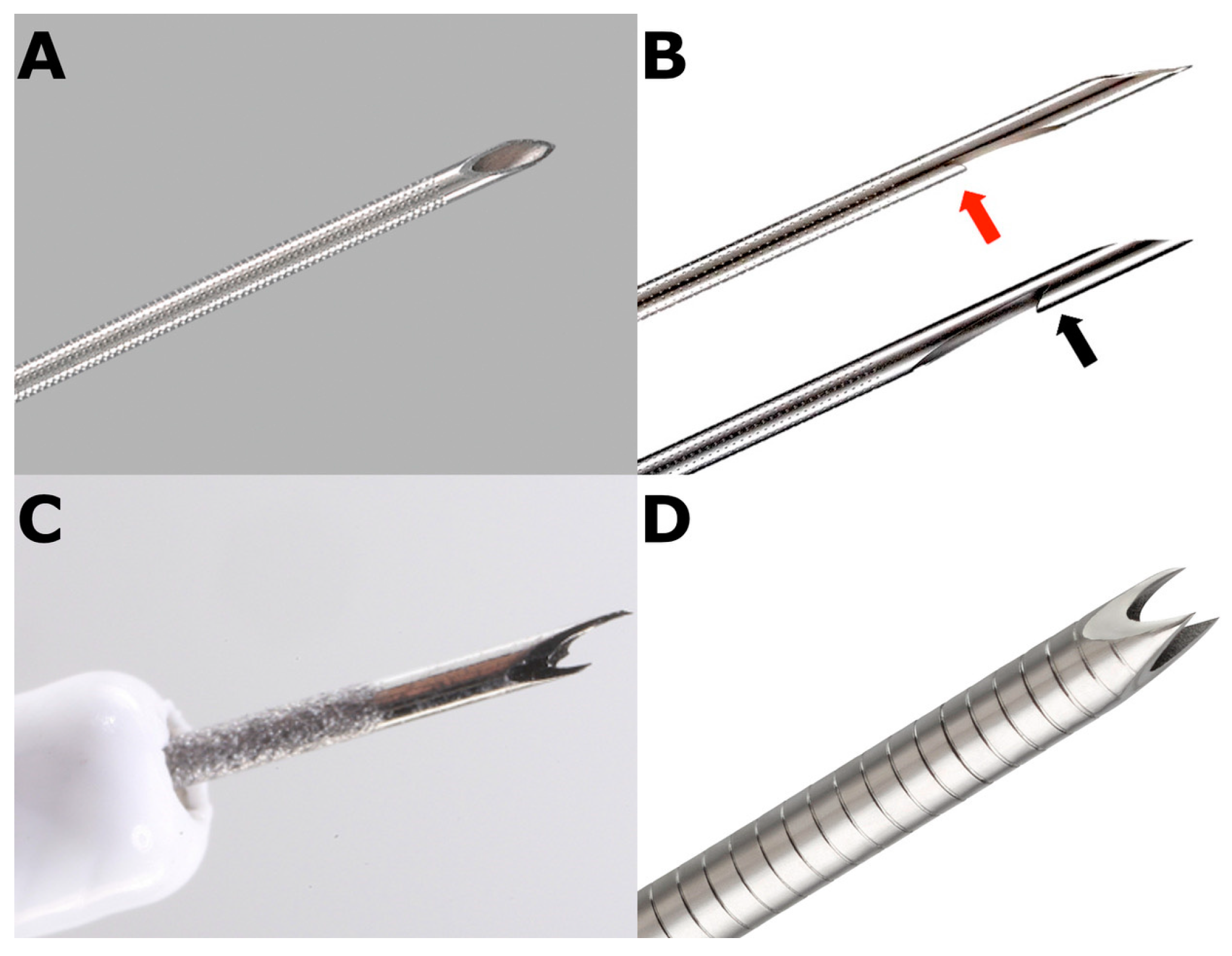
2.2. FNB in AIP
- (a)
- Franseen-tip needles;
- (b)
- Side-fenestrated forward-cutting beveled needle (20-gauge caliber available only);
- (c)
- Fork-tip needles;
- (d)
- Menghini-type needles.
