Does Adding Standard Systematic Biopsy to Targeted Prostate Biopsy in PI-RADS 3 to 5 Lesions Enhance the Detection of Clinically Significant Prostate Cancer? Should All Patients with PI-RADS 3 Undergo Targeted Biopsy?
Abstract
:1. Introduction
2. Materials and Methods
2.1. Population
- Indication of prostate biopsy because of a positive mp-MRI (PI-RADS ≥ 3).
- Patients had undergone a targeted + a standard biopsy.
- A pathology report based on the newly proposed grading prognostic group (ISUP grade group GGG)) was available [17].
- All the information about the variables selected for the study was available.
- All patients with an mp-MRI scan of poor quality, red, or those who were unavailable for review by an experienced uroradiologist were excluded.
2.2. Study Design and Objectives
- To analyze the diagnostic value of additional standard biopsy in the setting of targeted biopsy, in which the main endpoint was to evaluate the increase in the diagnoses of csPCa stratified according to the clinical setting and location of the lesion.
- To analyze the effectiveness of targeting PI-RADS 3 lesions, in which the main endpoint was to evaluate the percentage of csPCa detected at different lesion locations and clinical scenarios.
2.3. Mp-MRI Protocol and Characteristics
2.4. Biopsy Technique
2.5. Variables and Statistical Analysis
2.6. Main Variables
3. Results
3.1. Clinical Characteristics of the Cohort
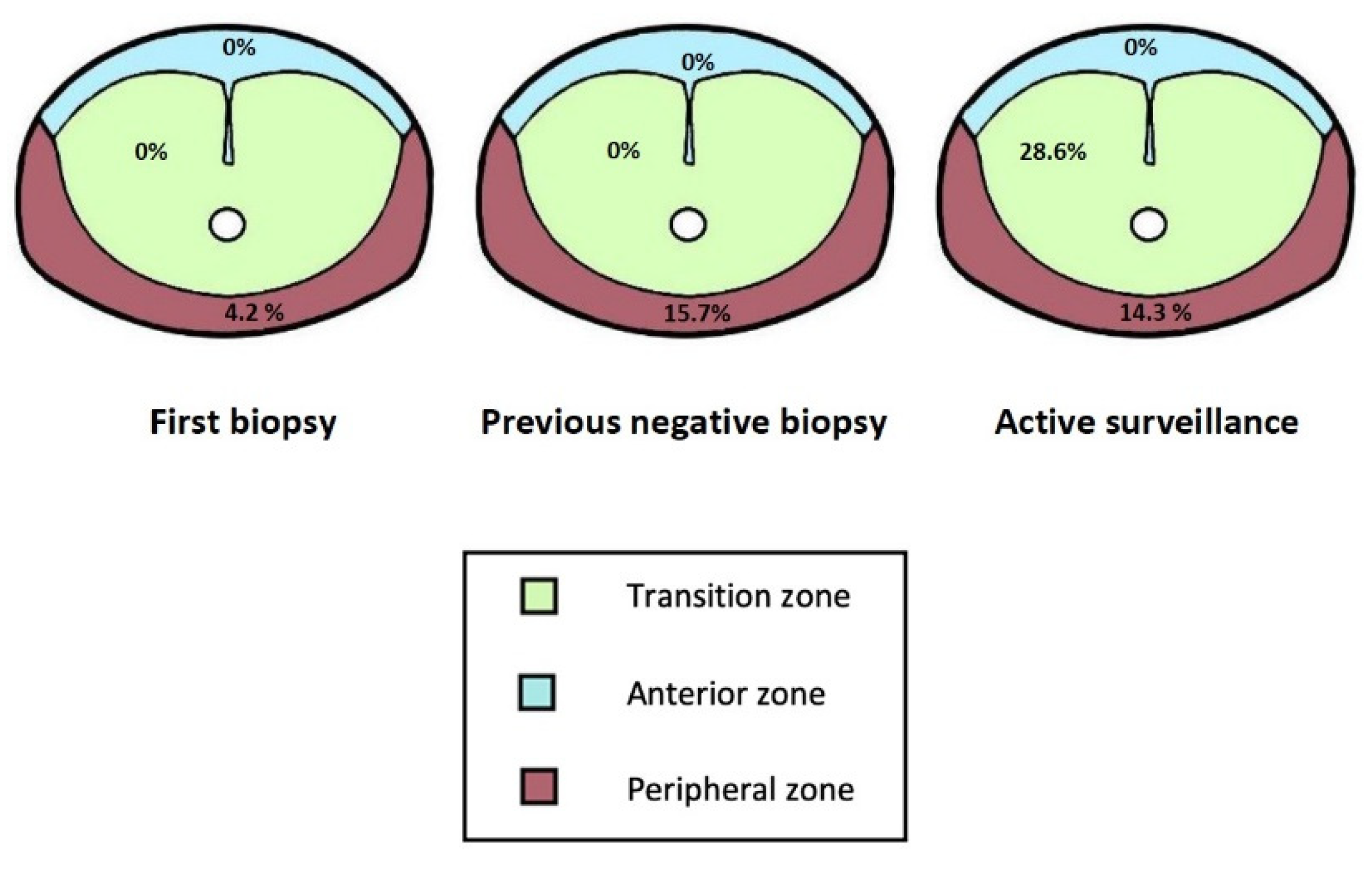
3.2. Value of Additional Standard Biopsy to Targeted Biopsy in PI-RADS 3–5
3.3. Value of Targeting PI-RADS 3 Lesions
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics. CA Cancer J. Clin. 2018, 68, 7–30. [Google Scholar] [CrossRef]
- Remmers, S.; Roobol, M.J. Personalized strategies in population screening for prostate cancer. Int. J. Cancer 2020, 147, 2977–2987. [Google Scholar] [CrossRef]
- Poyet, C.; Nieboer, D.; Bhindi, B.; Kulkarni, G.S.; Wiederkehr, C.; Wettstein, M.S.; Largo, R.; Wild, P.; Sulser, T.; Hermanns, T. Prostate cancer risk prediction using the novel versions of the European Randomised Study for Screening of Prostate Cancer (ERSPC) and Prostate Cancer Prevention Trial (PCPT) risk calculators: Independent validation and comparison in a contemporary Europe. BJU Int. 2015, 117, 401–408. [Google Scholar] [CrossRef] [Green Version]
- Ankerst, D.P.; Straubinger, J.; Selig, K.; Guerrios, L.; De Hoedt, A.; Hernandez, J.; Liss, M.A.; Leach, R.J.; Freedland, S.J.; Kattan, M.; et al. A Contemporary Prostate Biopsy Risk Calculator Based on Multiple Heterogeneous Cohorts. Eur. Urol. 2018, 74, 197–203. [Google Scholar] [CrossRef]
