Artificial Intelligence for Alzheimer’s Disease: Promise or Challenge?
Abstract
1. Introduction
1.1. AI and the Biomedical Research
1.2. Public Databases and Biobanks
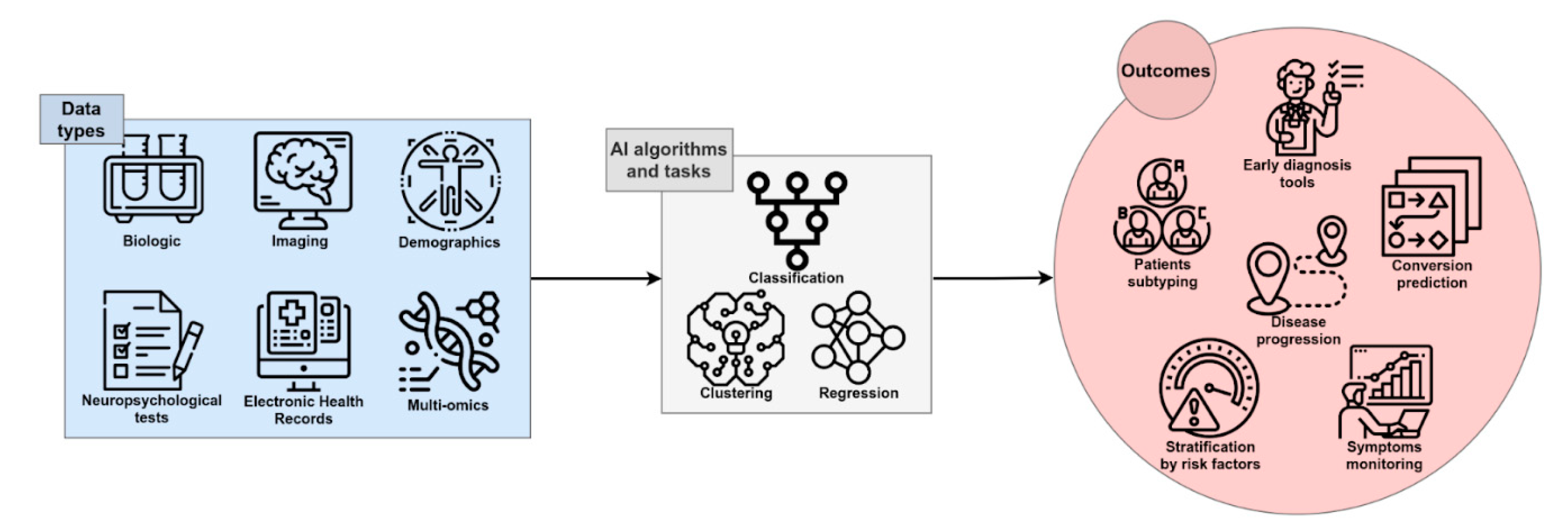
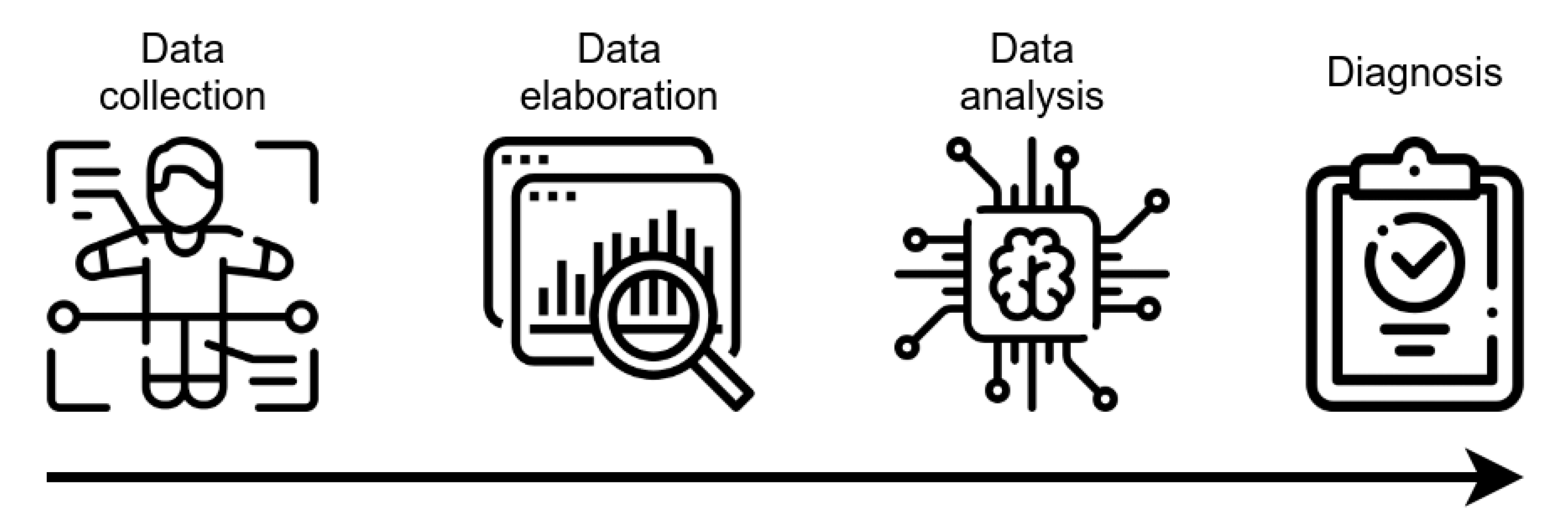
2. AI for AD Diagnosis: Is It Possible to Make an Early Diagnosis of AD with AI?
3. Prediction of MCI-to-AD Conversion: Will AI Be Able to Identify Those MCI Subjects Who Will Convert to AD?
4. Patient Stratification: Will AI Be Able to Predict the Course and Progression of the Disease?
5. AI for Precision Medicine in AD: Can AI Allow for AD Patients Sub-Typing?
6. Future Perspectives
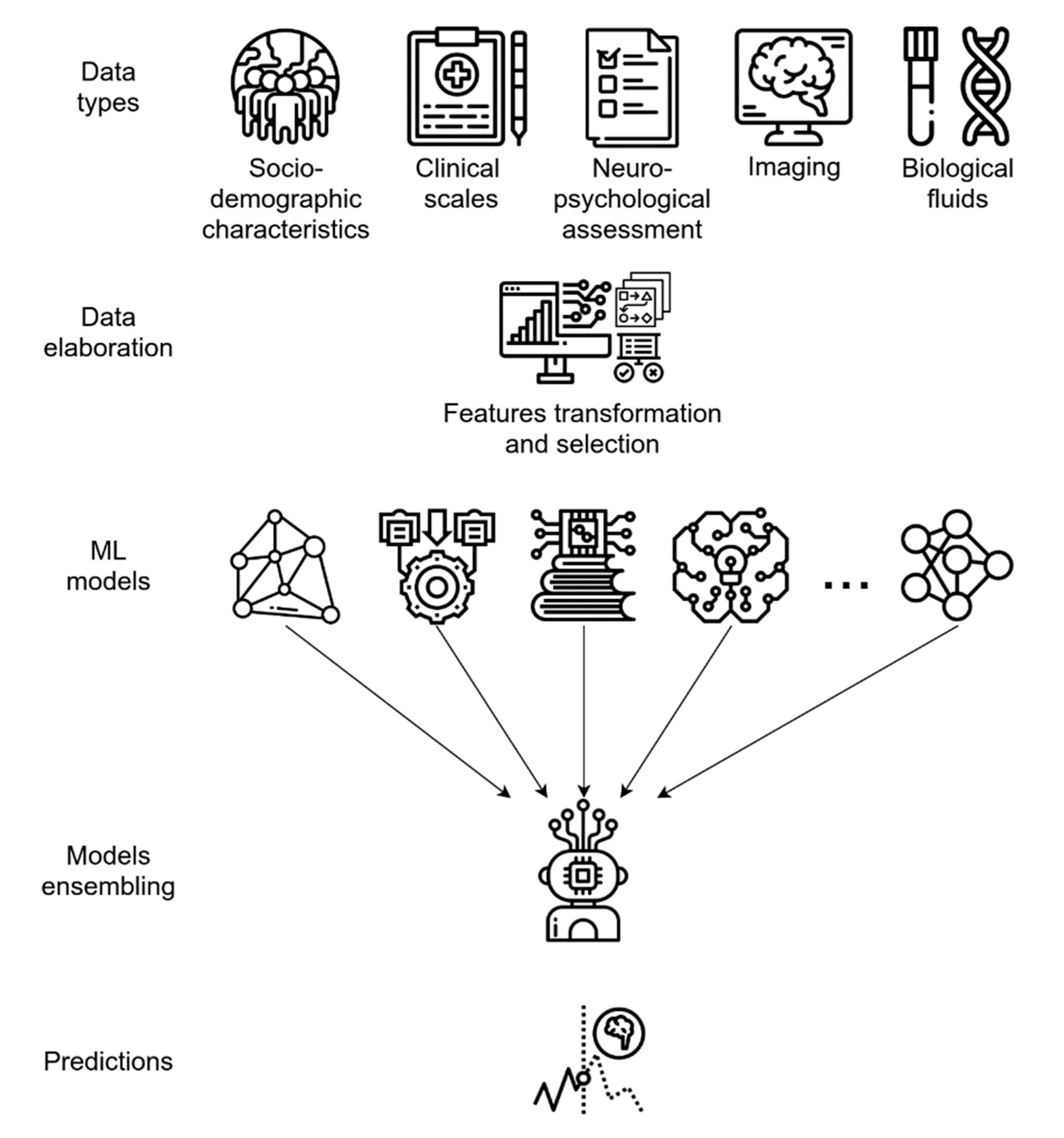
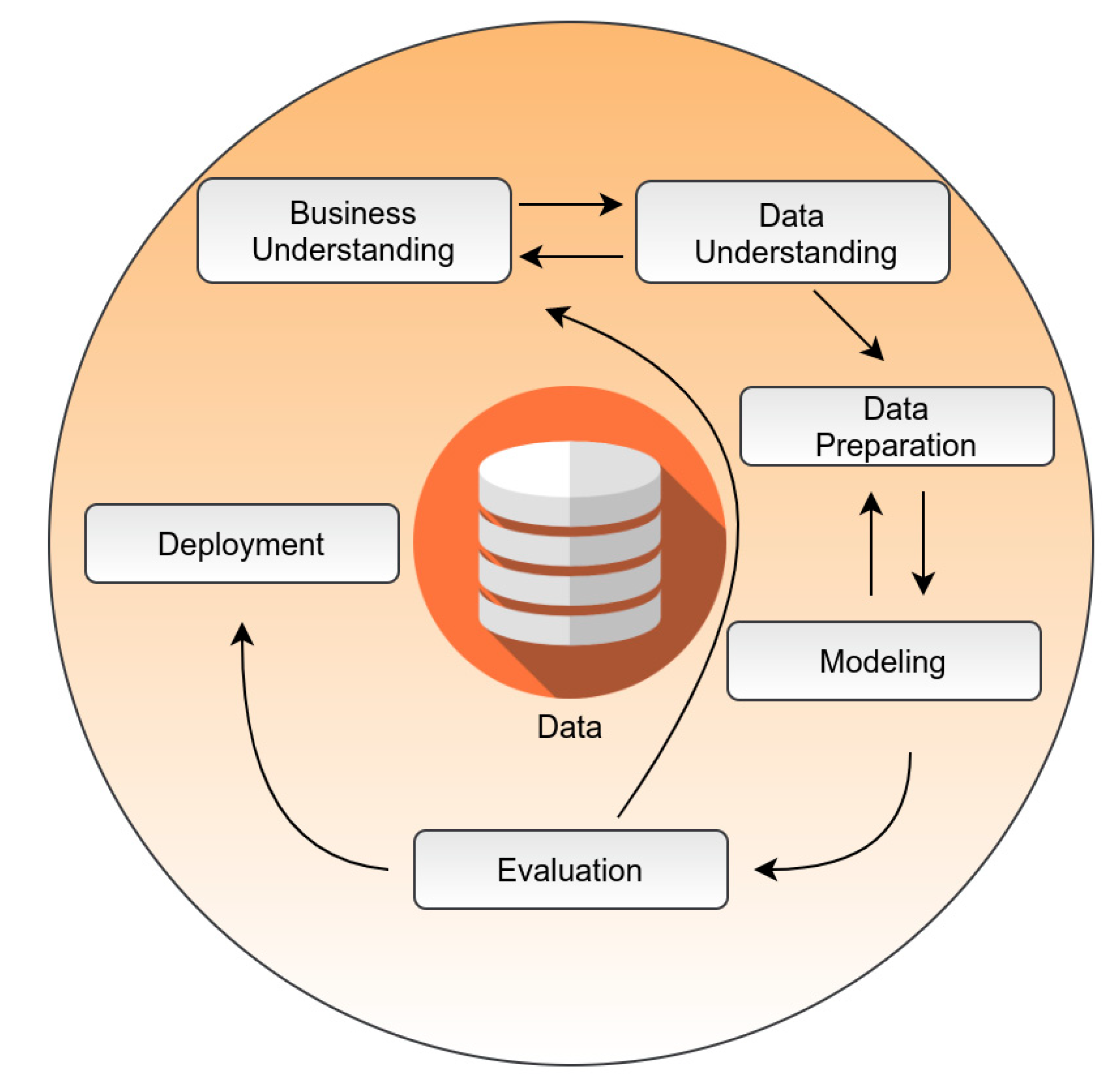
7. A Framework Overview for Pipeline Architecture in Healthcare
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Knopman, D.S.; Amieva, H.; Petersen, R.C.; Chételat, G.; Holtzman, D.M.; Hyman, B.T.; Nixon, R.A.; Jones, D.T. Alzheimer Disease. Nat. Rev. Dis. Primers 2021, 7, 33. [Google Scholar] [CrossRef]
- McKeith, I.G.; Boeve, B.F.; Dickson, D.W.; Halliday, G.; Taylor, J.-P.; Weintraub, D.; Aarsland, D.; Galvin, J.; Attems, J.; Ballard, C.G.; et al. Diagnosis and Management of Dementia with Lewy Bodies: Fourth Consensus Report of the DLB Consortium. Neurology 2017, 89, 88–100. [Google Scholar] [CrossRef]
- Rascovsky, K.; Hodges, J.R.; Knopman, D.; Mendez, M.F.; Kramer, J.H.; Neuhaus, J.; van Swieten, J.C.; Seelaar, H.; Dopper, E.G.P.; Onyike, C.U.; et al. Sensitivity of Revised Diagnostic Criteria for the Behavioural Variant of Frontotemporal Dementia. Brain 2011, 134, 2456–2477. [Google Scholar] [CrossRef]
- Beach, T.G.; Monsell, S.E.; Phillips, L.E.; Kukull, W. Accuracy of the Clinical Diagnosis of Alzheimer Disease at National Institute on Aging Alzheimer’s Disease Centers, 2005–2010. J. Neuropathol. Exp. Neurol. 2012, 71, 266–273. [Google Scholar] [CrossRef] [PubMed]
