Postmortem Cardiopulmonary Pathology in Patients with COVID-19 Infection: Single-Center Report of 12 Autopsies from Lausanne, Switzerland
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Postmortem Examination
2.3. Quantitative RT-qPCR for SARS-CoV-2 Detection in FFPE Tissues
2.4. Immunohistochemical Staining
2.5. In Situ Detection of SARS-CoV-2 mRNA in FFPE Tissues
2.6. Immunofluorescence Staining
3. Results
3.1. Patient Cohort
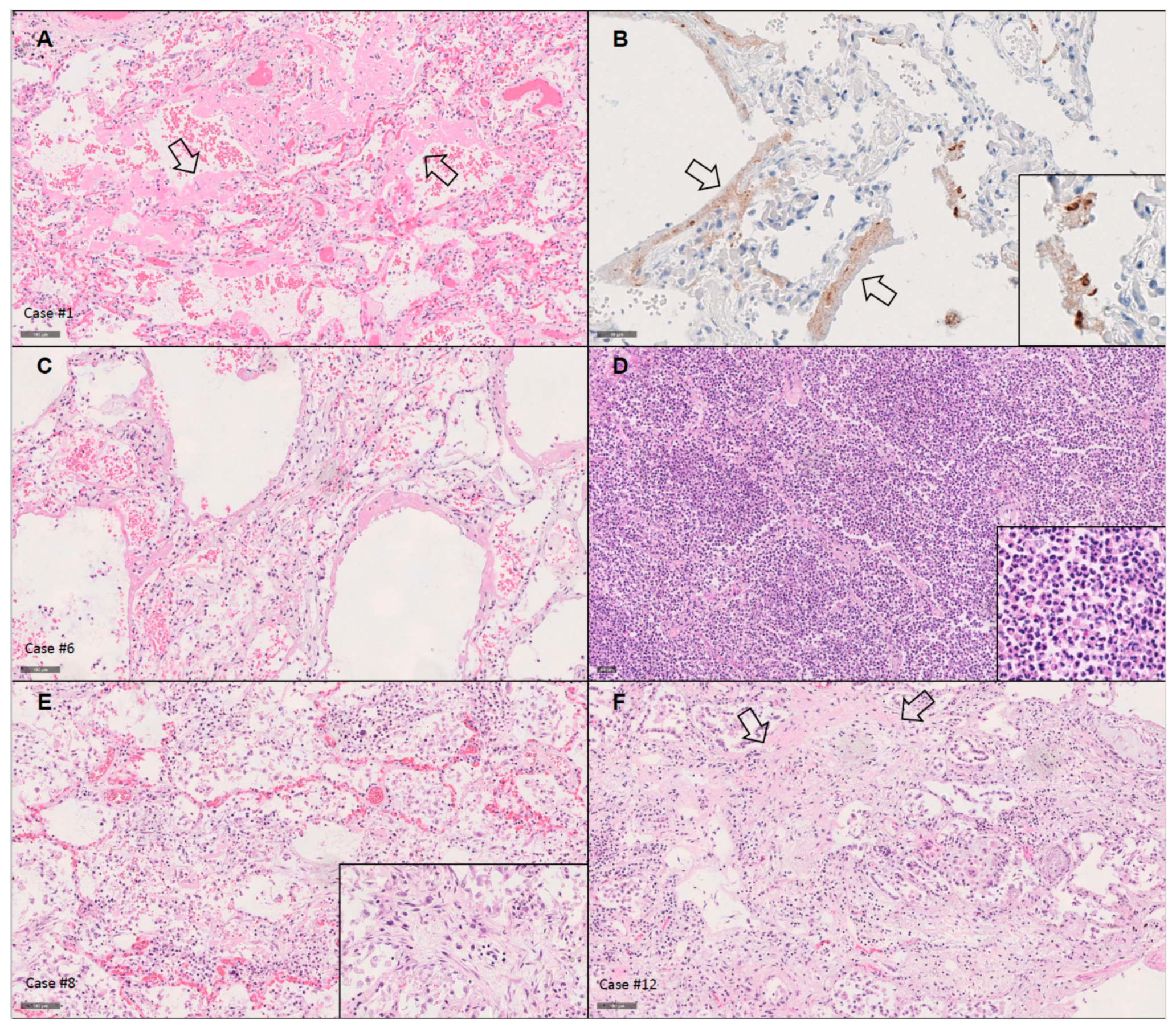
3.2. Histopathological Findings
3.2.1. Lungs
3.2.2. Heart
3.2.3. Other Significant Findings
3.3. Detection of SARS-CoV-2 Using RT-qPCR
3.3.1. Establishing the Methodology
3.3.2. Virus Detection Using RT-qPCR in Lung and Heart
3.4. Detection of SARS-CoV-2 Using Immunohistochemistry
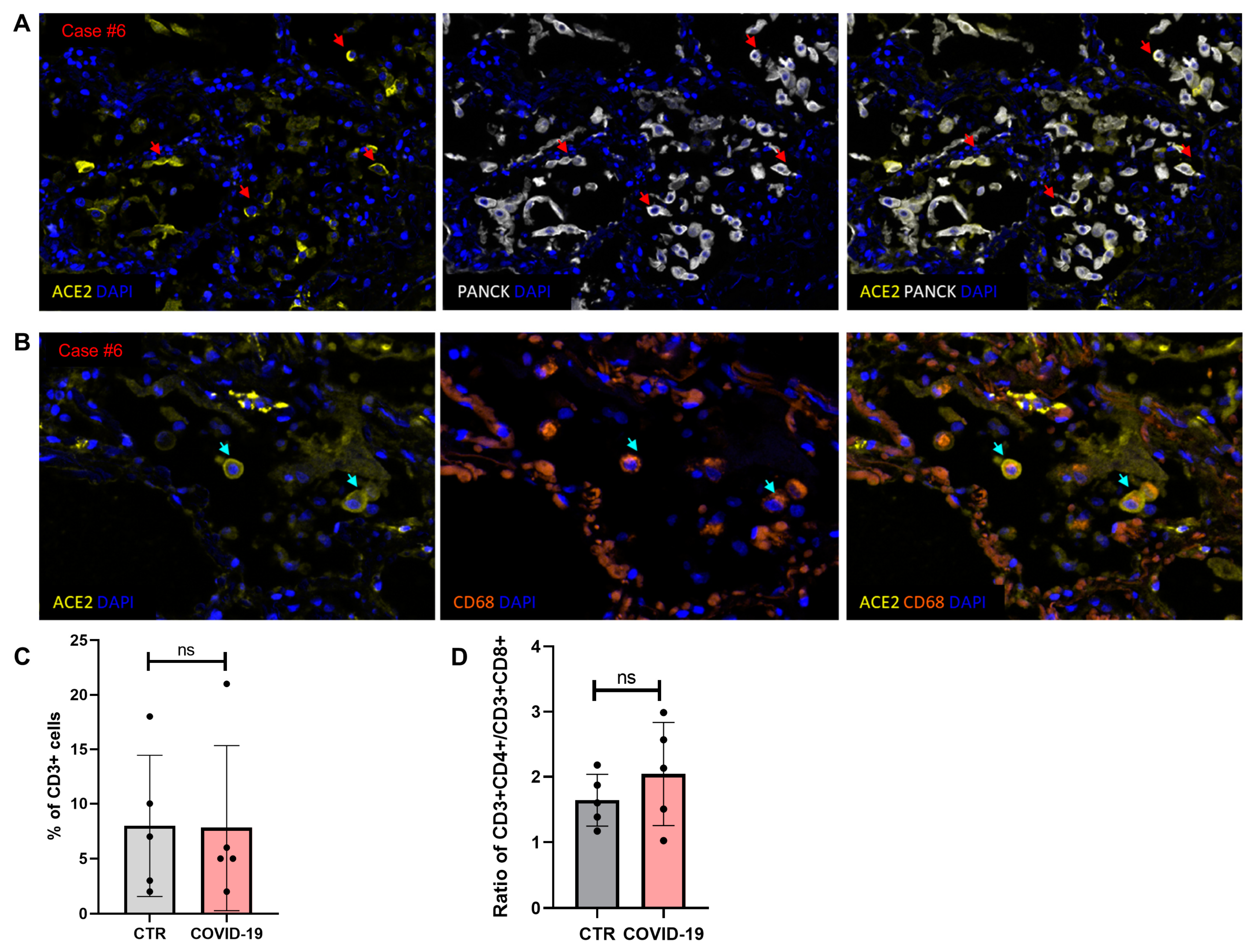
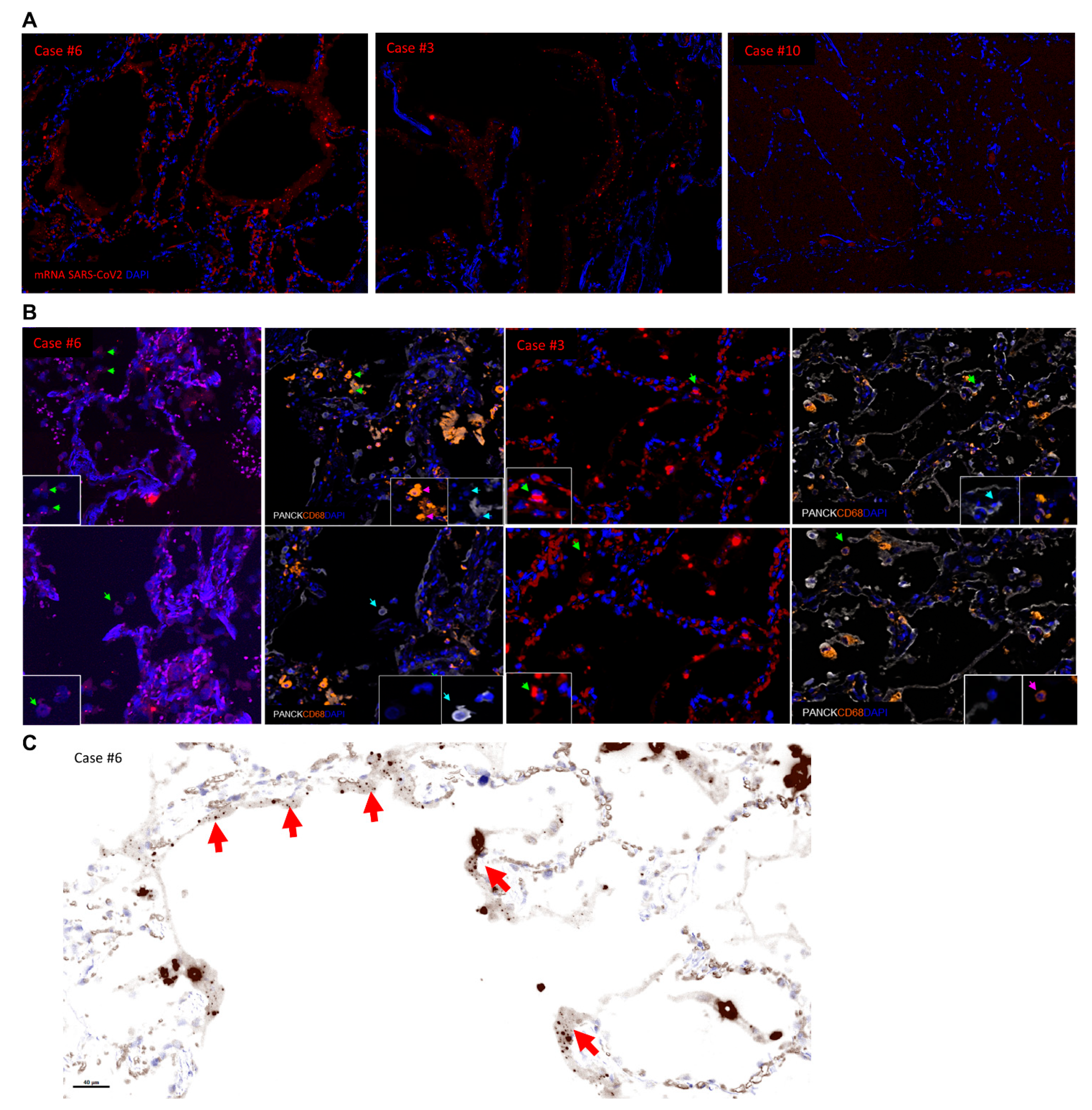
3.5. Multiplex Imaging and In Situ Detection of SARS-CoV-2 mRNA
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wu, Z.; McGoogan, J.M. Characteristics of and Important Lessons From the Coronavirus Disease 2019 (COVID-19) Outbreak in China: Summary of a Report of 72314 Cases From the Chinese Center for Disease Control and Prevention. JAMA 2020, 323, 1239–1242. [Google Scholar] [CrossRef]
- Available online: https://coronavirus.jhu.edu/map.html (accessed on 19 May 2021).