- Frantzi, M.; Gomez-Gomez, E.; Mischak, H. Noninvasive biomarkers to guide intervention: Toward personalized patient management in prostate cancer. Expert Rev. Precis. Med. Drug Dev. 2020, 5, 383–400. [Google Scholar] [CrossRef]
- Ahmed, H.U.; Bosaily, A.E.-S.; Brown, L.C.; Gabe, R.; Kaplan, R.S.; Parmar, M.K.; PROMIS Study Group. Diagnostic accuracy of multi-parametric MRI and TRUS biopsy in prostate cancer (PROMIS): A paired validating confirmatory study. Lancet 2017, 389, 815–822. [Google Scholar] [CrossRef] [Green Version]
- Kasivisvanathan, V.; Rannikko, A.S.; Borghi, M.; Panebianco, V.; Mynderse, L.A.; Vaarala, M.H.; Moore, C.M. MRI-Targeted or Standard Biopsy for Prostate-Cancer Diagnosis. N. Engl. J. Med. 2018, 378, 1767–1777. [Google Scholar] [CrossRef]
- Rouviere, O.; Puech, P.; Renard-Penna, R.; Claudon, M.; Roy, C.; Mège-Lechevallier, F.; Decaussin-Petrucci, M.; Dubreuil-Chambardel, M.; Magaud, L.; Remontet, L.; et al. Use of prostate systematic and targeted biopsy on the basis of multiparametric MRI in biopsy-naive patients (MRI-FIRST): A prospective, multicentre, paired diagnostic study. Lancet Oncol. 2019, 20, 100–109. [Google Scholar] [CrossRef]
- Sathianathen, N.J.; Omer, A.; Harriss, E.; Davies, L.; Kasivisvanathan, V.; Punwani, S.; Moore, C.M.; Kastner, C.; Barrett, T.; Bergh, R.C.V.D.; et al. Negative Predictive Value of Multiparametric Magnetic Resonance Imaging in the Detection of Clinically Significant Prostate Cancer in the Prostate Imaging Reporting and Data System Era: A Systematic Review and Meta-analysis. Eur. Urol. 2020, 78, 402–414. [Google Scholar] [CrossRef] [PubMed]
- Westphalen, A.C.; McCulloch, C.E.; Anaokar, J.M.; Arora, S.; Barashi, N.S.; Barentsz, J.O.; Bathala, T.K.; Bittencourt, L.K.; Booker, M.T.; Braxton, V.G.; et al. Variability of the Positive Predictive Value of PI-RADS for Prostate MRI across 26 Centers: Experience of the Society of Abdominal Radiology Prostate Cancer Disease-focused Panel. Radiology 2020, 296, 76–84. [Google Scholar] [CrossRef] [PubMed]
- Maggi, M.; Panebianco, V.; Mosca, A.; Salciccia, S.; Gentilucci, A.; Di Pierro, G.; Busetto, G.M.; Barchetti, G.; Campa, R.; Sperduti, I.; et al. Prostate Imaging Reporting and Data System 3 Category Cases at Multiparametric Magnetic Resonance for Prostate Cancer: A Systematic Review and Meta-analysis. Eur. Urol. Focus 2020, 6, 463–478. [Google Scholar] [CrossRef] [PubMed]
- Appayya, M.B.; Sidhu, H.S.; Dikaios, N.; Johnston, E.W.; Simmons, L.A.; Freeman, A.; Kirkham, A.P.; Ahmed, H.U.; Punwani, S. Characterizing indeterminate (Likert-score 3/5) peripheral zone prostate lesions with PSA density, PI-RADS scoring and qualitative descriptors on multiparametric MRI. Br. J. Radiol. 2018, 91, 20170645. [Google Scholar] [CrossRef]