- Sancesario, G.M.; Bernardini, S. Diagnosis of Neurodegenerative Dementia: Where Do We Stand, Now? Ann. Transl. Med. 2018, 6, 340. [Google Scholar] [CrossRef]
- Albert, M.S.; DeKosky, S.T.; Dickson, D.; Dubois, B.; Feldman, H.H.; Fox, N.C.; Gamst, A.; Holtzman, D.M.; Jagust, W.J.; Petersen, R.C.; et al. The Diagnosis of Mild Cognitive Impairment due to Alzheimer’s Disease: Recommendations from the National Institute on Aging-Alzheimer’s Association Workgroups on Diagnostic Guidelines for Alzheimer’s Disease. Alzheimer’s Dement. 2011, 7, 270–279. [Google Scholar] [CrossRef] [PubMed]
- Crutch, S.J.; Lehmann, M.; Schott, J.M.; Rabinovici, G.D.; Rossor, M.N.; Fox, N.C. Posterior Cortical Atrophy. Lancet Neurol. 2012, 11, 170–178. [Google Scholar] [CrossRef]
- Hampel, H.; Nisticò, R.; Seyfried, N.T.; Levey, A.I.; Modeste, E.; Lemercier, P.; Baldacci, F.; Toschi, N.; Garaci, F.; Perry, G.; et al. Omics Sciences for Systems Biology in Alzheimer’s Disease: State-of-the-Art of the Evidence. Ageing Res. Rev. 2021, 69, 101346. [Google Scholar] [CrossRef] [PubMed]
- Braak, H.; Braak, E. Neuropathological Stageing of Alzheimer-Related Changes. Acta Neuropathol. 1991, 82, 239–259. [Google Scholar] [CrossRef]
- Braak, H.; de Vos, R.A.; Jansen, E.N.; Bratzke, H.; Braak, E. Neuropathological Hallmarks of Alzheimer’s and Parkinson’s Diseases. Prog. Brain Res. 1998, 117, 267–285. [Google Scholar] [CrossRef]
- Livingston, G.; Huntley, J.; Sommerlad, A.; Ames, D.; Ballard, C.; Banerjee, S.; Brayne, C.; Burns, A.; Cohen-Mansfield, J.; Cooper, C.; et al. Dementia Prevention, Intervention, and Care: 2020 Report of the Lancet Commission. Lancet 2020, 396, 413–446. [Google Scholar] [CrossRef]
- Singh-Manoux, A.; Dugravot, A.; Fournier, A.; Abell, J.; Ebmeier, K.; Kivimäki, M.; Sabia, S. Trajectories of Depressive Symptoms Before Diagnosis of Dementia. JAMA Psychiatry 2017, 74, 712–718. [Google Scholar] [CrossRef]
