The Morphological and Dynamic Changes of Ultrasound in the Evaluation of Effects of Oral Steroids Treatment for Patients with Carpal Tunnel Syndrome
Abstract
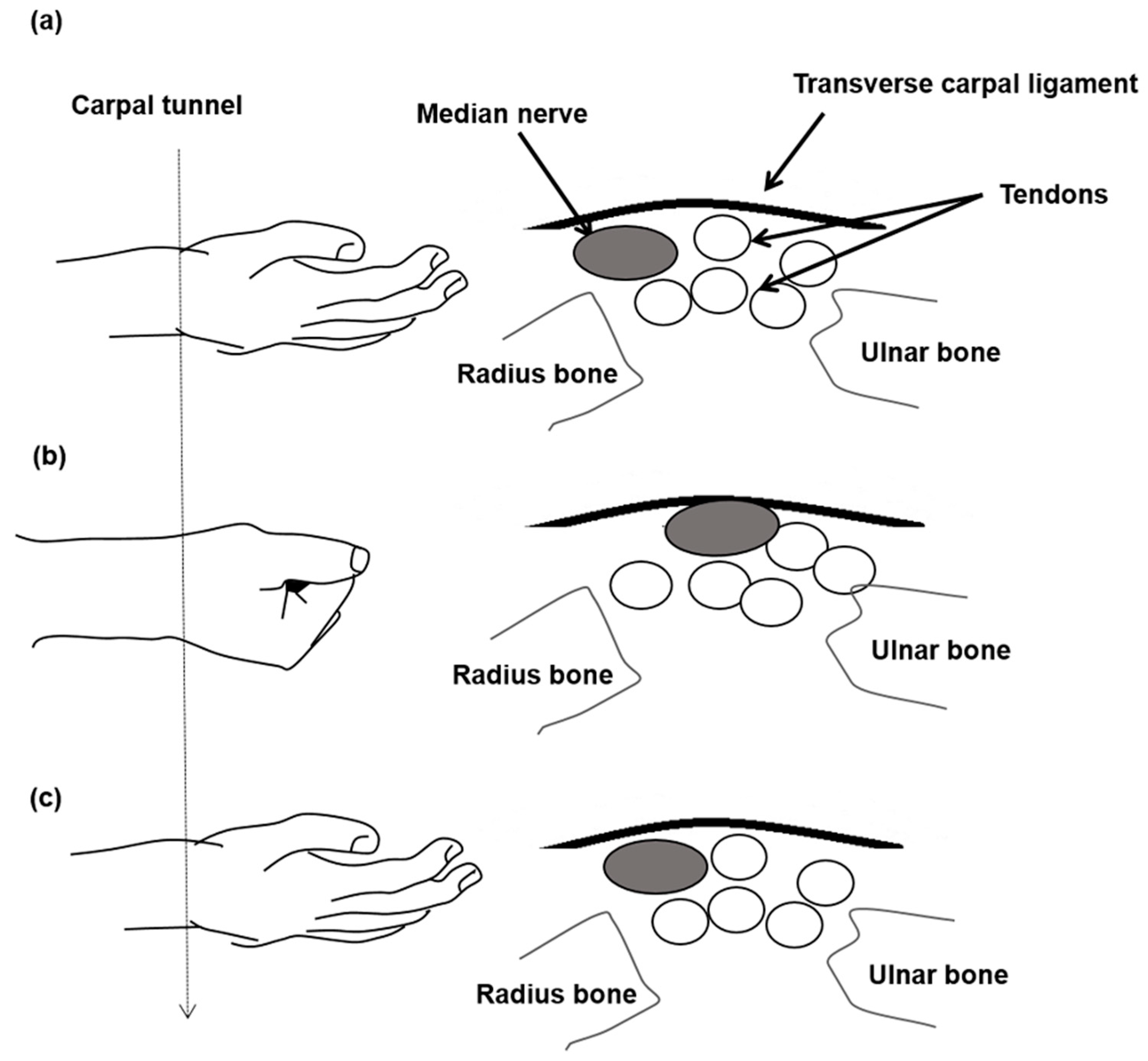
1. Introduction
2. Materials and Methods
2.1. Data Acquisition
2.2. Ultrasound Imaging
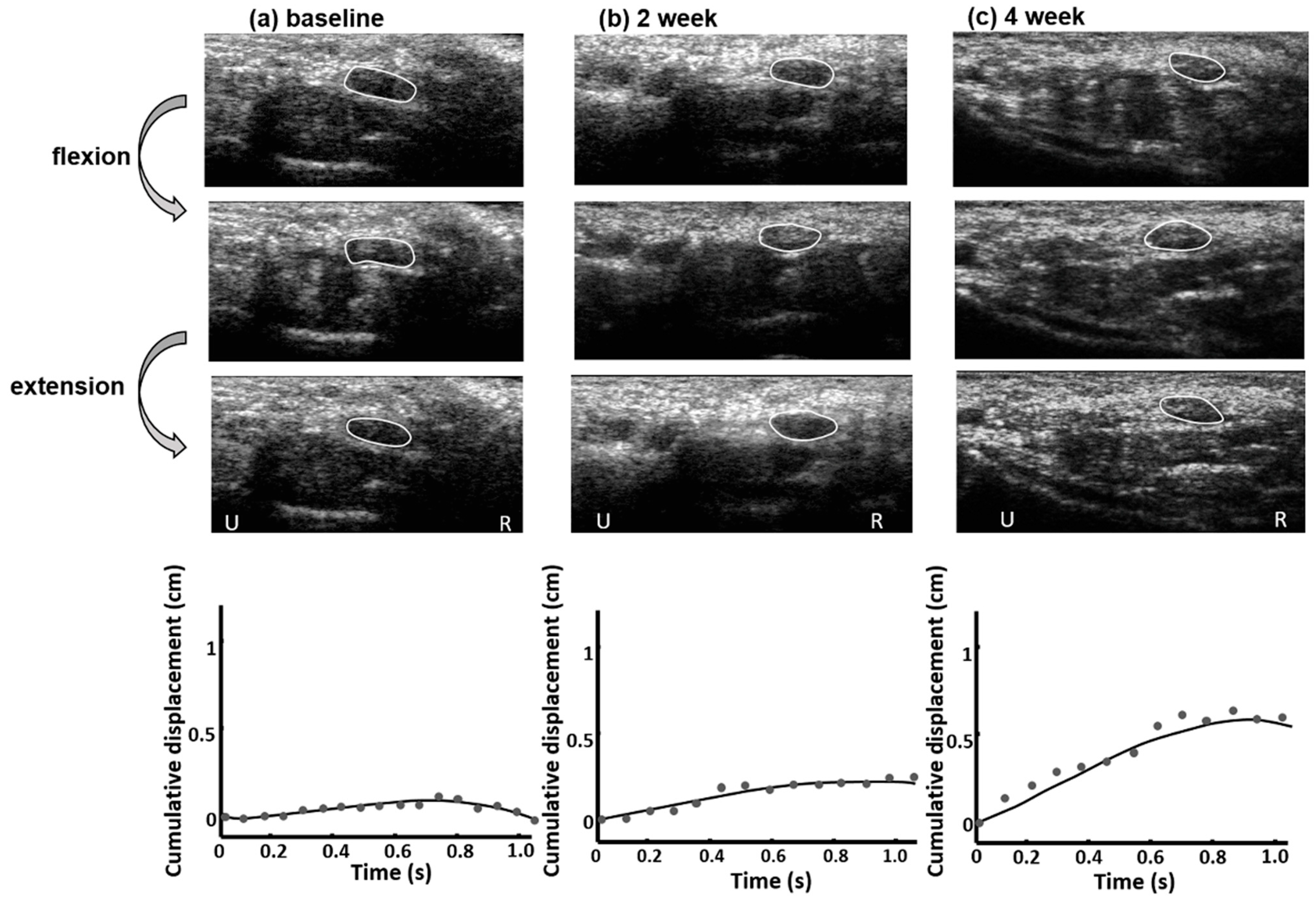
2.3. Median Nerve Mobility Pattern Estimation
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Werner, R.A.; Andary, M. Carpal tunnel syndrome: Pathophysiology and clinical neurophysiology. Clin. Neurophysiol. 2002, 113, 1373–1381. [Google Scholar] [CrossRef]
- Dale, A.M.; Harris-Adamson, C.; Rempel, D.; Gerr, F.; Hegmann, K.; Silverstein, B.; Burt, S.; Garg, A.; Kapellusch, J.; Merlino, L.; et al. Prevalence and incidence of carpal tunnel syndrome in US working populations: Pooled analysis of six prospective studies. Scand. J. Work. Environ. Health 2013, 39, 495–505. [Google Scholar] [CrossRef] [PubMed]
- Witt, J.C.; Hentz, J.G.; Stevens, J.C. Carpal tunnel syndrome with normal nerve conduction studies. Muscle Nerve 2004, 29, 515–522. [Google Scholar] [CrossRef]
- Atroshi, I.; Gummesson, C.; Johnsson, R.; Ornstein, E. Diagnostic properties of nerve conduction tests in population-based carpal tunnel syndrome. BMC Musculoskelet. Disord. 2003, 4, 9. [Google Scholar] [CrossRef] [PubMed]
- Keberle, M.; Jenett, M.; Kenn, W.; Reiners, K.; Peter, M.; Haerten, R.; Hahn, D. Technical advances in ultrasound and MR imaging of carpal tunnel syndrome. Eur. Radiol. 2000, 10, 1043–1050. [Google Scholar] [CrossRef]
- Nakamichi, K.-I.; Tachibana, S. Ultrasonographic measurement of median nerve cross-sectional area in idiopathic carpal tunnel syndrome: Diagnostic accuracy. Muscle Nerve 2002, 26, 798–803. [Google Scholar] [CrossRef]
- Lee, C.H.; Kim, T.K.; Yoon, E.S.; Dhong, E.S. Correlation of High-Resolution Ultrasonographic Findings with the Clinical Symptoms and Electrodiagnostic Data in Carpal Tunnel Syndrome. Ann. Plast. Surg. 2005, 54, 20–23. [Google Scholar] [CrossRef]
- Yoshii, Y.; Villarraga, H.R.; Henderson, J.; Zhao, C.; An, K.-N.; Amadio, P.C. Speckle Tracking Ultrasound for Assessment of the Relative Motion of Flexor Tendon and Subsynovial Connective Tissue in the Human Carpal Tunnel. Ultrasound Med. Biol. 2009, 35, 1973–1981. [Google Scholar] [CrossRef] [PubMed]