3. Complications
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kawa, S.; Kamisawa, T.; Notohara, K.; Fujinaga, Y.; Inoue, D.; Koyama, T.; Okazaki, K. Japanese clinical diagnostic criteria for Autoimmune Pancreatitis, 2018: Revision of Japanese clinical diagnostic criteria for Autoimmune Pancreatitis, 2011. Pancreas 2020, 49, e13–e14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shimosegawa, T.; Chari, S.T.; Frulloni, L.; Kamisawa, T.; Kawa, S.; Mino-Kenudson, M.; Kim, M.; Kloeppel, G.; Lerch, M.; Loehr, M.; et al. International Consensus Diagnostic Criteria for Autoimmune Pancreatitis. Pancreas 2011, 40, 352–358. [Google Scholar] [CrossRef] [PubMed]
- Loehr, M.; Beuers, U.; Vujasinovic, M.; Alvaro, D.; Frokjaer, J.B.; Buttgereit, F.; Capurso, G.; Culver, E.L.; de-Madaria, E.; Della Torre, E.; et al. European Guideline on IgG4-related digestive disease-UEG and SGF evidence-based recommendations. United Eur. Gastroenterol. J. 2020, 8, 637–666. [Google Scholar] [CrossRef]
- de Pretis, N.; Vieceli, F.; Brandolese, A.; Brozzi, L.; Amodio, A.; Frulloni, L. Autoimmune pancreatitis not otherwise specified (NOS): Clinical features and outcomes of the forgotten type. Hepatobiliary Pancreat. Dis. Int. 2019, 18, 576–579. [Google Scholar] [CrossRef] [PubMed]
- Kanno, A.; Ikeda, E.; Ando, K.; Nagai, H.; Miwata, T.; Kawasaki, Y.; Tada, Y.; Yokoyama, K.; Numao, N.; Ushio, J.; et al. The diagnosis of autoimmune pancreatitis using endoscopic ultrasonography. Diagnostics 2020, 10, 1005. [Google Scholar] [CrossRef] [PubMed]
- Notohara, K.; Kamisawa, T.; Fukushima, N.; Furukawa, N.; Tajiri, T.; Yamaguchi, H.; Aishima, S.; Fukumura, Y.; Hirabayashi, K.; Iwasaki, E.; et al. Guidance for diagnosing autoimmune pancreatitis with biopsy tissue. Pathol. Int. 2020, 70, 699–711. [Google Scholar] [CrossRef] [PubMed]
- Imai, K.; Matsubayashi, H.; Fukutomi, A.; Uesaka, K.; Sasaki, K.; Ono, H. Endoscopic ultrasonography-guided fine needle aspiration biopsy using 22-gauge needle in diagnosis of autoimmune pancreatitis. Dig. Liver Dis. 2011, 43, 869–874. [Google Scholar] [CrossRef] [PubMed]
- Sugimoto, M.; Takagi, T.; Suzuki, R.; Konno, N.; Asama, H.; Watanabe, K.; Nakamura, J.; Kikuchi, H.; Waragai, Y.; Takasumi, M.; et al. Endoscopic ultrasonography-guided fine needle aspiration can be used to rule out malignancy in autoimmune pancreatitis patients. J. Ultrasound Med. 2017, 36, 2237–2244. [Google Scholar] [CrossRef]
- Khalid, A.; Dewitt, J.; Ohori, N.P.; Chen, J.H.; Fasanella, K.E.; Sanders, M.; McGrath, K.M.; Nikiforova, M. EUS-FNA mutational analysis in differentiating autoimmune pancreatitis and pancreatic cancer. Pancreatology 2011, 11, 482–486. [Google Scholar] [CrossRef] [PubMed]
- Kanno, A.; Masamune, A.; Fujishima, F.; Iwashita, T.; Kodama, Y.; Katanuma, A.; Ohara, H.; Kitano, M.; Inoue, H.; Itoi, T.; et al. Diagnosis of autoimmune pancreatitis by EUS-guided FNA using a 22-gauge needle: A prospective multicenter study. Gastrointest. Endosc. 2016, 84, 797–804. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kanno, A.; Ishida, K.; Hamada, S.; Fujishima, F.; Unno, J.; Kume, K.; Kikuta, K.; Hirota, M.; Masamune, A.; Satoh, K.; et al. Diagnosis of autoimmune pancreatitis by EUS-FNA by using a 22-gauge needle based on the International Consensus Diagnostic Criteria. Gastrointest. Endosc. 2012, 76, 594–602. [Google Scholar] [CrossRef] [PubMed]