- Sancesario, G.M.; Bernardini, S. Alzheimer’s Disease in the Omics Era. Clin. Biochem. 2018, 59, 9–16. [Google Scholar] [CrossRef]
- Gunning, D.; Stefik, M.; Choi, J.; Miller, T.; Stumpf, S.; Yang, G.-Z. XAI—Explainable Artificial Intelligence. Sci. Robot. 2019, 4. [Google Scholar] [CrossRef] [PubMed]
- Quinn, J.F.; Raman, R.; Thomas, R.G.; Yurko-Mauro, K.; Nelson, E.B.; Van Dyck, C.; Galvin, J.E.; Emond, J.; Jack, C.R.; Weiner, M. Docosahexaenoic Acid Supplementation and Cognitive Decline in Alzheimer Disease: A Randomized Trial. JAMA 2010, 304, 1903–1911. [Google Scholar] [CrossRef]
- Sepulveda-Falla, D.; Chavez-Gutierrez, L.; Portelius, E.; Vélez, J.I.; Dujardin, S.; Barrera-Ocampo, A.; Dinkel, F.; Hagel, C.; Puig, B.; Mastronardi, C. A Multifactorial Model of Pathology for Age of Onset Heterogeneity in Familial Alzheimer’s Disease. Acta Neuropathol. 2021, 141, 217–233. [Google Scholar] [CrossRef] [PubMed]
- Richards, B.A.; Lillicrap, T.P.; Beaudoin, P.; Bengio, Y.; Bogacz, R.; Christensen, A.; Clopath, C.; Costa, R.P.; de Berker, A.; Ganguli, S. A Deep Learning Framework for Neuroscience. Nat. Neurosci. 2019, 22, 1761–1770. [Google Scholar] [CrossRef]
- Geerts, H.; Dacks, P.A.; Devanarayan, V.; Haas, M.; Khachaturian, Z.S.; Gordon, M.F.; Maudsley, S.; Romero, K.; Stephenson, D.; Initiative, B.H.M. Big Data to Smart Data in Alzheimer’s Disease: The Brain Health Modeling Initiative to Foster Actionable Knowledge. Alzheimer’s Dement. 2016, 12, 1014–1021. [Google Scholar] [CrossRef]
- Vinyals, O.; Babuschkin, I.; Czarnecki, W.M.; Mathieu, M.; Dudzik, A.; Chung, J.; Choi, D.H.; Powell, R.; Ewalds, T.; Georgiev, P.; et al. Grandmaster Level in StarCraft II Using Multi-Agent Reinforcement Learning. Nature 2019, 575, 350–354. [Google Scholar] [CrossRef]
- American Medical Association Augmented Intelligence in Health Care. 2019. Available online: https://www.ama-assn.org/system/files/2019-01/augmented-intelligence-policy-report.pdf (accessed on 30 April 2021).