- Akcar, N.; Özkan, S.; Mehmetoglu, Ö.; Calisir, C.; Adapinar, B. Value of Power Doppler and Gray-Scale US in the Diagnosis of Carpal Tunnel Syndrome: Contribution of Cross-Sectional Area just before the Tunnel Inlet as Compared with the Cross-Sectional Area at the Tunnel. Korean J. Radiol. 2010, 11, 632–639. [Google Scholar] [CrossRef]
- Kantarci, F.; Ustabasioglu, F.E.; Delil, S.; Olgun, D.C.; Korkmazer, B.; Dikici, A.S.; Tutar, O.; Nalbantoglu, M.; Uzun, N.; Mihmanli, I. Median nerve stiffness measurement by shear wave elastography: A potential sonographic method in the diagnosis of carpal tunnel syndrome. Eur. Radiol. 2013, 24, 434–440. [Google Scholar] [CrossRef]
- McDonagh, C.; Alexander, M.; Kane, D. The role of ultrasound in the diagnosis and management of carpal tunnel syndrome: A new paradigm. Rheumatology 2014, 54, 9–19. [Google Scholar] [CrossRef]
- Sarría, L.; Cabada, T.; Cozcolluela, R.; Martínez-Berganza, T.; García, S. Carpal tunnel syndrome: Usefulness of sonography. Eur. Radiol. 2000, 10, 1920–1925. [Google Scholar] [CrossRef]
- Wong, S.M.; Griffith, J.; Hui, A.C.F.; Lo, S.K.; Fu, M.; Wong, K.S.L. Carpal Tunnel Syndrome: Diagnostic Usefulness of Sonography. Radiology 2004, 232, 93–99. [Google Scholar] [CrossRef]
- Moran-Blanco, L.M.; Perez, M.; Esteban, A.; Bellon, J.; Arranz, B.; Del Cerro, M. Sonographic measurement of cross-sectional area of the median nerve in the diagnosis of carpal tunnel syndrome: Correlation with nerve conduction studies. J. Clin. Ultrasound 2009, 37, 125–131. [Google Scholar] [CrossRef]
- Fowler, J.R.; Gaughan, J.P.; Ilyas, A.M. The Sensitivity and Specificity of Ultrasound for the Diagnosis of Carpal Tunnel Syndrome: A Meta-analysis. Clin. Orthop. Relat. Res. 2011, 469, 1089–1094. [Google Scholar] [CrossRef]
- Billakota, S.; Hobson-Webb, L.D. Standard median nerve ultrasound in carpal tunnel syndrome: A retrospective review of 1021 cases. Clin. Neurophysiol. Pract. 2017, 2, 188–191. [Google Scholar] [CrossRef] [PubMed]
- Yoshii, Y.; Zhao, C.; Amadio, P.C. Recent Advances in Ultrasound Diagnosis of Carpal Tunnel Syndrome. Diagnostics 2020, 10, 596. [Google Scholar] [CrossRef] [PubMed]
