Ultrasound Measurement of Tumor-Free Distance from the Serosal Surface as the Alternative to Measuring the Depth of Myometrial Invasion in Predicting Lymph Node Metastases in Endometrial Cancer
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Ultrasound Examination
2.3. Surgical Procedures
2.4. Sentinel Lymph Node Identification
2.5. Specimens and Samples
2.6. Statistical Analysis
3. Results
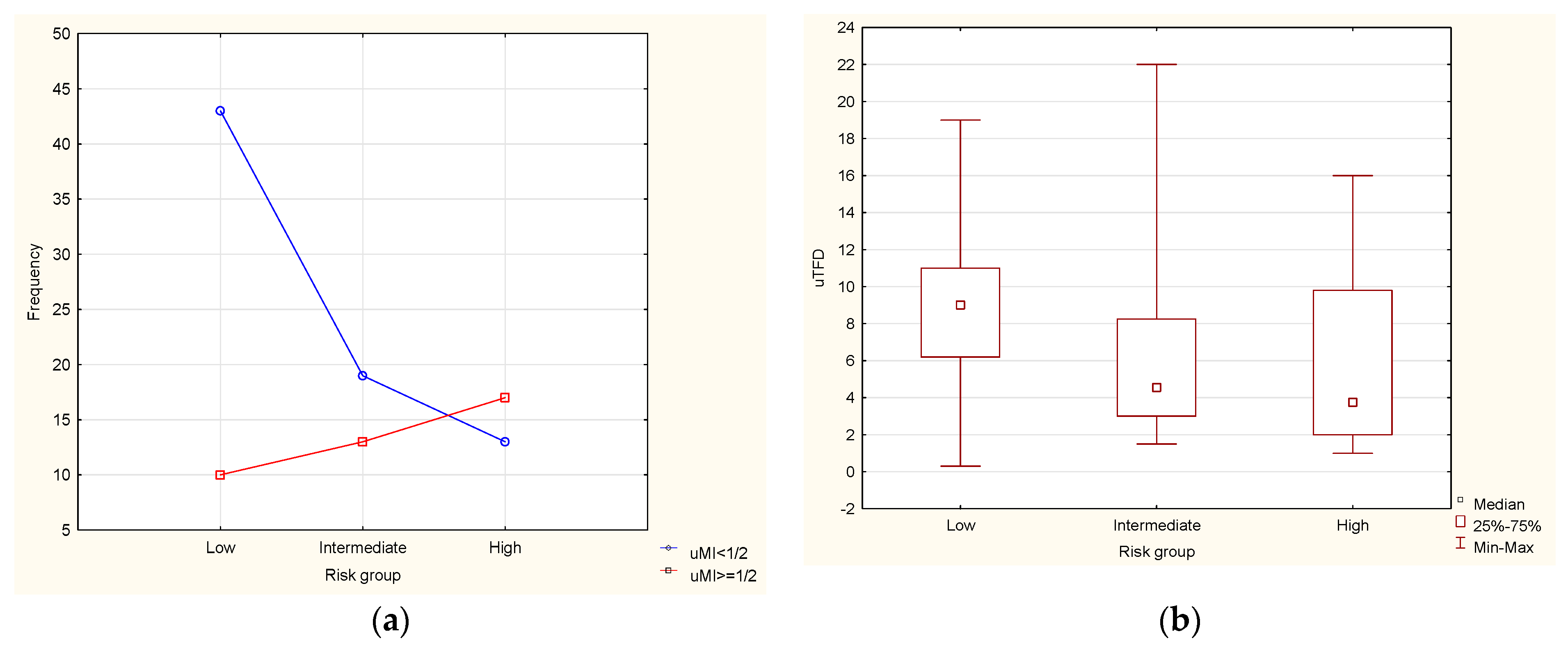
Survival Analysis and Ultrasound Biomarkers
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bray, F.; Jemal, A.; Grey, N.; Ferlay, J.; Forman, D. Global cancer transitions according to the Human Development Index (2008–2030): A population-based study. Lancet Oncol. 2012, 13, 790–801. [Google Scholar] [CrossRef]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [Green Version]
- Bokhman, J.V. Two pathogenetic types of endometrial carcinoma. Gynecol. Oncol. 1983, 15, 10–17. [Google Scholar] [CrossRef]
- Colombo, N.; Preti, E.; Landoni, F.; Carinelli, S.; Colombo, A.; Marini, C.; Sessa, C.; ESMO Guidelines Working Group. Endometrial cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2013, 24, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Colombo, N.; Creutzberg, C.; Amant, F.; Bosse, T.; González-Martín, A.; Ledermann, J.; Marth, C.; Nout, R.; Querleu, D.; Mirza, M.R.; et al. ESMO-ESGO-ESTRO Endometrial Consensus Conference Working Group Collaborators. ESMO-ESGO-ESTRO Consensus Conference on Endometrial Cancer: Diagnosis, treatment and follow-up. Ann. Oncol. 2016, 27, 16–41. [Google Scholar] [CrossRef]
- Concin, N.; Matias-Guiu, X.; Vergote, I.; Cibula, D.; Mirza, M.R.; Marnitz, S.; Ledermann, J.; Bosse, T.; Chargari, C.; Fagotti, A.; et al. ESGO/ESTRO/ESP guidelines for the management of patients with endometrial carcinoma. Radiother. Oncol. 2021, 154, 327–353. [Google Scholar] [CrossRef] [PubMed]