- Mitchell, T.M. Machine Learning; McGraw-Hill series in computer science; McGraw-Hill: New York, NY, USA, 1997; ISBN 978-0-07-042807-2. [Google Scholar]
- LeCun, Y.; Bengio, Y.; Hinton, G. Deep Learning. Nature 2015, 521, 436–444. [Google Scholar] [CrossRef]
- Tolar, M.; Hey, J.; Power, A.; Abushakra, S. Neurotoxic Soluble Amyloid Oligomers Drive Alzheimer’s Pathogenesis and Represent a Clinically Validated Target for Slowing Disease Progression. Int. J. Mol. Sci. 2021, 22, 6355. [Google Scholar] [CrossRef]
- Mishra, R.; Li, B. The Application of Artificial Intelligence in the Genetic Study of Alzheimer’s Disease. Aging Dis. 2020, 11, 1567–1584. [Google Scholar] [CrossRef] [PubMed]
- Schneider, G. Automating Drug Discovery. Nat. Rev. Drug Discov. 2018, 17, 97–113. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Schmitt-Ulms, G.; Sato, C.; Xi, Z.; Zhang, Y.; Zhou, Y.; St George-Hyslop, P.; Rogaeva, E. Drug Repositioning for Alzheimer’s Disease Based on Systematic ‘Omics’ Data Mining. PLoS ONE 2016, 11, e0168812. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Chowdhury, S.; Kumar, S. In Silico Repurposing of Antipsychotic Drugs for Alzheimer’s Disease. BMC Neurosci. 2017, 18, 76. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Kong, J.; Hu, P. Computational Drug Repurposing for Alzheimer’s Disease Using Risk Genes from GWAS and Single-Cell RNA Sequencing Studies. Front. Pharmacol. 2021, 12, 617537. [Google Scholar] [CrossRef]
- Harrer, S.; Shah, P.; Antony, B.; Hu, J. Artificial Intelligence for Clinical Trial Design. Trends Pharmacol. Sci. 2019, 40, 577–591. [Google Scholar] [CrossRef]
- He, J.; Baxter, S.L.; Xu, J.; Xu, J.; Zhou, X.; Zhang, K. The Practical Implementation of Artificial Intelligence Technologies in Medicine. Nat. Med. 2019, 25, 30–36. [Google Scholar] [CrossRef]
- Tran, B.X.; Vu, G.T.; Ha, G.H.; Vuong, Q.-H.; Ho, M.-T.; Vuong, T.-T.; La, V.-P.; Ho, M.-T.; Nghiem, K.-C.P.; Nguyen, H.L.T. Global Evolution of Research in Artificial Intelligence in Health and Medicine: A Bibliometric Study. J. Clin. Med. 2019, 8, 360. [Google Scholar] [CrossRef]
- Termine, A.; Fabrizio, C.; Strafella, C.; Caputo, V.; Petrosini, L.; Caltagirone, C.; Giardina, E.; Cascella, R. Multi-Layer Picture of Neurodegenerative Diseases: Lessons from the Use of Big Data through Artificial Intelligence. J. Pers. Med. 2021, 11, 280. [Google Scholar] [CrossRef]
- Ashish, N.; Bhatt, P.; Toga, A.W. Global Data Sharing in Alzheimer Disease Research. Alzheimer Dis. Assoc. Disord. 2016, 30, 160–168. [Google Scholar] [CrossRef][Green Version]
- Bertram, L.; McQueen, M.B.; Mullin, K.; Blacker, D.; Tanzi, R.E. Systematic meta-analyses of Alzheimer disease genetic association studies: The AlzGene database. Nat. Genet. 2007, 39, 17–23. [Google Scholar] [CrossRef]
- Beekly, D.L.; Ramos, E.M.; Lee, W.W.; Deitrich, W.D.; Jacka, M.E.; Wu, J.; Hubbard, J.L.; Koepsell, T.D.; Morris, J.C.; Kukull, W.A.; et al. The National Alzheimer’s Coordinating Center (NACC) Database: The Uniform Data Set. Alzheimer Dis. Assoc. Disord. 2007, 21, 249–258. [Google Scholar] [CrossRef] [PubMed]
- Haider, F.; de la Fuente, S.; Luz, S. An Assessment of Paralinguistic Acoustic Features for Detection of Alzheimer’s Dementia in Spontaneous Speech. IEEE J. Sel. Top. Signal Process. 2020, 14, 272–281. [Google Scholar] [CrossRef]
- Becker, J.T.; Boiler, F.; Lopez, O.L.; Saxton, J.; McGonigle, K.L. The Natural History of Alzheimer’s Disease: Description of Study Cohort and Accuracy of Diagnosis. Arch. Neurol. 1994, 51, 585–594. [Google Scholar] [CrossRef] [PubMed]
- Edgar, R.; Domrachev, M.; Lash, A.E. Gene Expression Omnibus: NCBI Gene Expression and Hybridization Array Data Repository. Nucleic Acids Res. 2002, 30, 207–210. [Google Scholar] [CrossRef]
- Sudlow, C.; Gallacher, J.; Allen, N.; Beral, V.; Burton, P.; Danesh, J.; Downey, P.; Elliott, P.; Green, J.; Landray, M.; et al. UK Biobank: An Open Access Resource for Identifying the Causes of a Wide Range of Complex Diseases of Middle and Old Age. PLoS Med. 2015, 12, e1001779. [Google Scholar] [CrossRef]
- Spalletta, G.; Piras, F.; Piras, F.; Sancesario, G.; Iorio, M.; Fratangeli, C.; Cacciari, C.; Caltagirone, C.; Orfei, M.D. Neuroanatomical Correlates of Awareness of Illness in Patients with Amnestic Mild Cognitive Impairment Who Will or Will Not Convert to Alzheimer’s Disease. Cortex 2014, 61, 183–195. [Google Scholar] [CrossRef]
- Giulietti, G.; Torso, M.; Serra, L.; Spanò, B.; Marra, C.; Caltagirone, C.; Cercignani, M.; Bozzali, M. Alzheimer’s Disease Neuroimaging Initiative (ADNI) Whole Brain White Matter Histogram Analysis of Diffusion Tensor Imaging Data Detects Microstructural Damage in Mild Cognitive Impairment and Alzheimer’s Disease Patients. J. Magn. Reson. Imaging 2018. [Google Scholar] [CrossRef]
- McKhann, G.M.; Knopman, D.S.; Chertkow, H.; Hyman, B.T.; Jack, C.R.; Kawas, C.H.; Klunk, W.E.; Koroshetz, W.J.; Manly, J.J.; Mayeux, R.; et al. The Diagnosis of Dementia due to Alzheimer’s Disease: Recommendations from the National Institute on Aging-Alzheimer’s Association Workgroups on Diagnostic Guidelines for Alzheimer’s Disease. Alzheimer’s Dement. 2011, 7, 263–269. [Google Scholar] [CrossRef]
- Sperling, R.A.; Aisen, P.S.; Beckett, L.A.; Bennett, D.A.; Craft, S.; Fagan, A.M.; Iwatsubo, T.; Jack, C.R.; Kaye, J.; Montine, T.J.; et al. Toward Defining the Preclinical Stages of Alzheimer’s Disease: Recommendations from the National Institute on Aging-Alzheimer’s Association Workgroups on Diagnostic Guidelines for Alzheimer’s Disease. Alzheimer’s Dement. 2011, 7, 280–292. [Google Scholar] [CrossRef]
- Jack, C.R.; Bennett, D.A.; Blennow, K.; Carrillo, M.C.; Dunn, B.; Haeberlein, S.B.; Holtzman, D.M.; Jagust, W.; Jessen, F.; Karlawish, J.; et al. NIA-AA Research Framework: Toward a Biological Definition of Alzheimer’s Disease. Alzheimer’s Dement. 2018, 14, 535–562. [Google Scholar] [CrossRef]
- Williamson, J.; Goldman, J.; Marder, K.S. Genetic Aspects of Alzheimer Disease. Neurologist 2009, 15, 80–86. [Google Scholar] [CrossRef]
- Salas-Gonzalez, D.; Górriz, J.M.; Ramírez, J.; López, M.; Álvarez, I.; Segovia, F.; Puntonet, C.G. Computer Aided Diagnosis of Alzheimer Disease Using Support Vector Machines and Classification Trees. In Advances in Neuro-Information Processing; Köppen, M., Kasabov, N., Coghill, G., Eds.; Springer: Berlin/Heidelberg, Germany, 2009; pp. 418–425. [Google Scholar]