- Yoshii, Y.; Villarraga, H.R.; Henderson, J.; Zhao, C.; An, K.-N.; Amadio, P.C. Ultrasound Assessment of the Displacement and Deformation of the Median Nerve in the Human Carpal Tunnel with Active Finger Motion. J. Bone Jt. Surg. Am. 2009, 91, 2922–2930. [Google Scholar] [CrossRef]
- Yao, Y.; Grandy, E.; Jenkins, L.; Hou, J.; Evans, P.J.; Seitz, W.H.; Li, Z.-M. Changes of median nerve conduction, cross-sectional area and mobility by radioulnar wrist compression intervention in patients with carpal tunnel syndrome. J. Orthop. Transl. 2019, 18, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Nakamichi, K.; Tachibana, S. Transverse Sliding of the Median Nerve Beneath the Flexor Retinaculum. J. Hand Surg. 1992, 17, 213–216. [Google Scholar] [CrossRef]
- Nakamichi, K.; Tachibana, S. Restricted Motion of the Median Nerve in Carpal Tunnel Syndrome. J. Hand Surg. 1995, 20, 460–464. [Google Scholar] [CrossRef]
- Wright, T.W.; Glowczewskie, F.; Wheeler, D.; Miller, G.; Cowin, D. Excursion and Strain of the Median Nerve. J. Bone Jt. Surg. Am. 1996, 78, 1897–1903. [Google Scholar] [CrossRef]
- Erel, E.; Dilley, A.; Greening, J.; Morris, V.; Cohen, B.; Lynn, B. Longitudinal sliding of the median nerve in patients with carpal tunnel syndrome. J. Hand Surg. 2003, 28, 439–443. [Google Scholar] [CrossRef]
- Osamura, N.; Zhao, C.; Zobitz, M.E.; An, K.-N.; Amadio, P.C. Evaluation of the material properties of the subsynovial connective tissue in carpal tunnel syndrome. Clin. Biomech. 2007, 22, 999–1003. [Google Scholar] [CrossRef]
- Liao, Y.-Y.; Lee, W.-N.; Lee, M.-R.; Chen, W.-S.; Chiou, H.-J.; Kuo, T.-T.; Yeh, C.-K. Carpal Tunnel Syndrome: US Strain Imaging for Diagnosis. Radiology 2015, 275, 205–214. [Google Scholar] [CrossRef]
- Moon, H.; Lee, B.J.; Park, D. Change to movement and morphology of the median nerve resulting from steroid injection in patients with mild carpal tunnel syndrome. Sci. Rep. 2020, 10, 1–9. [Google Scholar] [CrossRef]
- Gerritsen, A.A.M.; De Krom, M.C.T.F.M.; Struijs, M.A.; Scholten, R.J.P.M.; De Vet, H.C.W.; Bouter, L.M. Conservative treatment options for carpal tunnel syndrome: A systematic review of randomised controlled trials. J. Neurol. 2000, 249, 272–280. [Google Scholar] [CrossRef] [PubMed]
- Huisstede, B.M.; Hoogvliet, P.; Randsdorp, M.S.; Glerum, S.; van Middelkoop, M.; Koes, B. Carpal Tunnel Syndrome. Part I: Effectiveness of Nonsurgical Treatments—A Systematic Review. Arch. Phys. Med. Rehabil. 2010, 91, 981–1004. [Google Scholar] [CrossRef] [PubMed]