- Hermie, I.; Van Besien, J.; De Visschere, P.; Lumen, N.; Decaestecker, K. Which clinical and radiological characteristics can predict clinically significant prostate cancer in PI-RADS 3 lesions? A retrospective study in a high-volume academic center. Eur. J. Radiol. 2019, 114, 92–98. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Zhao, W.; Tan, S.; Zhang, Y.; Wei, C.; Chen, T.; Shen, J. Combining clinical and MRI data to manage PI-RADS 3 lesions and reduce excessive biopsy. Transl. Androl. Urol. 2020, 9, 1252–1261. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Ryu, H.; Lee, H.J.; Hwang, S.I.; Choe, G.; Hong, S.K. Who can safely evade a magnetic resonance imaging fusion-targeted biopsy (MRIFTB) for prostate imaging reporting and data system (PI-RADS) 3 lesion? World J. Urol. 2020, 39, 1463–1471. [Google Scholar] [CrossRef]
- Schoots, I.G.; Padhani, A.; Rouviere, O.; Barentsz, J.O.; Richenberg, J. Analysis of Magnetic Resonance Imaging–directed Biopsy Strategies for Changing the Paradigm of Prostate Cancer Diagnosis. Eur. Urol. Oncol. 2020, 3, 32–41. [Google Scholar] [CrossRef]
- Epstein, J.I.; Egevad, L.; Amin, M.B.; Delahunt, B.; Srigley, J.R.; Humphrey, P.A. The 2014 International Society of Urological Pathology (ISUP) Consensus Conference on Gleason Grading of Prostatic Carcinoma: Definition of Grading Patterns and Proposal for a New Grading System. Am. J. Surg. Pathol. 2016, 40, 244–252. [Google Scholar] [CrossRef]
- Gómez, E.G.; Rosa, J.V.; Valiente, J.C.; Tarradas, F.T.; Curado, F.A.; Ruiz, D.L.; Tapia, M.J.R. New Approach to Guide Target Prostate Biopsy: Technique and Initial Experience. Urology 2018, 121, 198–199. [Google Scholar] [CrossRef]
- Cash, H.; Maxeiner, A.; Stephan, C.; Fischer, T.; Durmus, T.; Holzmann, J.; Asbach, P.; Haas, M.; Hinz, S.; Neymeyer, J.; et al. The detection of significant prostate cancer is correlated with the Prostate Imaging Reporting and Data System (PI-RADS) in MRI/transrectal ultrasound fusion biopsy. World J. Urol. 2016, 34, 525–532. [Google Scholar] [CrossRef]
- Siddiqui, M.; Rais-Bahrami, S.; Turkbey, B.; George, A.K.; Rothwax, J.; Shakir, N.; Okoro, C.; Raskolnikov, D.; Parnes, H.L.; Linehan, W.M.; et al. Comparison of MR/Ultrasound Fusion–Guided Biopsy with Ultrasound-Guided Biopsy for the Diagnosis of Prostate Cancer. JAMA 2015, 313, 390–397. [Google Scholar] [CrossRef]