- Gorriz, J.M.; Ramirez, J.; Lassl, A.; Salas-Gonzalez, D.; Lang, E.W.; Puntonet, C.G.; Alvarez, I.; Lopez, M.; Gomez-Rio, M. Automatic Computer Aided Diagnosis Tool Using Component-Based SVM. In Proceedings of the 2008 IEEE Nuclear Science Symposium Conference Record, Dresden, Germany, 19–25 October 2008; pp. 4392–4395. [Google Scholar]
- Marcus, D.S.; Wang, T.H.; Parker, J.; Csernansky, J.G.; Morris, J.C.; Buckner, R.L. Open Access Series of Imaging Studies (OASIS): Cross-Sectional MRI Data in Young, Middle Aged, Nondemented, and Demented Older Adults. J. Cogn. Neurosci. 2007, 19, 1498–1507. [Google Scholar] [CrossRef]
- Zhang, Y.; Dong, Z.; Phillips, P.; Wang, S.; Ji, G.; Yang, J.; Yuan, T.-F. Detection of Subjects and Brain Regions Related to Alzheimer’s Disease Using 3D MRI Scans Based on Eigenbrain and Machine Learning. Front. Comput. Neurosci. 2015, 9. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Sohn, J.H.; Kawczynski, M.G.; Trivedi, H.; Harnish, R.; Jenkins, N.W.; Lituiev, D.; Copeland, T.P.; Aboian, M.S.; Mari Aparici, C.; et al. A Deep Learning Model to Predict a Diagnosis of Alzheimer Disease by Using 18F-FDG PET of the Brain. Radiology 2019, 290, 456–464. [Google Scholar] [CrossRef] [PubMed]
- Rathore, S.; Habes, M.; Iftikhar, M.A.; Shacklett, A.; Davatzikos, C. A Review on Neuroimaging-Based Classification Studies and Associated Feature Extraction Methods for Alzheimer’s Disease and Its Prodromal Stages. Neuroimage 2017, 155, 530–548. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.-H.; Lin, C.-H.; Lane, H.-Y. Machine Learning and Novel Biomarkers for the Diagnosis of Alzheimer’s Disease. Int. J. Mol. Sci. 2021, 22, 2761. [Google Scholar] [CrossRef] [PubMed]
- Stamate, D.; Kim, M.; Proitsi, P.; Westwood, S.; Baird, A.; Nevado-Holgado, A.; Hye, A.; Bos, I.; Vos, S.J.B.; Vandenberghe, R.; et al. A Metabolite-Based Machine Learning Approach to Diagnose Alzheimer-Type Dementia in Blood: Results from the European Medical Information Framework for Alzheimer Disease Biomarker Discovery Cohort. Alzheimer’s Dement. 2019, 5, 933–938. [Google Scholar] [CrossRef] [PubMed]
- Binaco, R.; Calzaretto, N.; Epifano, J.; McGuire, S.; Umer, M.; Emrani, S.; Wasserman, V.; Libon, D.J.; Polikar, R. Machine Learning Analysis of Digital Clock Drawing Test Performance for Differential Classification of Mild Cognitive Impairment Subtypes Versus Alzheimer’s Disease. J. Int. Neuropsychol. Soc. 2020, 26, 690–700. [Google Scholar] [CrossRef]
- Spasov, S.; Passamonti, L.; Duggento, A.; Liò, P.; Toschi, N. A Parameter-Efficient Deep Learning Approach to Predict Conversion from Mild Cognitive Impairment to Alzheimer’s Disease. NeuroImage 2019, 189, 276–287. [Google Scholar] [CrossRef]
- Bae, J.; Stocks, J.; Heywood, A.; Jung, Y.; Jenkins, L.; Hill, V.; Katsaggelos, A.; Popuri, K.; Rosen, H.; Beg, M.F.; et al. Transfer Learning for Predicting Conversion from Mild Cognitive Impairment to Dementia of Alzheimer’s Type Based on a Three-Dimensional Convolutional Neural Network. Neurobiol. Aging 2020, 99, 53–64. [Google Scholar] [CrossRef]
- Cabral, C.; Morgado, P.M.; Costa, D.C.; Silveira, M. Alzheimer’s Disease Neuroimaging Initiative Predicting Conversion from MCI to AD with FDG-PET Brain Images at Different Prodromal Stages. Comput. Biol. Med. 2015, 58, 101–109. [Google Scholar] [CrossRef]
- Cheng, B.; Liu, M.; Zhang, D.; Munsell, B.C.; Shen, D. Domain Transfer Learning for MCI Conversion Prediction. IEEE Trans. Biomed. Eng. 2015, 62, 1805–1817. [Google Scholar] [CrossRef]
- Cuingnet, R.; Gerardin, E.; Tessieras, J.; Auzias, G.; Lehéricy, S.; Habert, M.-O.; Chupin, M.; Benali, H.; Colliot, O. Alzheimer’s Disease Neuroimaging Initiative Automatic Classification of Patients with Alzheimer’s Disease from Structural MRI: A Comparison of Ten Methods Using the ADNI Database. Neuroimage 2011, 56, 766–781. [Google Scholar] [CrossRef]
- Davatzikos, C.; Bhatt, P.; Shaw, L.M.; Batmanghelich, K.N.; Trojanowski, J.Q. Prediction of MCI to AD Conversion, via MRI, CSF Biomarkers, Pattern Classification. Neurobiol. Aging 2011, 32, 2322.e19–>2322.e27. [Google Scholar] [CrossRef]
- Engedal, K.; Barca, M.L.; Høgh, P.; Bo Andersen, B.; Dombernowsky, N.W.; Naik, M.; Gudmundsson, T.E.; Øksengaard, A.-R.; Wahlund, L.-O.; Snaedal, J. The Power of EEG to Predict Conversion from Mild Cognitive Impairment and Subjective Cognitive Decline to Dementia. Dement. Geriatr. Cogn. Disord. 2020, 49, 38–47. [Google Scholar] [CrossRef]
- Grassi, M.; Rouleaux, N.; Caldirola, D.; Loewenstein, D.; Schruers, K.; Perna, G.; Dumontier, M. Alzheimer’s Disease Neuroimaging Initiative a Novel Ensemble-Based Machine Learning Algorithm to Predict the Conversion from Mild Cognitive Impairment to Alzheimer’s Disease Using Socio-Demographic Characteristics, Clinical Information, and Neuropsychological Measures. Front. Neurol. 2019, 10, 756. [Google Scholar] [CrossRef]
- Lee, G.; Nho, K.; Kang, B.; Sohn, K.-A.; Kim, D. Predicting Alzheimer’s Disease Progression Using Multi-Modal Deep Learning Approach. Sci. Rep. 2019, 9. [Google Scholar] [CrossRef]
- Lin, W.; Gao, Q.; Yuan, J.; Chen, Z.; Feng, C.; Chen, W.; Du, M.; Tong, T. Predicting Alzheimer’s Disease Conversion from Mild Cognitive Impairment Using an Extreme Learning Machine-Based Grading Method with Multimodal Data. Front. Aging Neurosci. 2020, 12. [Google Scholar] [CrossRef]
- Liu, S.; Liu, S.; Cai, W.; Che, H.; Pujol, S.; Kikinis, R.; Feng, D.; Fulham, M.J. ADNI Multimodal Neuroimaging Feature Learning for Multiclass Diagnosis of Alzheimer’s Disease. IEEE Trans. Biomed. Eng. 2015, 62, 1132–1140. [Google Scholar] [CrossRef] [PubMed]
- Moradi, E.; Pepe, A.; Gaser, C.; Huttunen, H.; Tohka, J. Machine Learning Framework for Early MRI-Based Alzheimer’s Conversion Prediction in MCI Subjects. NeuroImage 2015, 104, 398–412. [Google Scholar] [CrossRef] [PubMed]
- Pan, D.; Zeng, A.; Jia, L.; Huang, Y.; Frizzell, T.; Song, X. Early Detection of Alzheimer’s Disease Using Magnetic Resonance Imaging: A Novel Approach Combining Convolutional Neural Networks and Ensemble Learning. Front. Neurosci. 2020, 14, 259. [Google Scholar] [CrossRef] [PubMed]
- Platero, C.; Tobar, M.C. Alzheimer’s Disease Neuroimaging Initiative Predicting Alzheimer’s Conversion in Mild Cognitive Impairment Patients Using Longitudinal Neuroimaging and Clinical Markers. Brain Imaging Behav. 2020. [Google Scholar] [CrossRef]