- Morishima, T.; Kawashima, H.; Ohno, E.; Yamamura, T.; Funasaka, K.; Nakamura, M.; Miyahara, R.; Watanabe, O.; Ishigami, M.; Shimoyama, Y.; et al. Prospective multicenter study on the usefulness of EUS-guided FNA biopsy for the diagnosis of autoimmune pancreatitis. Gastrointest. Endosc. 2016, 84, 241–248. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.; Wang, Y.; Wang, J.; Guo, Q.; Chen, Q.; Wu, X.; Tang, S.; Cheng, B. The role of EUS-guided fine needle aspiration in autoimmune pancreatitis: A single center prospective study. Scand. J. Gastroenterol. 2018, 53, 1604–1610. [Google Scholar] [CrossRef]
- Ishikawa, T.; Itoh, A.; Kawashima, H.; Ohno, E.; Matsubara, H.; Itoh, Y.; Nakamura, Y.; Hiramatsu, T.; Nakamura, M.; Miyahara, R.; et al. Endoscopic ultrasound-guided fine needle aspiration in the differentiation of type 1 and type 2 autoimmune pancreatitis. World J. Gastroenterol. 2012, 18, 3883–3888. [Google Scholar] [CrossRef] [PubMed]
- Iwashita, T.; Yasuda, I.; Doi, S.; Ando, N.; Nakashima, M.; Adachi, S.; Hirose, Y.; Mukai, T.; Iwata, K.; Itoi, T.; et al. Use of samples from endoscopic ultrasound-guided 19-gauge fine-needle aspiration in diagnosis of autoimmune pancreatitis. Clin. Gastroenterol. Hepatol. 2012, 10, 316–322. [Google Scholar] [CrossRef] [PubMed]
- Sugimoto, M.; Takagi, T.; Suzuki, R.; Konno, N.; Asama, H.; Sato, Y.; Irie, H.; Watanabe, K.; Nakamura, J.; Kikuchi, H.; et al. Can the wet technique change the efficacy of endoscopic ultrasound-guided fine-needle aspiration for diagnosing autoimmune pancreatitis type 1? A prospective single-arm study. World J. Clin. Cases 2020, 8, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, Y.; Shimizu, A.; Ogawa, K.; Nakamura, M.; Hoki, S.; Kuroki, S.; Yano, Y.; Ikuta, K.; Shio, S. Late-onset type-2 autoimmune pancreatitis with two mass lesions diagnosed by endoscopic ultrasound fine-needle aspiration. Clin. J. Gastroenterol. 2021, 14, 899–904. [Google Scholar] [CrossRef] [PubMed]
- Levy, M.J.; Reddy, R.P.; Wiersema, M.J.; Smyrk, T.C.; Clain, J.E.; Harewood, G.C.; Pearson, R.K.; Rajan, E.; Topazian, M.D.; Yusuf, T.E.; et al. EUS-guided trucut biopsy in establishing autoimmune pancreatitis as the cause of obstructive jaundice. Gastrointest. Endosc. 2005, 61, 467–472. [Google Scholar] [CrossRef]
- Mizuno, N.; Bhatia, V.; Hososda, W.; Sawaki, A.; Hoki, N.; Hara, K.; Takagi, T.; Ko, S.; Yatabe, Y.; Goto, H.; et al. Histological diagnosis of autoimmune pancreatitis using EUS-guided trucut biopsy: A comparison study with EUS-FNA. J. Gastroenterol. 2009, 44, 742–750. [Google Scholar] [CrossRef] [PubMed]
- Detlefsen, S.; Drewes, A.M.; Vyberg, M.; Kloeppel, G. Diagnosis of autoimmune pancreatitis by core needle biopsy: Application of six microscopic criteria. Virchows Arch. 2009, 454, 531–539. [Google Scholar] [CrossRef] [PubMed]