- Leone, F.P.G.; Timmermann, D.; Bourne, T.; Valentin, L.; Epstein, E.; Goldstein, S.R.; Marret, H.; Parsons, A.K.; Gull, B.; Istre, O.; et al. Terms, definitions and measurements to describe the sonographic features of the endometrium and intrauterine lesions: A consensus opinion from the International Endometrial Tumor Analysis (IETA) group. Ultrasound Obstet. Gynecol. 2010, 35, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Van den Bosch, T.; Dueholm, M.; Leone, F.P.G.; Valentin, L.; Rasmussen, C.K.; Votino, A.; Van Schoubroeck, D.; Landolfo, C.; Installé, A.J.F.; Guerriero, S.; et al. Terms, definitions and measurements to describe sonographic features of myometrium and uterine masses: A consensus opinion from the Morphological Uterus Sonographic Assessment (MUSA) group. Ultrasound Obstet. Gynecol. 2015, 46, 284–298. [Google Scholar] [CrossRef]
- Creasman, W.T.; Morrow, C.P.; Bundy, B.N.; Homesley, H.D.; Graham, J.E.; Heller, P.B. Surgical pathologic spread patterns of endometrial cancer. A Gynecologic Oncology Group Study. Cancer 1987, 15, 2035–2041. [Google Scholar] [CrossRef]
- Lindauer, J.; Fowler, J.M.; Manolitsas, T.P.; Copeland, L.J.; Eaton, L.A.; Ramirez, L.A.; Cohn, D.E. Is there a prognostic difference between depth of myometrial invasion and the tumor-free distance from the uterine serosa in endometrial cancer? Gynecol. Oncol. 2003, 91, 547–951. [Google Scholar] [CrossRef]
- Gombolevskiy, V.; (Research and Practical Clinical Center for Diagnostics and Telemedicine Technologies of the Moscow Health Care Department, State Budget-Funded Health Care Institution of the City of Moscow, Moscow, Russia). Personal communication, 2021.