- Popescu, S.G.; Whittington, A.; Gunn, R.N.; Matthews, P.M.; Glocker, B.; Sharp, D.J.; Cole, J.H. Alzheimer’s Disease Neuroimaging Initiative Nonlinear Biomarker Interactions in Conversion from Mild Cognitive Impairment to Alzheimer’s Disease. Hum. Brain Mapp. 2020. [Google Scholar] [CrossRef] [PubMed]
- Pusil, S.; Dimitriadis, S.I.; López, M.E.; Pereda, E.; Maestú, F. Aberrant MEG Multi-Frequency Phase Temporal Synchronization Predicts Conversion from Mild Cognitive Impairment-to-Alzheimer’s Disease. Neuroimage Clin. 2019, 24, 101972. [Google Scholar] [CrossRef]
- Tong, T.; Gao, Q.; Guerrero, R.; Ledig, C.; Chen, L.; Rueckert, D. A Novel Grading Biomarker for the Prediction of Conversion from Mild Cognitive Impairment to Alzheimer’s Disease. IEEE Trans. Biomed. Eng. 2017, 64, 155–165. [Google Scholar] [CrossRef]
- Yan, Y.; Somer, E.; Grau, V. Classification of Amyloid PET Images Using Novel Features for Early Diagnosis of Alzheimer’s Disease and Mild Cognitive Impairment Conversion. Nucl. Med. Commun. 2019, 40, 242–248. [Google Scholar] [CrossRef]
- Petersen, R.C. How Early Can We Diagnose Alzheimer Disease (and Is It Sufficient)? The 2017 Wartenberg Lecture. Neurology 2018, 91, 395–402. [Google Scholar] [CrossRef]
- Grundman, M.; Petersen, R.C.; Ferris, S.H.; Thomas, R.G.; Aisen, P.S.; Bennett, D.A.; Foster, N.L.; Jack, C.R.; Galasko, D.R.; Doody, R.; et al. Mild Cognitive Impairment Can Be Distinguished from Alzheimer Disease and Normal Aging for Clinical Trials. Arch. Neurol. 2004, 61, 59–66. [Google Scholar] [CrossRef]
- Kohannim, O.; Hua, X.; Hibar, D.P.; Lee, S.; Chou, Y.-Y.; Toga, A.W.; Jack, C.R., Jr.; Weiner, M.W.; Thompson, P.M. Boosting Power for Clinical Trials Using Classifiers Based on Multiple Biomarkers. Neurobiol. Aging 2010, 31, 1429–1442. [Google Scholar] [CrossRef]
- Walhovd, K.B.; Fjell, A.M.; Brewer, J.; McEvoy, L.K.; Fennema-Notestine, C.; Hagler, D.J.; Jennings, R.G.; Karow, D.; Dale, A.M. Combining MR Imaging, Positron-Emission Tomography, and CSF Biomarkers in the Diagnosis and Prognosis of Alzheimer Disease. Am. J. Neuroradiol. 2010, 31, 347–354. [Google Scholar] [CrossRef] [PubMed]
- Westman, E.; Muehlboeck, J.-S.; Simmons, A. Combining MRI and CSF Measures for Classification of Alzheimer’s Disease and Prediction of Mild Cognitive Impairment Conversion. Neuroimage 2012, 62, 229–238. [Google Scholar] [CrossRef]
- Gao, J.; Li, P.; Chen, Z.; Zhang, J. A Survey on Deep Learning for Multimodal Data Fusion. Neural Comput. 2020, 32, 829–864. [Google Scholar] [CrossRef]
- El-Sappagh, S.; Alonso, J.M.; Islam, S.M.R.; Sultan, A.M.; Kwak, K.S. A Multilayer Multimodal Detection and Prediction Model Based on Explainable Artificial Intelligence for Alzheimer’s Disease. Sci. Rep. 2021, 11, 2660. [Google Scholar] [CrossRef]
- Sancesario, G.M.; Toniolo, S.; Chiasserini, D.; Di Santo, S.G.; Zegeer, J.; Bernardi, G.; Musicco, M.; Caltagirone, C.; Parnetti, L.; Bernardini, S. The Clinical Use of Cerebrospinal Fluid Biomarkers for Alzheimer’s Disease Diagnosis: The Italian Selfie. J. Alzheimer’s Dis. 2017, 55, 1659–1666. [Google Scholar] [CrossRef]
- Mattsson, N.; Palmqvist, S.; Stomrud, E.; Vogel, J.; Hansson, O. Staging β-Amyloid Pathology with Amyloid Positron Emission Tomography. JAMA Neurol. 2019, 76, 1319–1329. [Google Scholar] [CrossRef]
- Rabinovici, G.D.; Gatsonis, C.; Apgar, C.; Chaudhary, K.; Gareen, I.; Hanna, L.; Hendrix, J.; Hillner, B.E.; Olson, C.; Lesman-Segev, O.H.; et al. Association of Amyloid Positron Emission Tomography with Subsequent Change in Clinical Management Among Medicare Beneficiaries with Mild Cognitive Impairment or Dementia. JAMA 2019, 321, 1286–1294. [Google Scholar] [CrossRef]
- Ossenkoppele, R.; Rabinovici, G.D.; Smith, R.; Cho, H.; Schöll, M.; Strandberg, O.; Palmqvist, S.; Mattsson, N.; Janelidze, S.; Santillo, A.; et al. Discriminative Accuracy of [18F]Flortaucipir Positron Emission Tomography for Alzheimer Disease vs. Other Neurodegenerative Disorders. JAMA 2018, 320, 1151–1162. [Google Scholar] [CrossRef]
- Preische, O.; Schultz, S.A.; Apel, A.; Kuhle, J.; Kaeser, S.A.; Barro, C.; Gräber, S.; Kuder-Buletta, E.; LaFougere, C.; Laske, C.; et al. Serum Neurofilament Dynamics Predicts Neurodegeneration and Clinical Progression in Presymptomatic Alzheimer’s Disease. Nat. Med. 2019, 25, 277–283. [Google Scholar] [CrossRef]
- Palmqvist, S.; Janelidze, S.; Stomrud, E.; Zetterberg, H.; Karl, J.; Zink, K.; Bittner, T.; Mattsson, N.; Eichenlaub, U.; Blennow, K.; et al. Performance of Fully Automated Plasma Assays as Screening Tests for Alzheimer Disease-Related β-Amyloid Status. JAMA Neurol. 2019, 76, 1060–1069. [Google Scholar] [CrossRef]
- Schindler, S.E.; Bollinger, J.G.; Ovod, V.; Mawuenyega, K.G.; Li, Y.; Gordon, B.A.; Holtzman, D.M.; Morris, J.C.; Benzinger, T.L.S.; Xiong, C.; et al. High-Precision Plasma β-Amyloid 42/40 Predicts Current and Future Brain Amyloidosis. Neurology 2019, 93, e1647–e1659. [Google Scholar] [CrossRef]
- Janelidze, S.; Mattsson, N.; Palmqvist, S.; Smith, R.; Beach, T.G.; Serrano, G.E.; Chai, X.; Proctor, N.K.; Eichenlaub, U.; Zetterberg, H.; et al. Plasma P-Tau181 in Alzheimer’s Disease: Relationship to Other Biomarkers, Differential Diagnosis, Neuropathology and Longitudinal Progression to Alzheimer’s Dementia. Nat. Med. 2020, 26, 379–386. [Google Scholar] [CrossRef]
- Palmqvist, S.; Tideman, P.; Cullen, N.; Zetterberg, H.; Blennow, K.; Dage, J.L.; Stomrud, E.; Janelidze, S.; Mattsson-Carlgren, N.; Hansson, O. Prediction of Future Alzheimer’s Disease Dementia Using Plasma Phospho-Tau Combined with Other Accessible Measures. Nat. Med. 2021. [Google Scholar] [CrossRef]
- De Simone, M.S.; De Tollis, M.; Fadda, L.; Perri, R.; Caltagirone, C.; Carlesimo, G.A. Lost or Unavailable? Exploring Mechanisms That Affect Retrograde Memory in Mild Cognitive Impairment and Alzheimer’s Disease Patients. J. Neurol. 2020, 267, 113–124. [Google Scholar] [CrossRef] [PubMed]
- De Simone, M.S.; Perri, R.; Fadda, L.; De Tollis, M.; Turchetta, C.S.; Caltagirone, C.; Carlesimo, G.A. Different Deficit Patterns on Word Lists and Short Stories Predict Conversion to Alzheimer’s Disease in Patients with Amnestic Mild Cognitive Impairment. J. Neurol. 2017, 264, 2258–2267. [Google Scholar] [CrossRef]
- De Simone, M.S.; Perri, R.; Fadda, L.; Caltagirone, C.; Carlesimo, G.A. Predicting Progression to Alzheimer’s Disease in Subjects with Amnestic Mild Cognitive Impairment Using Performance on Recall and Recognition Tests. J. Neurol. 2019, 266, 102–111. [Google Scholar] [CrossRef] [PubMed]