- Görtz, M.; Radtke, J.P.; Hatiboglu, G.; Schütz, V.; Tosev, G.; Güttlein, M.; Leichsenring, J.; Stenzinger, A.; Bonekamp, D.; Schlemmer, H.-P.; et al. The Value of Prostate-specific Antigen Density for Prostate Imaging-Reporting and Data System 3 Lesions on Multiparametric Magnetic Resonance Imaging: A Strategy to Avoid Unnecessary Prostate Biopsies. Eur. Urol. Focus 2021, 7, 325–331. [Google Scholar] [CrossRef] [PubMed]
- Awamlh, B.A.H.A.; Marks, L.S.; Sonn, G.A.; Natarajan, S.; Fan, R.E.; Gross, M.D.; Mauer, E.; Banerjee, S.; Hectors, S.; Carlsson, S.; et al. Multicenter analysis of clinical and MRI characteristics associated with detecting clinically significant prostate cancer in PI-RADS (v2.0) category 3 lesions. Urol. Oncol. Semin. Orig. Investig. 2020, 38, 637.e9–637.e15. [Google Scholar] [CrossRef]
- Zhang, Y.; Zeng, N.; Zhang, F.B.; Huang, Y.X.R.; Tian, Y. Performing Precise Biopsy in Naive Patients With Equivocal PI-RADS, Version 2, Score 3, Lesions: An MRI-based Nomogram to Avoid Unnecessary Surgical Intervention. Clin. Genitourin. Cancer 2020, 18, 367–377. [Google Scholar] [CrossRef] [PubMed]
- Venderink, W.; van Luijtelaar, A.; Bomers, J.G.; van der Leest, M.; de Kaa, C.H.-V.; Barentsz, J.O.; Sedelaar, J.M.; Fütterer, J.J. Results of Targeted Biopsy in Men with Magnetic Resonance Imaging Lesions Classified Equivocal, Likely or Highly Likely to Be Clinically Significant Prostate Cancer. Eur. Urol. 2018, 73, 353–360. [Google Scholar] [CrossRef]
- Chu, C.E.; Lonergan, P.E.; Washington, S.L.; Cowan, J.E.; Shinohara, K.; Westphalen, A.C.; Carroll, P.R.; Cooperberg, M.R. Multiparametric Magnetic Resonance Imaging Alone is Insufficient to Detect Grade Reclassification in Active Surveillance for Prostate Cancer. Eur. Urol. 2020, 78, 515–517. [Google Scholar] [CrossRef]
- Salguero, J.; Gómez-Gómez, E.; Valero-Rosa, J.; Carrasco-Valiente, J.; Mesa, J.; Martin, C.; Campos-Hernández, J.P.; Rubio, J.M.; López, D.; Requena, M.J. Role of Multiparametric Prostate Magnetic Resonance Imaging before Confirmatory Biopsy in Assessing the Risk of Prostate Cancer Progression during Active Surveillance. Korean J. Radiol. 2020, 21, 559–567. [Google Scholar] [CrossRef]
- Baccaglini, W.; Glina, F.A.; Pazeto, C.L.; Medina, L.G.; Korkes, F.; Bernardo, W.M.; Sotelo, R.; Glina, S.; Marra, G.; Moschini, M.; et al. Accuracy of MRI-guided Versus Systematic Prostate Biopsy in Patients Under Active Surveillance: A Systematic Review and Meta-analysis. Clin. Genitourin. Cancer 2021, 19, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Arabi, A.; Deebajah, M.; Yaguchi, G.; Pantelic, M.; Williamson, S.; Gupta, N.; Park, H.; Peabody, J.; Menon, M.; Dabaja, A.; et al. Systematic Biopsy Does Not Contribute to Disease Upgrading in Patients Undergoing Targeted Biopsy for PI-RADS 5 Lesions Identified on Magnetic Resonance Imaging in the Course of Active Surveillance for Prostate Cancer. Urolpgy 2019, 134, 168–172. [Google Scholar] [CrossRef]