- Fox, S.E.; Akmatbekov, A.; Harbert, J.L.; Li, G.; Quincy Brown, J.; Vander Heide, R.S. Pulmonary and cardiac pathology in African American patients with COVID-19: An autopsy series from New Orleans. Lancet Respir. Med. 2020, 8, 681–686. [Google Scholar] [CrossRef]
- Borczuk, A.C.; Salvatore, S.P.; Seshan, S.V.; Patel, S.S.; Bussel, J.B.; Mostyka, M.; Elsoukkary, S.; He, B.; Del Vecchio, C.; Fortarezza, F.; et al. COVID-19 pulmonary pathology: A multi-institutional autopsy cohort from Italy and New York City. Mod. Pathol. 2020, 33, 2156–2168. [Google Scholar] [CrossRef]
- Calabrese, F.; Pezzuto, F.; Fortarezza, F.; Hofman, P.; Kern, I.; Panizo, A.; von der Thusen, J.; Timofeev, S.; Gorkiewicz, G.; Lunardi, F. Pulmonary pathology and COVID-19: Lessons from autopsy. The experience of European Pulmonary Pathologists. Virchows Arch. 2020, 477, 359–372. [Google Scholar] [CrossRef]
- Konopka, K.E.; Nguyen, T.; Jentzen, J.M.; Rayes, O.; Schmidt, C.J.; Wilson, A.M.; Farver, C.F.; Myers, J.L. Diffuse alveolar damage (DAD) resulting from coronavirus disease 2019 Infection is Morphologically Indistinguishable from Other Causes of DAD. Histopathology 2020, 77, 570–578. [Google Scholar] [CrossRef]
- McMullen, P.; Pytel, P.; Snyder, A.; Smith, H.; Vickery, J.; Brainer, J.; Guzy, R.; Wu, D.; Schoettler, N.; Adegunsoye, A.; et al. A series of COVID-19 autopsies with clinical and pathologic comparisons to both seasonal and pandemic influenza. J. Pathol. Clin. Res. 2021. [Google Scholar] [CrossRef]
- Satturwar, S.; Fowkes, M.; Farver, C.; Wilson, A.M.; Eccher, A.; Girolami, I.; Pujadas, E.; Bryce, C.; Salem, F.; El Jamal, S.M.; et al. Postmortem Findings Associated With SARS-CoV-2: Systematic Review and Meta-analysis. Am. J. Surg. Pathol. 2021, 45, 587–603. [Google Scholar] [CrossRef] [PubMed]
- Grosse, C.; Grosse, A.; Salzer, H.J.F.; Dunser, M.W.; Motz, R.; Langer, R. Analysis of cardiopulmonary findings in COVID-19 fatalities: High incidence of pulmonary artery thrombi and acute suppurative bronchopneumonia. Cardiovasc. Pathol. 2020, 49, 107–263. [Google Scholar] [CrossRef]
- Remmelink, M.; De Mendonca, R.; D’Haene, N.; De Clercq, S.; Verocq, C.; Lebrun, L.; Lavis, P.; Racu, M.L.; Trepant, A.L.; Maris, C.; et al. Unspecific post-mortem findings despite multiorgan viral spread in COVID-19 patients. Crit. Care 2020, 24, 495. [Google Scholar] [CrossRef] [PubMed]
- Sauter, J.L.; Baine, M.K.; Butnor, K.J.; Buonocore, D.J.; Chang, J.C.; Jungbluth, A.A.; Szabolcs, M.J.; Morjaria, S.; Mount, S.L.; Rekhtman, N.; et al. Insights into pathogenesis of fatal COVID-19 pneumonia from histopathology with immunohistochemical and viral RNA studies. Histopathology 2020, 77, 915–925. [Google Scholar] [CrossRef] [PubMed]
- Yao, X.H.; Luo, T.; Shi, Y.; He, Z.C.; Tang, R.; Zhang, P.P.; Cai, J.; Zhou, X.D.; Jiang, D.P.; Fei, X.C.; et al. A cohort autopsy study defines COVID-19 systemic pathogenesis. Cell Res. 2021. [Google Scholar] [CrossRef]
- Buja, L.M.; Zhao, B.; McDonald, M.; Ottaviani, G.; Wolf, D.A. Commentary on the spectrum of cardiopulmonary pathology in COVID-19. Cardiovasc Pathol 2021, 53, 107339. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Drosten, C.; Seifried, E.; Roth, W.K. TaqMan 5′-nuclease human immunodeficiency virus type 1 PCR assay with phage-packaged competitive internal control for high-throughput blood donor screening. J. Clin. Microbiol. 2001, 39, 43024308. [Google Scholar] [CrossRef]