- Di Lorenzo, F.; Motta, C.; Casula, E.P.; Bonnì, S.; Assogna, M.; Caltagirone, C.; Martorana, A.; Koch, G. LTP-like Cortical Plasticity Predicts Conversion to Dementia in Patients with Memory Impairment. Brain Stimul. 2020, 13, 1175–1182. [Google Scholar] [CrossRef]
- Giorgio, J.; Landau, S.M.; Jagust, W.J.; Tino, P.; Kourtzi, Z. Alzheimer’s Disease Neuroimaging Initiative Modelling Prognostic Trajectories of Cognitive Decline due to Alzheimer’s Disease. Neuroimage Clin. 2020, 26, 102199. [Google Scholar] [CrossRef]
- Thung, K.-H.; Yap, P.-T.; Adeli, E.; Lee, S.-W.; Shen, D. Conversion and Time-to-Conversion Predictions of Mild Cognitive Impairment Using Low-Rank Affinity Pursuit Denoising and Matrix Completion. Med. Image Anal. 2018, 45, 68–82. [Google Scholar] [CrossRef]
- Cox, D.R. Regression Models and Life-Tables. Breakthroughs in Statistics. R. Stat. Soc. 1992, 372, 527–541. [Google Scholar]
- Cox, D.R.; Oakes, D. Analysis of Survival Data; CRC Press: Boca Raton, FL, USA, 1984; Volume 21, ISBN 0-412-24490-X. [Google Scholar]
- Liu, K.; Chen, K.; Yao, L.; Guo, X. Prediction of Mild Cognitive Impairment Conversion Using a Combination of Independent Component Analysis and the Cox Model. Front. Hum. Neurosci. 2017, 11. [Google Scholar] [CrossRef]
- Li, S.; Okonkwo, O.; Albert, M.; Wang, M.-C. Variation in Variables That Predict Progression from MCI to AD Dementia over Duration of Follow-Up. Am. J. Alzheimer’s Dis. 2013, 2, 12–28. [Google Scholar] [CrossRef]
- Franzmeier, N.; Koutsouleris, N.; Benzinger, T.; Goate, A.; Karch, C.M.; Fagan, A.M.; McDade, E.; Duering, M.; Dichgans, M.; Levin, J.; et al. Predicting Sporadic Alzheimer’s Disease Progression via Inherited Alzheimer’s Disease-Informed Machine-Learning. Alzheimer’s Dement. 2020, 16, 501–511. [Google Scholar] [CrossRef]
- Koch, G.; Belli, L.; Giudice, T.L.; Lorenzo, F.D.; Sancesario, G.M.; Sorge, R.; Bernardini, S.; Martorana, A. Frailty among Alzheimer’s Disease Patients. CNS Neurol. Disord. Drug Targets 2013, 12, 507–511. [Google Scholar] [CrossRef]
- Langa, K.M.; Levine, D.A. The Diagnosis and Management of Mild Cognitive Impairment: A Clinical Review. JAMA 2014, 312, 2551–2561. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, P.B.; Lyketsos, C. Mild Cognitive Impairment: Searching for the Prodrome of Alzheimer’s Disease. World Psychiatry 2008, 7, 72–78. [Google Scholar] [CrossRef] [PubMed]
- Gamberger, D.; Lavrač, N.; Srivatsa, S.; Tanzi, R.E.; Doraiswamy, P.M. Identification of Clusters of Rapid and Slow Decliners among Subjects at Risk for Alzheimer’s Disease. Sci. Rep. 2017, 7. [Google Scholar] [CrossRef] [PubMed]
- Gamberger, D.; Ženko, B.; Mitelpunkt, A.; Lavrač, N. Homogeneous Clusters of Alzheimer’s Disease Patient Population. Biomed. Eng. Online 2016, 15. [Google Scholar] [CrossRef]
- Mitelpunkt, A.; Galili, T.; Kozlovski, T.; Bregman, N.; Shachar, N.; Markus-Kalish, M.; Benjamini, Y. Novel Alzheimer’s Disease Subtypes Identified Using a Data and Knowledge Driven Strategy. Sci. Rep. 2020, 10, 1327. [Google Scholar] [CrossRef]
- Young, A.L.; Marinescu, R.V.; Oxtoby, N.P.; Bocchetta, M.; Yong, K.; Firth, N.C.; Cash, D.M.; Thomas, D.L.; Dick, K.M.; Cardoso, J.; et al. Uncovering the Heterogeneity and Temporal Complexity of Neurodegenerative Diseases with Subtype and Stage Inference. Nat. Commun. 2018, 9. [Google Scholar] [CrossRef] [PubMed]
- Hampel, H.; Vergallo, A.; Perry, G.; Lista, S.; Alzheimer Precision Medicine Initiative. The Alzheimer Precision Medicine Initiative. J. Alzheimer’s Dis. 2019, 68, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Forloni, G. Alzheimer’s Disease: From Basic Science to Precision Medicine Approach. BMJ Neurol. Open 2020, 2, e000079. [Google Scholar] [CrossRef]
- Neff, R.A.; Wang, M.; Vatansever, S.; Guo, L.; Ming, C.; Wang, Q.; Wang, E.; Horgusluoglu-Moloch, E.; Song, W.-M.; Li, A.; et al. Molecular Subtyping of Alzheimer’s Disease Using RNA Sequencing Data Reveals Novel Mechanisms and Targets. Sci. Adv. 2021, 7. [Google Scholar] [CrossRef]
- Maudsley, S.; Devanarayan, V.; Martin, B.; Geerts, H. Intelligent and Effective Informatic Deconvolution of “Big Data” and Its Future Impact on the Quantitative Nature of Neurodegenerative Disease Therapy. Alzheimer’s Dement. 2018, 14, 961–975. [Google Scholar] [CrossRef]
- Valliani, A.A.-A.; Ranti, D.; Oermann, E.K. Deep Learning and Neurology: A Systematic Review. Neurol. Ther. 2019, 8, 351–365. [Google Scholar] [CrossRef]
- Singh, A.; Sengupta, S.; Lakshminarayanan, V. Explainable Deep Learning Models in Medical Image Analysis. J. Imaging 2020, 6, 52. [Google Scholar] [CrossRef]
- Feng, X.; Yang, J.; Lipton, Z.C.; Small, S.A.; Provenzano, F.A.; Alzheimer’s Disease Neuroimaging Initiative. Deep Learning on MRI Affirms the Prominence of the Hippocampal Formation in Alzheimer’s Disease Classification. bioRxiv 2018, 456277. [Google Scholar] [CrossRef]
- Lundberg, S.; Lee, S.-I. A Unified Approach to Interpreting Model Predictions. arXiv 2017, arXiv:1705.07874. [Google Scholar]
- Selvaraju, R.R.; Cogswell, M.; Das, A.; Vedantam, R.; Parikh, D.; Batra, D. Grad-CAM: Visual Explanations from Deep Networks via Gradient-Based Localization. In Proceedings of the 2017 IEEE International Conference on Computer Vision (ICCV), Venice, Italy, 22–29 October 2017; pp. 618–626. [Google Scholar]
- Grapov, D.; Fahrmann, J.; Wanichthanarak, K.; Khoomrung, S. Rise of Deep Learning for Genomic, Proteomic, and Metabolomic Data Integration in Precision Medicine. OMICS 2018, 22, 630–636. [Google Scholar] [CrossRef]
- Rajkomar, A.; Dean, J.; Kohane, I. Machine Learning in Medicine. N. Engl. J. Med. 2019, 380, 1347–1358. [Google Scholar] [CrossRef]
- Wolpert, D.H.; Macready, W.G. No Free Lunch Theorems for Optimization. IEEE Trans. Evol. Comput. 1997, 1, 67–82. [Google Scholar] [CrossRef]
- Waring, J.; Lindvall, C.; Umeton, R. Automated Machine Learning: Review of the State-of-the-Art and Opportunities for Healthcare. Artif. Intell. Med. 2020, 104, 101822. [Google Scholar] [CrossRef]
- Wirth, R.; Hipp, J. CRISP-DM: Towards a Standard Process Model for Data Mining. In Proceedings of the 4th International Conference on the Practical Applications of Knowledge Discovery and Data Mining, Manchester, UK, 11–13 April 2000. [Google Scholar]