- Falagario, U.; Jambor, I.; Taimen, P.; Syvanen, K.T.; Kahkonen, E.; Merisaari, H.; Perez, I.M.; Knaapila, J.; Steiner, A.; Verho, J.; et al. Added value of systematic biopsy in men with a clinical suspicion of prostate cancer undergoing biparametric MRI-targeted biopsy: Multi-institutional external validation study. World J. Urol. 2021, 39, 1879–1887. [Google Scholar] [CrossRef]
- Cash, H.; Günzel, K.; Maxeiner, A.; Stephan, C.; Fischer, T.; Durmus, T.; Miller, K.; Asbach, P.; Haas, M.; Kempkensteffen, C. Prostate cancer detection on transrectal ultrasonography-guided random biopsy despite negative real-time magnetic resonance imaging/ultrasonography fusion-guided targeted biopsy: Reasons for targeted biopsy failure. BJU Int. 2016, 118, 35–43. [Google Scholar] [CrossRef]
- Hansen, N.L.; Barrett, T.; Lloyd, T.; Warren, A.; Samel, C.; Bratt, O.; Kastner, C. Optimising the number of cores for magnetic resonance imaging-guided targeted and systematic transperineal prostate biopsy. BJU Int. 2020, 125, 260–269. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Variable | Total | csPCa | No csPCa |
|---|---|---|---|
| n = 483 | n = 252 | n = 231 | |
| Age; years | 65 (59–71) | 68 (63–73) | 63 (58–68) |
| PSA; ng/mL | 6.39 (4.8–9.5) | 6.7 (511–10.5) | 5.85 (4.4–8.7) |
| Prostate volume; cc | 55 (40–76) | 50 (36–69) | 60 (44–80.1) |
| PSAD | 0.12 (0.08–0.18) | 0.14 (0.10–0.23) | 0.10 (0.07–0.15) |
| Clinical scenario | |||
| 1° Biopsy | 45 (9.3) | 31 (12.3) | 14 (6.1) |
| Previous negative biopsy | 337 (69.8) | 159 (63.1) | 178 (77.1) |
| Active surveillance | 101 (20.9) | 62 (24.6) | 39 (16.9) |
| mp-MRI I-PI-RADSv.2 score | |||
| 3 | 105 (21.7) | 21 (8.3) | 84 (36.4) |
| 4 | 289 (59.8) | 153 (60.7) | 136 (58.9) |
| 5 | 89 (18.4) | 78 (31) | 11 (4.8) |
| mp-MRI PI-RADSv.2 score | |||
| 2 | 50 (10.4) | 6 (2.4) | 44 (19) |
| 3 | 102 (21.1) | 19 (7.5) | 83 (35.9) |
| 4 | 245 (50.7) | 148 (58.7) | 97 (42) |
| 5 | 86 (17.8) | 79 (31.3) | 7 (3) |
| Targeted lesion mm | 10 (7–14) | 14 (10–22) | 10 (7–13) |
| Targeted location | |||
| Peripheral | 325 (67.3) | 155 (61.5) | 170 (73.6) |
| Transition/central zone | 66 (13.7) | 26 (10.3) | 40 (17.3) |
| Anterior fibromuscular stroma | 92 (19) | 71 (28.2) | 21 (9.1) |
| Number of targeted cores | 4 (4–5) | 4 (3–4) | 4 (4–5) |
| Variable | Total |
|---|---|
| n = 102 | |
| Age; years | 62 (57–68) |
| PSA; ng/mL | 5.8 (4.3–8) |
| Prostate volume; cc | 57 (41–81) |
| PSAD | 0.10 (0.07–0.16) |
| Clinical scenario | |
| 1° Biopsy | 9 (8.8) |