- Alcázar, J.L.; Galván, R.; Albela, S.; Martinez, S.; Pahisa, J.; Jurado, M.; López-García, G. Assessing myometrial infiltration by endometrial cancer: Uterine virtual navigation with three-dimensional US. Radiology 2009, 250, 776–783. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schwab, K.V.; O’Malley, D.M.; Fowler, J.M.; Copeland, L.J.; Cohn, D.E. Prospective evaluation of prognostic significance of the tumor-free distance from uterine serosa in surgically staged endometrial adenocarcinoma. Gynecol. Oncol. 2009, 112, 146–149. [Google Scholar] [CrossRef]
- Kondalsamy-Chennakesavan, S.; van Vugt, S.; Sanday, K.; Nicklin, J.; Land, R.; Perrin, L.; Crandon, A.; Obermair, A. Evaluation of tumor-free distance and depth of myometrial invasion as prognostic factors for lymph node metastases in endometrial cancer. Int. J. Gynecol. Cancer 2010, 20, 1217–1221. [Google Scholar] [CrossRef] [PubMed]
- Geels, Y.P.; Pijnenborg, J.M.; van den Berg-van Erp, S.H.; Snijders, M.P.; Bulten, J.; Massuger, L.F. Absolute depth of myometrial invasion in endometrial cancer is superior to the currently used cut-off value of 50%. Gynecol. Oncol. 2013, 129, 285–291. [Google Scholar] [CrossRef] [PubMed]
- Mariani, A.; Dowdy, S.C.; Keeney, G.L.; Long, H.J.; Lesnick, T.G.; Podratz, K.C. High-risk endometrial cancer subgroups: Candidates for target-based adjuvant therapy. Gynecol. Oncol. 2004, 95, 120–126. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, M.; Aoki, Y.; Kase, H.; Fujita, K.; Tanaka, K. Low risk endometrial cancer: A study of pelvic lymph node metastasis. Int. J. Gynecol. Cancer 2003, 13, 38–41. [Google Scholar] [CrossRef] [PubMed]
- Hertel, J.D.; Heuttner, P.C.; Pfeifer, J.D. Lymphovascular space invasion in microcystic elongated and fragmented (MELF)-pattern well-differentiated endometrioid adenocarcinoma is associated with a higher rate of lymph node metastasis. Int. J. Gynecol. Pathol. 2014, 33, 127–134. [Google Scholar] [CrossRef]
- Korkmaz, V.; Meydanli, M.M.; Yalçın, I.; Sarı, M.E.; Sahin, H.; Kocaman, E.; Haberal, A.; Dursun, P.; Güngör, T.; Ayhan, A. Comparison of three different risk-stratification models for predicting lymph node involvement in endometrioid endometrial cancer clinically confined to the uterus. J. Gynecol. Oncol. 2017, 28, e78. [Google Scholar] [CrossRef] [Green Version]
- Meydanli, M.M.; Aslan, K.; Oz, M.; Muftuoglu, K.H.; Yalçin, I.; Engin-Ustun, Y. A novel multivariable prediction model for lymphatic dissemination in endometrioid endometrial cancer: The lymph node Metastasis Risk Index. Eur. J. Obstet. Gynecol. Reprod. Biol. 2019, 240, 310–315. [Google Scholar] [CrossRef]
- Reyes-Baez, F.E.; Garzon, S.; Mariani, A. Lumping and splitting: The need for precision medicine and “personomics” in endometrial cancer. J. Gynecol. Oncol. 2021, 32, e38. [Google Scholar] [CrossRef]