- Wipperman, J.; Goerl, K. Carpal tunnel syndrome: Diagnosis and management. Am. Fam. Physician 2016, 94, 993–999. [Google Scholar] [PubMed]
- Herskovitz, S.; Berger, A.R.; Lipton, R.B. Low-dose, short-term oral prednisone in the treatment of carpal tunnel syndrome. Neurology 1995, 45, 1923–1925. [Google Scholar] [CrossRef]
- Chang, M.H.; Chiang, H.T.; Lee, S.S.-J.; Ger, L.P.; Lo, Y.K. Oral drug of choice in carpal tunnel syndrome. Neurology 1998, 51, 390–393. [Google Scholar] [CrossRef]
- Hui, A.C.F.; Wong, S.M.; Wong, K.S.L.; Li, E.; Kay, R.; Yung, P.S.-H.; Hung, L.K.; Yu, L.M. Oral steroid in the treatment of carpal tunnel syndrome. Ann. Rheum. Dis. 2001, 60, 813–814. [Google Scholar] [CrossRef]
- Chang, M.-H.; Ger, L.-P.; Hsieh, P.F.; Huang, S.-Y. A randomised clinical trial of oral steroids in the treatment of carpal tunnel syndrome: A long term follow up. J. Neurol. Neurosurg. Psychiatry 2002, 73, 710–714. [Google Scholar] [CrossRef]
- Viera, A.J. Management of carpal tunnel syndrome. Am. Fam. Physician 2003, 68, 265–272. [Google Scholar] [PubMed]
- Yang, C.-P.; Hsieh, C.-L.; Wang, N.-H.; Li, T.-C.; Hwang, K.-L.; Yu, S.-C.; Chang, M.-H. Acupuncture in Patients with Carpal Tunnel Syndrome. Clin. J. Pain 2009, 25, 327–333. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.-P.; Wang, N.-H.; Li, T.-C.; Hsieh, C.-L.; Chang, H.-H.; Hwang, K.-L.; Ko, W.-S.; Chang, M.-H. A Randomized Clinical Trial of Acupuncture Versus Oral Steroids for Carpal Tunnel Syndrome: A Long-Term Follow-Up. J. Pain 2011, 12, 272–279. [Google Scholar] [CrossRef]
- Stevens, J.C. AAEM minimonograph #26: The electrodiagnosis of carpal tunnel syndrome. American Association of Elec-trodiagnostic Medicine. Muscle Nerve 1997, 20, 1477–1486. [Google Scholar] [CrossRef] [PubMed]
- Kuo, T.-T.; Lee, M.-R.; Liao, Y.-Y.; Chen, J.-P.; Hsu, Y.-W.; Yeh, C.-K. Assessment of Median Nerve Mobility by Ultrasound Dynamic Imaging for Diagnosing Carpal Tunnel Syndrome. PLoS ONE 2016, 11, e0147051. [Google Scholar] [CrossRef]
- Aboonq, M.S. Pathophysiology of carpal tunnel syndrome. Neurosciences 2015, 20, 4–9. [Google Scholar]