| Previous negative biopsy | 73 (71.6) |
| Active surveillance | 20 (19.6) |
| Targeted lesion mm | 8 (6–10) |
| Targeted location | |
| Peripheral | 79 (77.5) |
| Transition/central zone | 16 (15.7) |
| Anterior fibromuscular stroma | 7 (6.9) |
| Number of targeted cores | 4(3–4) |
| VARIABLE | Multivariate Analysis for csPCa, n = 102 | ||
|---|---|---|---|
| OR | p | 95% CI (OR) | |
| PSAD | 1.389 | 0.692 | 0.272–7.085 |
| Transition/central zone vs. anterior fibromuscular stroma | 0.036 | 0.046 | 0.001–0.936 |
| Peripheral zone vs. anterior fibromuscular stroma | 0.082 | 0.039 | 0.008–0.885 |
| Previous negative biopsy vs. biopsy-naïve setting | 0.033 | 0.001 | 0.004–0.262 |
| Active surveillance vs. biopsy-naïve setting | 0.342 | 0.266 | 0.052–2.266 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gomez-Gomez, E.; Moreno Sorribas, S.; Valero-Rosa, J.; Blanca, A.; Mesa, J.; Salguero, J.; Carrasco-Valiente, J.; López-Ruiz, D.; Anglada-Curado, F.J. Does Adding Standard Systematic Biopsy to Targeted Prostate Biopsy in PI-RADS 3 to 5 Lesions Enhance the Detection of Clinically Significant Prostate Cancer? Should All Patients with PI-RADS 3 Undergo Targeted Biopsy? Diagnostics 2021, 11, 1335. https://doi.org/10.3390/diagnostics11081335
Gomez-Gomez E, Moreno Sorribas S, Valero-Rosa J, Blanca A, Mesa J, Salguero J, Carrasco-Valiente J, López-Ruiz D, Anglada-Curado FJ. Does Adding Standard Systematic Biopsy to Targeted Prostate Biopsy in PI-RADS 3 to 5 Lesions Enhance the Detection of Clinically Significant Prostate Cancer? Should All Patients with PI-RADS 3 Undergo Targeted Biopsy? Diagnostics. 2021; 11(8):1335. https://doi.org/10.3390/diagnostics11081335
Chicago/Turabian StyleGomez-Gomez, Enrique, Sara Moreno Sorribas, Jose Valero-Rosa, Ana Blanca, Juan Mesa, Joseba Salguero, Julia Carrasco-Valiente, Daniel López-Ruiz, and Francisco José Anglada-Curado. 2021. "Does Adding Standard Systematic Biopsy to Targeted Prostate Biopsy in PI-RADS 3 to 5 Lesions Enhance the Detection of Clinically Significant Prostate Cancer? Should All Patients with PI-RADS 3 Undergo Targeted Biopsy?" Diagnostics 11, no. 8: 1335. https://doi.org/10.3390/diagnostics11081335
APA StyleGomez-Gomez, E., Moreno Sorribas, S., Valero-Rosa, J., Blanca, A., Mesa, J., Salguero, J., Carrasco-Valiente, J., López-Ruiz, D., & Anglada-Curado, F. J. (2021). Does Adding Standard Systematic Biopsy to Targeted Prostate Biopsy in PI-RADS 3 to 5 Lesions Enhance the Detection of Clinically Significant Prostate Cancer? Should All Patients with PI-RADS 3 Undergo Targeted Biopsy? Diagnostics, 11(8), 1335. https://doi.org/10.3390/diagnostics11081335