- Ioannidou, K.; Ndiaye, D.R.; Noto, A.; Fenwick, C.; Fortis, S.P.; Pantaleo, G.; Petrovas, C.; de Leval, L. In Situ Characterization of Follicular Helper CD4 T Cells Using Multiplexed Imaging. Front. Immunol. 2020, 11, 607–626. [Google Scholar] [CrossRef]
- Koehler, P.; Bassetti, M.; Chakrabarti, A.; Chen, S.C.A.; Colombo, A.L.; Hoenigl, M.; Klimko, N.; Lass-Florl, C.; Oladele, R.O.; Vinh, D.C.; et al. Defining and managing COVID-19-associated pulmonary aspergillosis: The 2020 ECMM/ISHAM consensus criteria for research and clinical guidance. Lancet. Infect. Dis. 2020, 21, 149–162. [Google Scholar] [CrossRef]
- Corman, V.M.; Landt, O.; Kaiser, M.; Molenkamp, R.; Meijer, A.; Chu, D.K.; Bleicker, T.; Brunink, S.; Schneider, J.; Schmidt, M.L.; et al. Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. Euro. Surveill. 2020, 25, 2000045. [Google Scholar] [CrossRef]
- Nalla, A.K.; Casto, A.M.; Huang, M.W.; Perchetti, G.A.; Sampoleo, R.; Shrestha, L.; Wei, Y.; Zhu, H.; Jerome, K.R.; Greninger, A.L. Comparative Performance of SARS-CoV-2 Detection Assays Using Seven Different Primer-Probe Sets and One Assay Kit. J. Clin. Microbiol. 2020, 58, e00557-20. [Google Scholar] [CrossRef]
- Vogels, C.B.F.; Brito, A.F.; Wyllie, A.L.; Fauver, J.R.; Ott, I.M.; Kalinich, C.C.; Petrone, M.E.; Casanovas-Massana, A.; Catherine Muenker, M.; Moore, A.J.; et al. Analytical sensitivity and efficiency comparisons of SARS-CoV-2 RT-qPCR primer-probe sets. Nat. Microbiol. 2020, 5, 1299–1305. [Google Scholar] [CrossRef]
- von Stillfried, S.; Villwock, S.; Bulow, R.D.; Djudjaj, S.; Buhl, E.M.; Maurer, A.; Ortiz-Bruchle, N.; Celec, P.; Klinkhammer, B.M.; Wong, D.W.L.; et al. SARS-CoV-2 RNA screening in routine pathology specimens. Microb. Biotechnol. 2021. [Google Scholar] [CrossRef]
- Schaefer, I.M.; Padera, R.F.; Solomon, I.H.; Kanjilal, S.; Hammer, M.M.; Hornick, J.L.; Sholl, L.M. In situ detection of SARS-CoV-2 in lungs and airways of patients with COVID-19. Mod. Pathol. 2020, 33, 2104–2114. [Google Scholar] [CrossRef] [PubMed]
- Lean, F.Z.X.; Lamers, M.M.; Smith, S.P.; Shipley, R.; Schipper, D.; Temperton, N.; Haagmans, B.L.; Banyard, A.C.; Bewley, K.R.; Carroll, M.W.; et al. Development of immunohistochemistry and in situ hybridisation for the detection of SARS-CoV and SARS-CoV-2 in formalin-fixed paraffin-embedded specimens. Sci. Rep. 2020, 10, 21894. [Google Scholar] [CrossRef]
- Hoffmann, M.; Kleine-Weber, H.; Schroeder, S.; Kruger, N.; Herrler, T.; Erichsen, S.; Schiergens, T.S.; Herrler, G.; Wu, N.H.; Nitsche, A.; et al. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell 2020, 181, 271–280 e278. [Google Scholar] [CrossRef]
- Putlyaeva, L.V.; Lukyanov, K.A. Studying SARS-CoV-2 with Fluorescence Microscopy. Int. J. Mol. Sci. 2021, 22, 6558. [Google Scholar] [CrossRef]
- Xu, Z.; Shi, L.; Wang, Y.; Zhang, J.; Huang, L.; Zhang, C.; Liu, S.; Zhao, P.; Liu, H.; Zhu, L.; et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet. Respir. Med. 2020, 8, 420–422. [Google Scholar] [CrossRef]
- Zhang, H.; Zhou, P.; Wei, Y.; Yue, H.; Wang, Y.; Hu, M.; Zhang, S.; Cao, T.; Yang, C.; Li, M.; et al. Histopathologic Changes and SARS-CoV-2 Immunostaining in the Lung of a Patient With COVID-19. Ann. Intern. Med. 2020, 172, 629–632. [Google Scholar] [CrossRef] [PubMed]