- Vögelin, E.; Nüesch, E.; Jüni, P.; Reichenbach, S.; Eser, P.; Ziswiler, H.-R. Sonographic Follow-Up of Patients with Carpal Tunnel Syndrome Undergoing Surgical or Nonsurgical Treatment: Prospective Cohort Study. J. Hand Surg. 2010, 35, 1401–1409. [Google Scholar] [CrossRef] [PubMed]
- Asadov, R.; Erdal, A.; Buğdaycı, O.; Gündüz, O.H.; Ekinci, G. The effectiveness of ultrasonography and ultrasonographic elastography in the diagnosis of carpal tunnel syndrome and evaluation of treatment response after steroid injection. Eur. J. Radiol. 2018, 108, 172–176. [Google Scholar] [CrossRef] [PubMed]
- Cingoz, M.; Kandemirli, S.G.; Alis, D.C.; Samanci, C.; Kandemirli, G.C.; Adatepe, N.U. Evaluation of median nerve by shear wave elastography and diffusion tensor imaging in carpal tunnel syndrome. Eur. J. Radiol. 2018, 101, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Kurt, S.; Kisacik, B.; Kaplan, Y.; Yildirim, B.; Etikan, I.; Karaer, H. Obesity and Carpal Tunnel Syndrome: Is There a Causal Relationship? Eur. Neurol. 2008, 59, 253–257. [Google Scholar] [CrossRef] [PubMed]
- Komurcu, H.F.; Kilic, S.; Anlar, O. Relationship of Age, Body Mass Index, Wrist and Waist Circumferences to Carpal Tunnel Syndrome Severity. Neurol. Med. Chir. 2014, 54, 395–400. [Google Scholar] [CrossRef] [PubMed][Green Version]


| Variable | Steroid (n = 14, 22 Wrists) | Nicergoline (n = 22, 35 Wrists) | p-Value |
|---|---|---|---|
| Age (years), mean ± SD | 58.00 ± 11.29 | 52.95 ± 7.16 | 0.109 |
| Gender, n (%) | |||
| Male | 5 (35.71%) | 3 (13.63%) | 0.217 |
| Female | 9 (64.28%) | 19 (86.36%) | |
| Side of hands, n (%) | |||
| Left | 1 (7.14%) | 4 (18.18) | 0.528 |
| Right | 5 (35.71%) | 5 (22.73%) | |
| Both | 8 (57.14%) | 13 (53.09%) | |
| Severity, n (%) | |||
| Mild | 4 (28.57%) | 2 (9.09%) | 0.181 |
| Moderate | 10 (71.43%) | 20 (90.91%) | |
| AMP (cm), mean ± SD | 0.25 ± 0.16 | 0.25 ± 0.09 | 0.912 |
| CSA (mm2), mean ± SD | 12.50 ± 3.39 | 12.25 ± 2.08 | 0.807 |
| GSS, mean ± SD | 10.86 ± 13.11 | 20.36 ± 9.48 | 0.692 |
| DML (ms), mean ± SD | 5.68 ± 1.29 | 5.63 ± 1.20 | 0.899 |
| Variable | Steroid | Nicergoline | Difference in Change over Time | p-Value | ||
|---|---|---|---|---|---|---|
| Mean (SE) | within Group Change | Mean (SE) | within Group Change | |||
| AMP (cm) | ||||||
| baseline | 0.27 (0.04) | reference | 0.22(0.04) | reference | reference | |