- Liro, M.; Śniadecki, M.; Wycinka, E.; Wojtylak, S.; Bianek, A.; Sawicki, S.; Wydra, D. The ultrasound-measured tumor-free distance (uTDF) is a valuable predictor of of lymph node status in endometrial cancer. Supplement: Abstracts of the 24th World Congress on Ultrasound in Obstetrics and Gynecology, Barcelona, Spain, 14–17 September 2014. Ultrasound. Obstet. Gynecol. 2014, 44, 15, Abstract Number OC06.06. [Google Scholar] [CrossRef]
- World Medical Association Declaration of Helsinki. Recommendations guiding physicians in biomedical research involving human subjects. JAMA 1997, 277, 925–926. [Google Scholar] [CrossRef]
- Cohen, J.F.; Korevaar, D.A.; Altman, D.G.; Bruns, D.E.; Gatsonis, C.A.; Hooft, L.; Irwig, L.; Levine, D.; Reitsma, J.B.; De Vet, H.C.W.; et al. STARD 2015 guidelines for reporting diagnostic accuracy studies: Explanation and elaboration. BMJ Open 2016, 6, e012799. [Google Scholar] [CrossRef] [PubMed]
- Pecorelli, S.; FIGO Committee on Gynecologic Oncology. Revised FIGO staging for carcinoma of the vulva, cervix and endometrium. Int. J. Gynaecol. Obstet. 2009, 105, 103–104. [Google Scholar] [CrossRef] [PubMed]
- Mariani, A.; Dowdy, S.C.; Cliby, W.A.; Gostout, B.S.; Jones, M.B.; Wilson, T.O.; Podratz, K.C. Prospective assessment of lymphatic dissemination in endometrial cancer: A paradigm shift in surgical staging. Gynecol. Oncol. 2008, 109, 11–18. [Google Scholar] [CrossRef] [Green Version]
- Savelli, L.; Ceccarini, M.; Ludovisi, M.; Fruscella, E.; De Iaco, P.A.; Salizzoni, E.; Mabrouk, M.; Manfredi, R.; Testa, A.C.; Ferrandina, G. Preoperative local staging of endometrial cancer: Transvaginal sonography versus magnetic resonance imaging. Ultrasound Obstet. Gynecol. 2008, 31, 560–568. [Google Scholar] [CrossRef] [PubMed]
- Naftalin, J.; Nunes, N.; Hoo, W.; Arora, R.; Jurkovic, D. Endometrial cancer and ultrasound: Why measuring endometrial thickness is sometimes not enough. Ultrasound Obstet. Gynecol. 2012, 39, 106–109. [Google Scholar] [CrossRef]
- Barretta, R.; Merisio, C.; Piantelli, G.; Rolla, M.; Giordano, G.; Melpignano, M.; Nardelli, G.B. Preoperative transvaginal ultrasonography and intraoperative gross examination for assessing myometrial invasion by endometrial cancer. J. Ultrasound Med. 2008, 27, 349–355. [Google Scholar] [CrossRef]
- Su, M.T.; Su, R.M.; Yue, C.T.; Chou, C.Y.; Hsu, C.C.; Chang, F.M. Three-dimensional transvaginal ultrasound provides clearer delineation of myometrial invasion in a patient with endometrial cancer and uterine leiomyoma. Ultrasound Obstet. Gynecol. 2003, 22, 434–436. [Google Scholar] [CrossRef]
- Eriksson, L.S.; Lindqvist, P.G.; Flöter Rådestad, A.; Dueholm, M.; Fischerova, D.; Franchi, D.; Jokubkiene, L.; Leone, F.P.; Savelli, L.; Sladkevicius, P.; et al. Transvaginal ultrasound assessment of myometrial and cervical stroma invasion in women with endometrial cancer: Interobserver reproducibility among ultrasound experts and gynecologists. Ultrasound Obstet. Gynecol. 2015, 45, 476–482. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Williams, J.W.; Hirschowitz, L. Assessment of uterine wall thickness and position of the vascular plexus in the deep myometrium: Implications for the measurement of depth of myometrial invasion of endometrial carcinomas. Int. J. Gynecol. Pathol. 2006, 25, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Pineda, L.; Alcázar, J.L.; Caparrós, M.; Mínguez, J.A.; Idoate, M.A.; Quiceno, H.; Solórzano, J.L.; Jurado, M. Agreement between preoperative transvaginal ultrasound and intraoperative macroscopic examination for assessing myometrial infiltration in low-risk endometrioid carcinoma. Ultrasound Obstet. Gynecol. 2016, 47, 369–373. [Google Scholar] [CrossRef] [Green Version]
- Liu, Z.Z.; Jiang, Y.X.; Dai, Q.; Yang, M.; Zhu, Q.L.; Zhao, D.C.; Gao, P. Imaging of endometrial carcinoma using contrast-enhanced sonography. J. Ultrasound Med. 2011, 30, 1519–1527. [Google Scholar] [CrossRef] [PubMed]
- Alcázar, J.L.; Galán, M.J.; Jurado, M.; López-García, G. Intratumoral blood flow analysis in endometrial carcinoma: Correlation with tumor characteristics and risk for recurrence. Gynecol. Oncol. 2002, 84, 258–262. [Google Scholar] [CrossRef] [PubMed]
- Fischerova, D.; Frühauf, F.; Zikan, M.; Kocián, R.; Dundr, P.; Nemejcova, K.; Dusek, L.; Cibula, D. Factors affecting sonographic preoperative local staging of endometrial cancer. Ultrasound Obstet. Gynecol. 2014, 43, 575–585. [Google Scholar] [CrossRef] [PubMed]
- De Smet, F.; De Brabanter, J.; Van den Bosch, T.; Pochet, N.; Amant, F.; Van Holsbeke, C.; Moerman, P.; De Moor, B.; Vergote, I.; Timmerman, D. New models to predict depth of infiltration in endometrial carcinoma based on transvaginal sonography. Ultrasound Obstet. Gynecol. 2006, 27, 664–671. [Google Scholar] [CrossRef] [PubMed]
- Van Gestel, T.; Suykens, J.; Baesens, B.; Viaene, S.; Vanthienen, J.; Dedene, G.; De Moor, B.; Vandewalle, J. Benchmarking least squares support vector machine classifiers. Mach. Learn. 2004, 54, 5–32. [Google Scholar] [CrossRef]
- Van Holsbeke, C.; Ameye, L.; Testa, A.C.; Mascilini, F.; Lindqvist, P.; Fischerova, D.; Frühauf, F.; Fransis, S.; Jonge, E.; Timmerman, D.; et al. Development and external validation of new mathematical models for preoperative prediction of high-risk endometrial cancer. Ultrasound Obstet. Gynecol. 2014, 43, 586–595. [Google Scholar] [CrossRef] [Green Version]
- Karlsson, B.; Norström, A.; Granberg, S.; Wikland, M. The use of endovaginal ultrasound to diagnose invasion of endometrial cancer. Ultrasound Obstet. Gynecol. 1992, 2, 35–39. [Google Scholar] [CrossRef]
- Valentin, L. Ultrasound deserves to play a prominent role in the diagnosis and management of endometrial cancer. Ultrasound Obstet. Gynecol. 2014, 43, 483–487. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Antonsen, S.L.; Jensen, L.N.; Loft, A.; Berthelsen, A.K.; Costa, J.; Tabor, A.; Qvist, I.; Hansen, M.R.; Fisker, R.; Andersen, E.S.; et al. MRI, PET/CT and ultrasound in the preoperative staging of endometrial cancer—A multicenter prospective comparative study. Gynecol. Oncol. 2013, 128, 300–308. [Google Scholar] [CrossRef] [Green Version]