- American Thoracic, S.; European Respiratory, S. American Thoracic Society/European Respiratory Society International Multidisciplinary Consensus Classification of the Idiopathic Interstitial Pneumonias. This joint statement of the American Thoracic Society (ATS), and the European Respiratory Society (ERS) was adopted by the ATS board of directors, June 2001 and by the ERS Executive Committee, June 2001. Am. J. Respir. Crit. Care. Med. 2002, 165, 277–304. [Google Scholar] [CrossRef]
- Travis, W.D.; Costabel, U.; Hansell, D.M.; King, T.E., Jr.; Lynch, D.A.; Nicholson, A.G.; Ryerson, C.J.; Ryu, J.H.; Selman, M.; Wells, A.U.; et al. An official American Thoracic Society/European Respiratory Society statement: Update of the international multidisciplinary classification of the idiopathic interstitial pneumonias. Am. J. Respir. Crit. Care Med. 2013, 188, 733–748. [Google Scholar] [CrossRef]
- Nicholson, A.G.; Osborn, M.; Devaraj, A.; Wells, A.U. COVID-19 related lung pathology: Old patterns in new clothing? Histopathology 2020, 77, 169–172. [Google Scholar] [CrossRef]
- von Stillfried, S.; Boor, P. Detection methods for SARS-CoV-2 in tissue. Pathologe 2021. [Google Scholar] [CrossRef]
- Grant, R.A.; Morales-Nebreda, L.; Markov, N.S.; Swaminathan, S.; Querrey, M.; Guzman, E.R.; Abbott, D.A.; Donnelly, H.K.; Donayre, A.; Goldberg, I.A.; et al. Circuits between infected macrophages and T cells in SARS-CoV-2 pneumonia. Nature 2021, 590, 635–641. [Google Scholar] [CrossRef]
- Abassi, Z.; Knaney, Y.; Karram, T.; Heyman, S.N. The Lung Macrophage in SARS-CoV-2 Infection: A Friend or a Foe? Front. Immunol. 2020, 11, 1312. [Google Scholar] [CrossRef]
- Katzenstein, A.L.; Bloor, C.M.; Leibow, A.A. Diffuse alveolar damage—The role of oxygen, shock, and related factors. A review. Am. J. Pathol. 1976, 85, 209–228. [Google Scholar]
- Varga, Z.; Flammer, A.J.; Steiger, P.; Haberecker, M.; Andermatt, R.; Zinkernagel, A.S.; Mehra, M.R.; Schuepbach, R.A.; Ruschitzka, F.; Moch, H. Endothelial cell infection and endotheliitis in COVID-19. Lancet 2020, 395, 1417–1418. [Google Scholar] [CrossRef]
- Ackermann, M.; Verleden, S.E.; Kuehnel, M.; Haverich, A.; Welte, T.; Laenger, F.; Vanstapel, A.; Werlein, C.; Stark, H.; Tzankov, A.; et al. Pulmonary Vascular Endothelialitis, Thrombosis, and Angiogenesis in Covid-19. N. Engl. J. Med. 2020, 383, 120–128. [Google Scholar] [CrossRef]
- Fortarezza, F.; Boscolo, A.; Pezzuto, F.; Lunardi, F.; Jesus Acosta, M.; Giraudo, C.; Del Vecchio, C.; Sella, N.; Tiberio, I.; Godi, I.; et al. Proven COVID-19-associated pulmonary aspergillosis in patients with severe respiratory failure. Mycoses 2021. [Google Scholar] [CrossRef] [PubMed]
- Kula, B.E.; Clancy, C.J.; Hong Nguyen, M.; Schwartz, I.S. Invasive mould disease in fatal COVID-19: A systematic review of autopsies. Lancet Microbe 2021. [Google Scholar] [CrossRef]
- Menter, T.; Haslbauer, J.D.; Nienhold, R.; Savic, S.; Hopfer, H.; Deigendesch, N.; Frank, S.; Turek, D.; Willi, N.; Pargger, H.; et al. Postmortem examination of COVID-19 patients reveals diffuse alveolar damage with severe capillary congestion and variegated findings in lungs and other organs suggesting vascular dysfunction. Histopathology 2020, 77, 198–209. [Google Scholar] [CrossRef]