- Fujii, L.; Chari, S.T.; El-Youssef, M.; Takahashi, N.; Topazian, M.D.; Zhang, L.; Levy, M.J. Pediatric pancreatic EUS-guided trucut biopsy for evaluation of autoimmune pancreatitis. Gastrointest. Endosc. 2013, 78, 824–828. [Google Scholar] [CrossRef] [PubMed]
- Facciorusso, A.; Wani, S.; Triantafyllou, K.; Tziatzios, G.; Cannizzaro, R.; Muscatiello, N.; Singh, S. Comparative accuracy of needle sizes and designs for EUS tissue sampling of solid pancreatic masses: A network meta-analysis. Gastrointest. Endosc. 2019, 90, 893–903. [Google Scholar] [CrossRef] [PubMed]
- Oppong, K.W.; Maheshwari, P.; Nayar, M.K.; Darne, A.; Parkinson, D.; Leeds, J.S.; Haugk, B. Utility of endoscopic ultrasound-guided fine-needle biopsy in the diagnosis of type 1 autoimmune pancreatitis. Endosc. Int. Open 2020, 8, 1855–1861. [Google Scholar]
- Bang, J.Y.; Hebert-Magee, S.; Navaneethan, U.; Hasan, M.K.; Hawes, R.; Varadarajulu, S. EUS-guided fine needle biopsy of pancreatic masses can yield true histology. Gut 2018, 67, 2081–2084. [Google Scholar] [CrossRef] [PubMed]
- Crinò, S.F.; Le Grazie, M.; Manfrin, E.; Conti Bellocchi, M.C.; Bernardoni, L.; Granato, A.; Locatelli, F.; Parisi, A.; Di Stefano, S.; Frulloni, L.; et al. Randomized trial comparing fork-tip and side-fenestrated needles for EUS-guided fine-needle biopsy of solid pancreatic lesions. Gastrointest. Endosc. 2020, 92, 648–658. [Google Scholar] [CrossRef] [PubMed]
- Rimbaş, M.; Crino, S.F.; Gasbarrini, A.; Costamagna, G.; Scarpa, A.; Larghi, A. EUS-guided fine-needle tissue acquisition for solid pancreatic lesions: Finally moving from fine-needle aspiration to fine-needle biopsy? Endosc. Ultrasound. 2018, 7, 137–140. [Google Scholar]
- Kurita, A.; Yasukawa, S.; Zen, Y.; Yoshimura, K.; Ogura, T.; Ozawa, E.; Okabe, Y.; Asada, M.; Nebiki, H.; Shigekawa, M.; et al. Comparison of a 22-gauge Franseen-tip needle with a 20-gauge forward-bevel needle fort he diagnosis of type 1 autoimmune pancreatitis: A prospective, randomized, controlled, multicenter study (COMPAS study). Gastrointest. Endosc. 2020, 91, 373–381. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, T.; Kawashima, H.; Ohno, E.; Suhara, H.; Hayashi, D.; Hiramatsu, T.; Matsubara, H.; Suzuki, T.; Kuwahara, T.; Ishikawa, E.; et al. Usefulness of endoscopic ultrasound-guided fine-needle biopsy fort he diagnosis of autoimmune pancreatitis using a 22-gauge Franseen needle: A prospective multicenter study. Endoscopy 2020, 52, 978–985. [Google Scholar] [PubMed]
- Palazzo, L. Second-generation fine-needle biopsy for autoimmune pancreatitis: Ready for prime time? Endoscopy 2020, 52, 986–987. [Google Scholar] [CrossRef] [PubMed]
- Estrada, P.; Pfau, P. Diagnosing autoimmune pancreatitis: Choosing your weapon. Gastrointest. Endosc. 2020, 91, 382–384. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Notohara, K.; Kamisawa, T.; Kanno, A.; Naitoh, I.; Iwasaki, E.; Shimizu, K.; Kuraishi, Y.; Motoya, M.; Kodama, Y.; Kasashima, S.; et al. Efficacy and limitations of the histological diagnosis of type 1 autoimmune pancreatitis with endoscopic ultrasound-guided fine needle biopsy with large tissue amounts. Pancreatology 2020, 20, 834–843. [Google Scholar] [CrossRef] [PubMed]