- Koplay, M.; Dogan, N.U.; Erdogan, H.; Sivri, M.; Erol, C.; Nayman, A.; Karabagli, P.; Paksoy, Y.; Celik, C. Diagnostic efficacy of diffusion-weighted MRI for pre-operative assessment of myometrial and cervical invasion and pelvic lymph node metastasis in endometrial carcinoma. J. Med. Imaging Radiat. Oncol. 2014, 58, 538–546. [Google Scholar] [CrossRef]
- Signorelli, M.; Crivellaro, C.; Buda, A.; Guerra, L.; Fruscio, R.; Elisei, F.; Dolci, C.; Cuzzocrea, M.; Milani, R.; Messa, C. Staging of high-risk endometrial cancer with PET/CT and sentinel lymph node mapping. Clin. Nucl. Med. 2015, 40, 780–785. [Google Scholar] [CrossRef]
- Akbayir, O.; Corbacioglu, A.; Goksedef, B.P.C.; Numanoglu, C.; Akca, A.; Guraslan, H.; Bakir, L.V.; Cetin, A. The novel criteria for predicting pelvic lymph node metastasis in endometrioid adenocarcinoma of endometrium. Gynecol. Oncol. 2012, 125, 400–403. [Google Scholar] [CrossRef]
- Dueholm, M.; Møller, C.; Rydbjerg, S.; Hansen, E.S.; Ørtoft, G. An ultrasound algorithm for identification of endometrial cancer. Ultrasound Obstet. Gynecol. 2014, 43, 557–568. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Yamashita, T.; Ishikawa, M. Regulation of tumor invasion by HOXB13 gene overexpressed in human endometrial cancer. Oncol. Rep. 2005, 13, 721–726. [Google Scholar] [CrossRef]
- Gibson, D.A.; Saunders, P.T. Endocrine disruption of oestrogen action and female reproductive tract cancers. Endocr. Relat. Cancer 2014, 21, T13–T31. [Google Scholar] [CrossRef] [Green Version]
- Mitamura, T.; Watari, H.; Todo, Y.; Kato, T.; Konno, Y.; Hosaka, M.; Sakuragi, N. Lymphadenectomy can be omitted for low-risk endometrial cancer based on preoperative assessments. J. Gynecol. Oncol. 2014, 25, 301–305. [Google Scholar] [CrossRef] [Green Version]
- Imai, K.; Kato, H.; Katayama, K.; Nakanishi, K.; Kawano, A.; Iura, A.; Konnai, K.; Onose, R.; Hirahara, F.; Miyagi, E. A preoperative risk-scoring system to predict lymph node metastasis in endometrial cancer and stratify patients for lymphadenectomy. Gynecol. Oncol. 2016, 142, 273–277. [Google Scholar] [CrossRef] [PubMed]
- Todo, Y.; Watari, H.; Okamoto, K.; Hareyama, H.; Minobe, S.; Kato, H.; Sakuragi, N. Tumor volume successively reflects the state of disease progression in endometrial cancer. Gynecol. Oncol. 2013, 129, 472–477. [Google Scholar] [CrossRef] [PubMed]
- Todo, Y.; Choi, H.J.; Kang, S.; Kim, J.W.; Nam, J.H.; Watari, H.; Tamakoshi, A.; Sakuragi, N. Clinical significance of tumor volume in endometrial cancer: A Japan-Korea cooperative study. Gynecol. Oncol. 2013, 131, 294–298. [Google Scholar] [CrossRef] [PubMed]
- Murali, R.; Soslow, R.A.; Weigelt, B. Classification of endometrial carcinoma: More than two types. Lancet Oncol. 2014, 15, e268–e278. [Google Scholar] [CrossRef]