- Van Hemelrijck, M.; Adolfsson, J.; Garmo, H.; Bill-Axelson, A.; Bratt, O.; Ingelsson, E.; Lambe, M.; Stattin, P.; Holmberg, L. Risk of thromboembolic diseases in men with prostate cancer: Results from the population-based PCBaSe Sweden. Lancet Oncol. 2010, 11, 450–458. [Google Scholar] [CrossRef]
- Lie, J.T.; Hammond, P.I. Pathology of the senescent heart: Anatomic observations on 237 autopsy studies of patients 90 to 105 years old. Mayo Clin. Proc. 1988, 63, 552–564. [Google Scholar] [CrossRef]
- Kholova, I.; Niessen, H.W. Amyloid in the cardiovascular system: A review. J. Clin. Pathol. 2005, 58, 125–133. [Google Scholar] [CrossRef] [PubMed]
- Ueda, M.; Horibata, Y.; Shono, M.; Misumi, Y.; Oshima, T.; Su, Y.; Tasaki, M.; Shinriki, S.; Kawahara, S.; Jono, H.; et al. Clinicopathological features of senile systemic amyloidosis: An ante- and post-mortem study. Mod. Pathol. 2011, 24, 1533–1544. [Google Scholar] [CrossRef] [PubMed]
| Antibody | Clone | Tissue Specificity | Species | Source | Dilution |
|---|---|---|---|---|---|
| ACE2 | CL4035 | Membranous | Mouse | Atlas Antibodies/ AMAB91262 | 1:1000 |
| CD3 | 2GV6 | Cytoplasmic/ Membranous | Rabbit | Roche/dispenser | Prediluted |
| CD4 | SP35 | Membranous | Rabbit | Roche/dispenser | Prediluted |
| CD8 | C8/144B | Membranous | Mouse | DAKO/M7103 | 1:30 |
| CD68 | PG-M1 | Cytoplasmic | Mouse | DAKO/M0876 | 1:200 |
| PANCK | AE1/AE3 | Cytoplasmic | Mouse | DAKO/M3515 | 1:100 |
| Panel | Antibodies | Opal Fluorophores | Dilution |
|---|---|---|---|
| Multiplexed IF Panel 1 | CD3 | Opal 520 | 1/400 |
| CD4 | Opal 690 | 1/150 | |
| CD8 | Opal 620 | 1/150 | |
| CD68 | Opal 480 | 1/700 | |
| PANCK | Opal 780 | 1/25 | |
| DAPI | Spectral DAPI | ||
| Multiplexed IF Panel 2 | ACE2 | Opal 620 | 1/150 |
| CD3 | Opal 520 | 1/400 | |
| CD68 | Opal 480 | 1/700 | |
| PANCK | Opal 780 | 1/25 | |
| DAPI | Spectral DAPI |
| Case | Gender, Age (Years) | Duration of Symptoms {Mechanical Ventilation} (Days) | Time Interval between Death and Autopsy (h) | Lung Findings * | Virus Detection (Lung) | Cardiac Findings | Virus Detection (Heart) | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Diffuse Alveolar Damage (DAD) | Acute Broncho-pneumonia | RT-qPCR (Viral Copy Number per Reaction) | Viral RNA (RNA Scope) | IHC § | RT-qPCR (Viral Copy Number per Reaction) | |||||||
| RdRp LoD: 20.8c/r | E Gene LoD: 5.4c/r | RdRp LoD: 20.8c/r | E Gene LoD: 5.4c/r | |||||||||
| 1 | F, 72 | 6 {-} | 14.5 | exudative phase | - | 64,345 | 482,526 | present | positive | heart 480 g; hypertrophy, patchy fibrosis | <LoD ‡ | <LoD ‡ |
| 2 | F, 72 | 8 {-} | 13 | exudative phase | upper lobe predominant, focally with aspirate | 3129 | 26,161 | ND | positive | heart 420 g; biventricular hypertrophy; CAD with stenosis up to 70%; fibrotic scar left ventricle (0.7 cm) | <LoD ‡ | 48 ‡ |
| 3 | M, 96 | 8 {-} | 6.5 | late exudative phase | present in all lobes, necrotizing | 18,858 | 316,089 | present | positive | heart 400 g; hypertrophy; CAD with stenosis up to 30%; patchy fibrosis | <LoD | 479 |
| 4 | M, 86 | 10 {3} | 70 | late exudative/early proliferative phase | - | 76,722 | 537,302 | present | positive | heart 420 g; hypertrophy; CAD with stenosis up to 50%; low-grade diffuse interstitial fibrosis | <LoD | 50 |