- Nayak, H.K.; Chouhan, M.I. Does large tissue amount by endoscopic ultrasound-guided fine needle biopsy affect efficacy of diagnosing type 1 autoimmune pancreatitis? Pancreatology 2020, 21, 320–321. [Google Scholar] [CrossRef] [PubMed]
- Hedenstroem, P.; Lindkvist, B. EUS-guided neelde biopsy sampling in autoimmune pancreatitis: Is needle tip design more important than needle size? Endosc. Int. Open 2020, 8, 1862–1864. [Google Scholar]
- Tsutsumi, K.; Ueki, T.; Noma, Y.; Omonishi, K.; Ohno, K.; Kawahara, S.; Oda, T.; Kato, H.; Okada, H. Utility of a 21-gauge Menghini-type biopsy needle with the rolling method for an endoscopic ultrasound-guided histological diagnosis of autoimmune pancreatitis: A retrospective study. BMC Gastroenterol. 2021, 21, 21. [Google Scholar] [CrossRef] [PubMed]
- Detlefsen, S.; Joergensen, M.T.; Mortensen, M.B. Microscopic findings in EUS-guided fine needle (SharkCore) biopsies with type 1 and type 2 autoimmune pancreatitis. Pathol. Int. 2017, 67, 514–520. [Google Scholar] [CrossRef]
- Arora, K.; Rivera, M.; Ting, D.T.; Desphande, V. The histological diagnosis of IgG4-related disease on small biopsies: Challenges and pitfalls. Histopathology 2019, 74, 688–698. [Google Scholar] [CrossRef] [PubMed]
- Crinò, S.F.; Di Mitri, R.; Nguyen, N.Q.; Tarantino, I.; de Nucci, G.; Deprez, P.H.; Carrara, S.; Kitano, M.; Shami, V.M.; Fernández-Esparrach, G.; et al. Endoscopic ultrasound-guided fine-neelde biopsy with or without rapid on-site evaluation for diagnosis of solid pancreatic lesionns: A randomized controlled non-inferiority trial. Gastroenterology 2021, 161, 899–909.e5. [Google Scholar] [CrossRef] [PubMed]
- Facciorusso, A.; Barresi, L.; Cannizzaro, R.; Antonini, F.; Triantafyllou, K.; Tziatzios, G.; Muscatiello, N.; Hart, P.A.; Wani, S. Diagnostic yield of endoscopic ultrasound-guided tissue acquisition in autoimmune pancreatitis: A systematic review and meta-analysis. Endosc. Int. Open 2021, 9, E66–E75. [Google Scholar] [CrossRef]
- Yoon, S.B.; Moon, S.H.; Song, T.J.; Kim, J.H.; Kim, M.H. Endoscopic ultrasound-guided fine needle aspiration versus biopsy for diagnosis of autoimmune pancreatitis: Systematic review and comparative meta-analysis. Dig. Endosc. 2020. [Google Scholar] [CrossRef] [PubMed]
- Chhoda, A.; Rustagi, T. EUS-guided needle biopsy for autoimmune pancreatitis. Clin. J. Gastroenterol. 2020, 13, 669–677. [Google Scholar] [CrossRef] [PubMed]
- Nakai, Y.; Isayama, H.; Chang, K.J.; Yamamoto, N.; Mizuno, S.; Mohri, D.; Kogure, H.; Matsubara, S.; Tada, M.; Koike, K. A pilot study of EUS-guided through-the-needle forceps biopsy (with video). Gastrointest. Endosc. 2016, 84, 158–162. [Google Scholar] [CrossRef] [PubMed]

| Imai 2011 | Ishikawa 2012 | Kanno 2012 | Kanno 2016 | Morishima 2016 | Cao 2018 | All | |
|---|---|---|---|---|---|---|---|