- Vougas, K.; Sakellaropoulos, T.; Kotsinas, A.; Foukas, G.-R.P.; Ntargaras, A.; Koinis, F.; Polyzos, A.; Myrianthopoulos, V.; Zhou, H.; Narang, S. Machine Learning and Data Mining Frameworks for Predicting Drug Response in Cancer: An Overview and a Novel in Silico Screening Process Based on Association Rule Mining. Pharmacol. Ther. 2019, 203, 107395. [Google Scholar] [CrossRef] [PubMed]

| Method | Definition | Details |
|---|---|---|
| Machine Learning | A collection of data analysis techniques that aim to generate predictive models by learning from data and improving their ability to make predictions through experience. | ML models are considered shallow learners, working on data with hand-crafted features defined through expert-based knowledge. Raw data must be pre-processed before constructing a ML system, requiring domain expertise to proceed with feature extraction and engineering, in order to train the algorithm appropriately. As an example of a ML algorithm, a Support Vector Machine (SVM) accomplishes the classification task by finding the hyperplane that, in the multi-dimensional feature space, optimally separates the data into two (or more) classes. |
| Deep Learning | A sub-field of ML that uses methods that are able to learn relationships between inputs and outputs by modeling highly non-linear interactions. | DL models are different from shallow learners and can elaborate raw data, thus requiring little or no feature engineering, thanks to their ability to model complex functions and identify relevant aspects in the data distribution. DL algorithms are based on Artificial Neural Networks (ANNs), which are inspired by the human brain and can model very complex functions, identifying important aspects in the features and suppressing irrelevant ones. As an example of a DL algorithm, a Convolutional Neural Network (CNN) is composed of nodes organized into layers. It can take an image as input, elaborate the features of the image through its layers, and assign a class attribution as output, thus differentiating between two or more groups. |
| Supervised learning | A ML task defined through the use of labeled data sets for algorithm training. | The algorithms learn to give the right answer, as defined by the ground truth set, which has labels assigned to the data.An SVM performing the classification task is an example of an algorithm trained by supervised learning. |
| Unsupervised learning | A set of algorithms aimed to discover hidden patterns or data groupings without the need for human intervention. | In unsupervised learning, unlike supervised learning, there are no correct answers and the algorithm’s aim is to discover structures within variables. The algorithms work with unlabeled data.Common unsupervised methods include clustering algorithms and Principal Component Analysis (PCA). |
| Classification task | The algorithm is trained to predict a class label. | A classical example is the classification of patients affected by a disease vs. normal controls. The algorithm learns to associate input data with an output label in a supervised manner, and its results can be evaluated by metrics such as accuracy score. |
| Regression task | The algorithm is trained to predict the value of a continuous variable. | An example is the prediction of hippocampus volume as a numerical quantity. The algorithm learns to associate input data with an output value in a supervised manner, and its results can be evaluated by metrics such as the Root Mean Squared Error (RMSE). |
| Clustering | Clustering consists of partitioning a data set, in order to find a grouping of the data points. | Clustering is one of the most important unsupervised learning techniques. Its main goal is to reveal sub-groups within heterogeneous data, in such a way that greater homogeneity is shown within clusters (rather than between clusters). Clustering algorithms can lead to the identification of patterns across subjects or patients that are difficult to find even for an expert clinician. |
| Overfitting | Overfitting occurs when the model is too dependent on training data to make accurate predictions on test data. | When a model is overfitted, its learned ability to separate between two classes does not generalize well to data it has never seen before, therefore limiting its usability for real-world applications. |
| Ensemble learning | Model ensembling consists of combining multiple ML models, in order to obtain better predictive performance than any of the constituents alone. | A single model alone can be weak in generating predictions. Combining multiple models can compensate for their individual weaknesses. |
| Transfer learning | A supervised learning technique in which the knowledge previously acquired from the model in one task is used to solve related ones. | In a transfer learning approach, the model is first pre-trained on a source task, then re-trained and tested on a target task. The source task should be related to the target task, with similar relations between the input and output data. In fact, in the pre-training phase, the model gains helpful knowledge for the target task. |
| Cox regression | The Cox proportional hazards model is a regression technique for investigating the association between the time of an event occurring and one or more predictor variables. | Cox regression gives hazard rates as measures of how factors influence the risk for an event occurrence (outcome), be it death or infection. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fabrizio, C.; Termine, A.; Caltagirone, C.; Sancesario, G. Artificial Intelligence for Alzheimer’s Disease: Promise or Challenge? Diagnostics 2021, 11, 1473. https://doi.org/10.3390/diagnostics11081473
Fabrizio C, Termine A, Caltagirone C, Sancesario G. Artificial Intelligence for Alzheimer’s Disease: Promise or Challenge? Diagnostics. 2021; 11(8):1473. https://doi.org/10.3390/diagnostics11081473
Chicago/Turabian StyleFabrizio, Carlo, Andrea Termine, Carlo Caltagirone, and Giulia Sancesario. 2021. "Artificial Intelligence for Alzheimer’s Disease: Promise or Challenge?" Diagnostics 11, no. 8: 1473. https://doi.org/10.3390/diagnostics11081473
APA StyleFabrizio, C., Termine, A., Caltagirone, C., & Sancesario, G. (2021). Artificial Intelligence for Alzheimer’s Disease: Promise or Challenge? Diagnostics, 11(8), 1473. https://doi.org/10.3390/diagnostics11081473