| 5 | F, 74 | 11 {5} | 70 | exudative phase | present in all lobes | 3669 | 26,874 | ND | positive | heart 330 g; hypertrophy; pacemaker in place | <LoD ‡ | <LoD ‡ |
| 6 | F, 71 | 14 {-} | 9.5 | late exudative phase and focally proliferative phase | present in all lobes | 5469 | 230,265 | present (high) | positive | heart 410 g; status post myocardial infarction with a 1.5 cm scar (apical posterior left ventricle) | <LoD | 16 |
| 7 | M, 35 | 15 {2} | 20 | - | present in all lobes, necrotizing (clinical/radio-logical picture: aspiration pneumonia) | <LoD ‡ | 178 ‡ | ND | negative | heart 360 g | <LoD ‡ | 135 ‡ |
| 8 | M, 79 | 16 {4} | 61.5 | proliferative phase | - | <LoD | 69 | ND | negative | heart 390 g; hypertrophy; focal fibrosis in one left papillary muscle | <LoD ‡ | <LoD ‡ |
| 9 | M, 75 | 17 {5} | 20.5 | late exudative phase | - | <LoD | 26 | ND | negative | heart 870 g; hypertrophy; signs of chronic ischemia with patchy fibrosis; mechanical aortic valve; pacemaker in place | <LoD ‡ | 111 ‡ |
| 10 | F, 73 | 21 {14} | 12 | AFOP | - (microbiology: proteus mirabilis) | <LoD ‡ | 11‡ | absent | negative | heart 510 g; hypertrophy; CAD with stenosis up to 30% | NC | NC |
| 11 | M, 60 | 18 # {17} | 13 | proliferative phase | focal, with bronchial ulceration (aspergillosis) | <LoD ‡ | 24 ‡ | ND | negative | heart 470 g; hypertrophy; CAD with stenosis up to 80% | <LoD ‡ | <LoD ‡ |
| 12 | M, 69 | 38 # {19} | 13.5 | proliferative phase | focal (microbiology: serratia marcescens) | <LoD ‡ | 49 ‡ | ND | negative | heart 540 g; hypertrophy; focal amyloidosis; CAD with stenosis up to 50% and two scar regions (2 cm each) and aneurysm; pacemaker in place | <LoD ‡ | <LoD ‡ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Berezowska, S.; Lefort, K.; Ioannidou, K.; Ndiaye, D.-R.; Maison, D.; Petrovas, C.; Rotman, S.; Piazzon, N.; Milowich, D.; Sala, N.; et al. Postmortem Cardiopulmonary Pathology in Patients with COVID-19 Infection: Single-Center Report of 12 Autopsies from Lausanne, Switzerland. Diagnostics 2021, 11, 1357. https://doi.org/10.3390/diagnostics11081357
Berezowska S, Lefort K, Ioannidou K, Ndiaye D-R, Maison D, Petrovas C, Rotman S, Piazzon N, Milowich D, Sala N, et al. Postmortem Cardiopulmonary Pathology in Patients with COVID-19 Infection: Single-Center Report of 12 Autopsies from Lausanne, Switzerland. Diagnostics. 2021; 11(8):1357. https://doi.org/10.3390/diagnostics11081357
Chicago/Turabian StyleBerezowska, Sabina, Karine Lefort, Kalliopi Ioannidou, Daba-Rokhya Ndiaye, Damien Maison, Constantinos Petrovas, Samuel Rotman, Nathalie Piazzon, Dina Milowich, Nathalie Sala, and et al. 2021. "Postmortem Cardiopulmonary Pathology in Patients with COVID-19 Infection: Single-Center Report of 12 Autopsies from Lausanne, Switzerland" Diagnostics 11, no. 8: 1357. https://doi.org/10.3390/diagnostics11081357
APA StyleBerezowska, S., Lefort, K., Ioannidou, K., Ndiaye, D.-R., Maison, D., Petrovas, C., Rotman, S., Piazzon, N., Milowich, D., Sala, N., Tsai, C.-Y., Multone, E., Bochud, P.-Y., Oddo, M., Bisig, B., & de Leval, L. (2021). Postmortem Cardiopulmonary Pathology in Patients with COVID-19 Infection: Single-Center Report of 12 Autopsies from Lausanne, Switzerland. Diagnostics, 11(8), 1357. https://doi.org/10.3390/diagnostics11081357