| Number of Patients | 21 | 47 | 25 | 78 | 50 | 27 | 248 |
| Plasma cell infiltration | 0 (0) | 16 (34) | 23 (92) | 43 (55) | 36 (72) | 18 (67) | 136 (55) |
| IgG4 + plasma cells | 0 (0) | 10 (21) | 9 (36) | 19 (24) | 27 (54) | 8 (30) | 73 (29) |
| Storiform fibrosis | 0 (0) | 34 (72) | 20 (80) | 49 (63) | 0 (0) | 18 (67) | 121 (49) |
| Obliterative phlebitis | 0 (0) | 0 (0) | 4 (16) | 38 (49) | 0 (0) | 0 (0) | 42 (17) |
| Level 1 for type 1 AIP | 0 (0) | 9 (19) | 14 (56) | 32 (41) | 0 (0) | 5 (19) | 60 (24) |
| Level 2 for type 1 AIP | 0 (0) | 5 (11) | 6 (24) | 13 (17) | 27 (54) | 12 (44) | 63 (25) |
| Level 1 or 2 for type 1 AIP | 0 (0) | 14 (30) | 20 (80) | 45 (58) | 27 (54) | 17 (63) | 123 (50) |
| Kurita 2020 | Kurita 2020 | Ishikawa 2020 | Notohara 2020 | Oppong 2020 | Oppong 2020 | Tsutsumi 2021 | All | |
|---|---|---|---|---|---|---|---|---|
| Number of Patients | 50 | 51 | 56 | 85 | 6 | 18 | 14 | 280 |
| Needle Type | Franseen | Forward bevel | Franseen | Not specified | Reverse bevel | Fork-tip | Menghini type | / |
| Needle diameter | 22-G | 20-G | 22-G | 19, 20, 22-G | 22, 20, 19-G | 25, 22-G | 21-G | / |
| Plasma cell infiltration | 42 (84) | 31 (61) | 55 (100) | 19 (22) | 0 (0) | 12 (67) | 12 (86) | 171 (61) |
| IgG4 + plasma cells | 38 (76) | 22 (43) | 36 (65) | 73 (86) | 1 (17) | 14 (78) | 9 (64) | 193 (69) |
| Storiform fibrosis | 28 (56) | 13 (25) | 40 (73) | 32 (38) | 0 (0) | 11 (61) | 5 (36) | 129 (46) |
| Obliterative phlebitis | 12 (24) | 7 (14) | 24 (44) | 24 (28) | 0 (0) | 8 (44) | 1 (7) | 76 (27) |
| Level 1 for type 1 AIP | 28 (56) | 13 (26) | 32 (58) | 22 (26) | 0 (0) | 13 (72) | 5 (36) | 113 (40) |
| Level 2 for type 1 AIP | 11 (22) | 10 (20) | 19 (34) | 23 (27) | 0 (0) | 1 (7) | 4 (29) | 68 (24) |
| Level 1 or 2 for type 1 AIP | 39 (78) | 23 (45) | 51 (93) | 45 (53) | 0 (0) | 14 (78) | 9 (64) | 181 (65) |
| Kanno 2012 | Ishikawa 2012 | Ishikawa 2020 | Detlefsen 2017 | Matsumoto 2021 | All | |
|---|---|---|---|---|---|---|
| Patients with type 2 AIP included | 1 | 3 | 1 | 2 | 1 | 8 |
| Type of Tissue Acquisition | FNA | FNA | FNB | FNB | FNA | / |
| GELs (level 1 for type 2 AIP) | 1 | 0 | 0 | 1 | 1 | 3 (37) |
| Granulocytic infiltrate (level 2 for type 2 AIP) | NA | 3 | 1 | 2 | 0 | 6 (75) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Pretis, N.; Crinò, S.F.; Frulloni, L. The Role of EUS-Guided FNA and FNB in Autoimmune Pancreatitis. Diagnostics 2021, 11, 1653. https://doi.org/10.3390/diagnostics11091653
de Pretis N, Crinò SF, Frulloni L. The Role of EUS-Guided FNA and FNB in Autoimmune Pancreatitis. Diagnostics. 2021; 11(9):1653. https://doi.org/10.3390/diagnostics11091653
Chicago/Turabian Stylede Pretis, Nicolò, Stefano Francesco Crinò, and Luca Frulloni. 2021. "The Role of EUS-Guided FNA and FNB in Autoimmune Pancreatitis" Diagnostics 11, no. 9: 1653. https://doi.org/10.3390/diagnostics11091653
APA Stylede Pretis, N., Crinò, S. F., & Frulloni, L. (2021). The Role of EUS-Guided FNA and FNB in Autoimmune Pancreatitis. Diagnostics, 11(9), 1653. https://doi.org/10.3390/diagnostics11091653