| 2 week | 0.73 (0.09) | 0.46 (0.10) | 0.24(0.04) | 0.01 (0.02) | 0.45 (0.10) | <0.001 |
| 4 week | 0.96 (0.11) | 0.69 (0.11) | 0.25 (0.04) | 0.03 (0.02) | 0.66 (0.11) | <0.001 |
| CSA (mm2) | ||||||
| baseline | 12.18 (0.86) | reference | 12.29 (0.42) | reference | reference | |
| 2 week | 10.94 (0.69) | −1.24 (0.50) | 12.16 (0.46) | −0.13 (0.34) | −1.12 (0.60) | 0.0665 |
| 4 week | 10.13 (0.72) | −2.05 (0.90) | 12.42 (0.45) | 0.13 (0.35) | −2.18 (0.96) | 0.0255 |
| GSS | ||||||
| baseline | 19.97 (3.86) | reference | 21.47 (2.35) | reference | reference | |
| 2 week | 9.38 (2.03) | −10.6 (3.09) | 20.59 (1.81) | −0.89 (1.10) | −9.71 (3.28) | 0.0037 |
| 4 week | 9.97 (3.09) | −10.0 (4.28) | 19.79 (2.12) | −1.69 (1.43) | −8.31 (4.51) | 0.0676 |
| DML (ms) | ||||||
| baseline | 5.61 (0.33) | reference | 5.68 (0.26) | reference | reference | |
| 4 week | 5.13 (0.23) | −0.47 (0.24) | 5.48 (0.25) | −0.19 (0.12) | −0.28 (0.27) | 0.297 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yau, Y.-C.; Yang, C.-P.; Lin, C.-P.; Tsai, I.-J.; Chang, C.-M.; Yang, C.-C.; Shih, P.-H.; Liao, Y.-Y. The Morphological and Dynamic Changes of Ultrasound in the Evaluation of Effects of Oral Steroids Treatment for Patients with Carpal Tunnel Syndrome. Diagnostics 2021, 11, 1336. https://doi.org/10.3390/diagnostics11081336
Yau Y-C, Yang C-P, Lin C-P, Tsai I-J, Chang C-M, Yang C-C, Shih P-H, Liao Y-Y. The Morphological and Dynamic Changes of Ultrasound in the Evaluation of Effects of Oral Steroids Treatment for Patients with Carpal Tunnel Syndrome. Diagnostics. 2021; 11(8):1336. https://doi.org/10.3390/diagnostics11081336
Chicago/Turabian StyleYau, Yun-Chain, Chun-Pai Yang, Ching-Po Lin, I-Ju Tsai, Ching-Mao Chang, Cheng-Chia Yang, Po-Hsuan Shih, and Yin-Yin Liao. 2021. "The Morphological and Dynamic Changes of Ultrasound in the Evaluation of Effects of Oral Steroids Treatment for Patients with Carpal Tunnel Syndrome" Diagnostics 11, no. 8: 1336. https://doi.org/10.3390/diagnostics11081336
APA StyleYau, Y.-C., Yang, C.-P., Lin, C.-P., Tsai, I.-J., Chang, C.-M., Yang, C.-C., Shih, P.-H., & Liao, Y.-Y. (2021). The Morphological and Dynamic Changes of Ultrasound in the Evaluation of Effects of Oral Steroids Treatment for Patients with Carpal Tunnel Syndrome. Diagnostics, 11(8), 1336. https://doi.org/10.3390/diagnostics11081336