- Wortman, B.G.; Creutzberg, C.L.; Putter, H.; Jürgenliemk-Schulz, I.M.; Jobsen, J.J.; Lutgens, L.C.H.W.; van der Steen-Banasik, E.M.; Mens, J.W.M.; Slot, A.; Kroese, M.C.S.; et al. Ten-year results of the PORTEC-2 trial for high-intermediate risk endometrial carcinoma: Improving patient selection for adjuvant therapy. Br. J. Cancer 2018, 119, 1067–1074. [Google Scholar] [CrossRef] [Green Version]
- De Boer, S.M.; Powell, M.E.; Mileshkin, L.; Katsaros, D.; Bessette, P.; Haie-Meder, C.; Ottevanger, P.B.; Ledermann, J.A.; Khaw, P.; D’Amico, R.; et al. Adjuvant chemoradiotherapy versus radiotherapy alone in women with high-risk endometrial cancer (PORTEC-3): Patterns of recurrence and post-hoc survival analysis of a randomised phase 3 trial. Lancet Oncol. 2019, 20, 1273–1285. [Google Scholar] [CrossRef] [Green Version]
- Rocco, N.; Montagna, G.; Di Micco, R.; Benson, J.; Criscitiello, C.; Chen, L.; Di Pace, B.; Esgueva Colmenarejo, A.J.; Harder, Y.; Karakatsanis, A.; et al. The Impact of the COVID-19 Pandemic on Surgical Management of Breast Cancer: Global Trends and Future Perspectives. Oncologist 2021, 26, 66–77. [Google Scholar] [CrossRef] [PubMed]
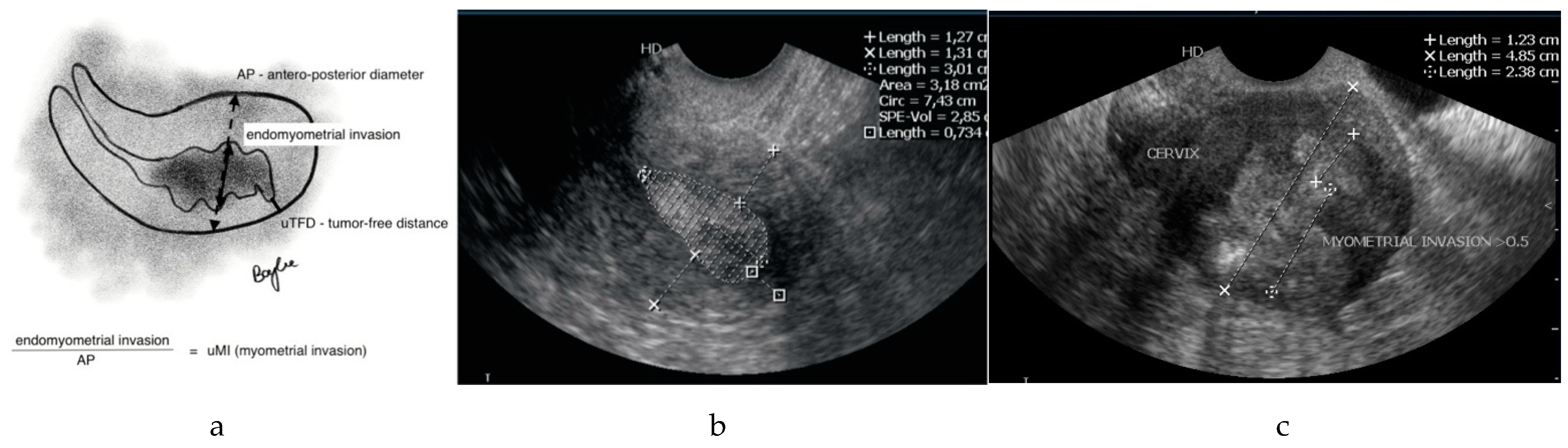

| Variable | Characteristic | Value |
|---|---|---|
| Age at diagnosis (range) | Mean ± SD (range) | 63 ± 8.3 (40–85) |
| FIGO stage * | Number (%) | |
| Ia | 69 (59) | |
| Ib | 35 (30) | |
| II | 5 (5) | |
| III | 7 (6) | |
| Histologic type | Number (%) | |
| Endometrioid | 82 (71) | |
| Endometrioid with epithelial differentiation | 20 (17) | |
| Serous carcinoma | 11 (9) | |
| Carcinosarcoma | 3 (3) | |
| Grade | Number (%) | |
| 1 | 41 (36) | |
| 2 | 45 (39) | |
| 3 | 28 (25) | |
| Risk grouping according to initial risk * | Number (%) | Number of patients with metastatic nodes (%) |
| Low | 54 (46) | 1 (2) |
| Intermediate | 32 (27.5) | 7 (22) |
| High | 30 (26) | 12 (40) |
| uTFD [mm] | Mean ± SD (range) | 7.39 ± 4.83 (0.3–22.0) |
| uMI | Number (%) | |
| <50% | 76 (66) | |
| ≥50% | 40 (34) | |
| Lymph node procedure | Number (%) | |
| SLNB only | 70 (60) | |
| LND (+SLNB) | 46 (40) | |
| Lymph nodes extracted | Number | 1298 |
| SLNB cases | Number (%) | 313 (24) |
| LND (+SLNB) cases | Number (%) | 985 (76) |
| Lymph nodes metastases | Number of patients (%) | 20 (17) |
| Distribution of nodes: | Number (%) | 34/1288 (2.64) |
| Obturator | 19 (7 SLN) | |
| Iliac nodes | 13 (2 SLN) | |
| Para-aortic | 2 | |
| Risk grouping according to ESGO–ESTRO–ESP guidelines * | Number (%) | Number of patients with metastatic nodes (%) |
| Low | 52 (45) | 0 (0) |
| Intermediate | 30 (26) | 4 (3) |
| High-intermediate | 21 (18) | 4 (3) |
| High | 13 (11) | 12 (10) |
| Advanced metastatic | 0 (0) | 0 (0) |
| Variable | OR (95% CI) | p Value | Significance (α = 0.05) | ACC | Specificity | Sensitivity | NPV | PPV | AUC | |
|---|---|---|---|---|---|---|---|---|---|---|
| Ultrasound parameter | (u)MI (≥50%) | 4.746 (1.710–13.174) | 0.0028 | Yes | 70.7% | 71.88% | 65.0% | 92.68% | 22.67% | 0.684 (0.568–0.801) |
| (u)TFD | 0.842 (0.736–0.963) | 0.0119 | Yes | 63.8% | 61.46% | 75.0% | 89.02% | 35.48% | 0.683 (0.563–0.803) | |
| Histologic parameter | (p)MI (≥50%) | 6.600 (2.196–19.833) | 0.0008 | Yes | 69.83% | 68.75% | 75.0% | 92.18% | 28.84% | 0.719 (0.611–0.827) |
| (p)TFD | 0.843 (0.747–0.950) | 0.0052 | Yes | 77.59% | 82.29% | 55.0% | 90.79% | 32.50% | 0.712 (0.577–0.846) | |
| Grading | G1 | 1 | 47.41% | 39.58% | 85.0% | 89.77% | 39.29% | |||
| G2 | 2.667 (0.657–10.825) | 0.1700 | No | 0.673 (0.550–0.791) | ||||||
| G3 | 5.700 (1.386–23.449) | 0.0159 | Yes | |||||||
| Cancer histology | Endometroid | 1 | 74.34% | 78.49% | 55.0% | 92.96% | 33.34% | |||
| Endometroid with squamous differentiation | 2.704 (0.793–9.216) | 0.111 | No | 0.685 (0.559–0.811) | ||||||
| Serous | 9.733 (2.463–38.459) | 0.001 | Yes | |||||||
| Variable | OR (95% CI) | p Value | Significance (α = 0.05) | ACC | Specificity | Sensitivity | NPV | PPV | AUC | |
|---|---|---|---|---|---|---|---|---|---|---|
| Multivariable analysis | ||||||||||
| Ultrasound parameter | (u)MI (≥50%) | 0.470 (0.060–3.667) | 0.471 | No | 74.13% | 77.08% | 60% | 90.24% | 35.29% | 0.722 (0.607–0.836) |
| (u)TFD | 0.950 (0.745–1.23) | 0.683 | No | |||||||
| (u)MI × (u)TFD | 0.938 (0.683–1.287) | 0.691 | No | |||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liro, M.; Śniadecki, M.; Wycinka, E.; Wojtylak, S.; Brzeziński, M.; Stańczak, A.; Wydra, D. Ultrasound Measurement of Tumor-Free Distance from the Serosal Surface as the Alternative to Measuring the Depth of Myometrial Invasion in Predicting Lymph Node Metastases in Endometrial Cancer. Diagnostics 2021, 11, 1472. https://doi.org/10.3390/diagnostics11081472
Liro M, Śniadecki M, Wycinka E, Wojtylak S, Brzeziński M, Stańczak A, Wydra D. Ultrasound Measurement of Tumor-Free Distance from the Serosal Surface as the Alternative to Measuring the Depth of Myometrial Invasion in Predicting Lymph Node Metastases in Endometrial Cancer. Diagnostics. 2021; 11(8):1472. https://doi.org/10.3390/diagnostics11081472
Chicago/Turabian StyleLiro, Marcin, Marcin Śniadecki, Ewa Wycinka, Szymon Wojtylak, Michał Brzeziński, Agata Stańczak, and Dariusz Wydra. 2021. "Ultrasound Measurement of Tumor-Free Distance from the Serosal Surface as the Alternative to Measuring the Depth of Myometrial Invasion in Predicting Lymph Node Metastases in Endometrial Cancer" Diagnostics 11, no. 8: 1472. https://doi.org/10.3390/diagnostics11081472
APA StyleLiro, M., Śniadecki, M., Wycinka, E., Wojtylak, S., Brzeziński, M., Stańczak, A., & Wydra, D. (2021). Ultrasound Measurement of Tumor-Free Distance from the Serosal Surface as the Alternative to Measuring the Depth of Myometrial Invasion in Predicting Lymph Node Metastases in Endometrial Cancer. Diagnostics, 11(8), 1472. https://doi.org/10.3390/diagnostics11081472







