Current Insights into Oral Cancer Diagnostics
Abstract
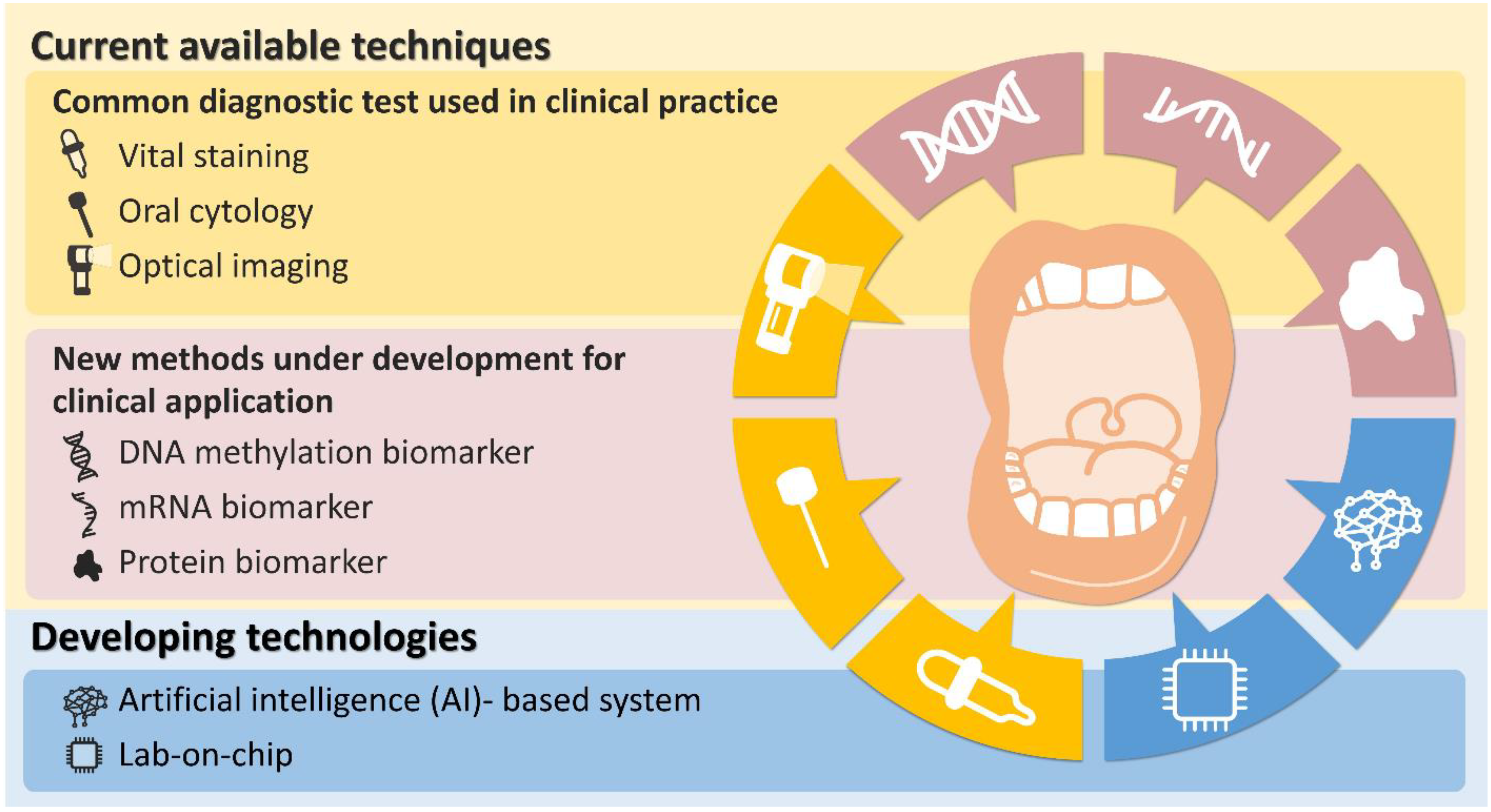
:1. Introduction
2. Current Available Techniques
2.1. Common Diagnostic Test Currently Available in Clinical Practice
2.1.1. Vital Staining
2.1.2. Oral Cytology
2.1.3. Optical Imaging
Autofluorescence-Based
Chemiluminesence-Based
Multispectral Fluorescence- and Reflectance-Based
2.2. New Methods under Development for Clinical Application
2.2.1. DNA Methylation Biomarker
ZNF582 and PAX1
2.2.2. mRNA Biomarker
Multipanel mRNA OAZ1, SAT and DUSP1
2.2.3. Protein Biomarker
CD44
S100A7
3. Developing Future Technologies
3.1. Artificial Intelligence (AI)-Based System
3.2. Lab-on-Chip
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kalavrezos, N.; Scully, C. Mouth cancer for clinicians. Part 2: Epidemiology. Dent. Update 2015, 42, 354–359. [Google Scholar] [CrossRef]
- Sankaranarayanan, R.; Ramadas, K.; Amarasinghe, H.; Subramanian, S.; Johnson, N. Oral Cancer: Prevention, Early Detection, and Treatment. In Cancer: Disease Control Priorities, 3rd ed.; Gelband, H., Jha, P., Sankaranarayanan, R., Horton, S., Eds.; The International Bank for Reconstruction and Development, The World Bank: Washington, DC, USA, 2015; Volume 3. [Google Scholar]
- WHO. WHO Classification of Tumours of Head and Neck, 4th ed.; WHO: Lyon, France, 2017. [Google Scholar]
- Chinn, S.B.; Myers, J.N. Oral Cavity Carcinoma: Current Management, Controversies, and Future Directions. J. Clin. Oncol. 2015, 33, 3269–3276. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silverman, S., Jr.; Kerr, A.R.; Epstein, J.B. Oral and pharyngeal cancer control and early detection. J. Cancer Educ. 2010, 25, 279–281. [Google Scholar] [CrossRef] [Green Version]
- McCullough, M.J.; Prasad, G.; Farah, C.S. Oral mucosal malignancy and potentially malignant lesions: An update on the epidemiology, risk factors, diagnosis and management. Aust. Dent. J. 2010, 55, 61–65. [Google Scholar] [CrossRef] [PubMed]
- Warnakulasuriya, S. Global epidemiology of oral and oropharyngeal cancer. Oral Oncol. 2009, 45, 309–316. [Google Scholar] [CrossRef] [PubMed]
- Chattopadhyay, I.; Verma, M.; Panda, M. Role of Oral Microbiome Signatures in Diagnosis and Prognosis of Oral Cancer. Technol. Cancer Res. Treat. 2019, 18, 1533033819867354. [Google Scholar] [CrossRef] [Green Version]
- Petersen, P.E. Oral cancer prevention and control—The approach of the World Health Organization. Oral Oncol. 2009, 45, 454–460. [Google Scholar] [CrossRef]
- Balaram, P.; Sridhar, H.; Rajkumar, T.; Vaccarella, S.; Herrero, R.; Nandakumar, A.; Ravichandran, K.; Ramdas, K.; Sankaranarayanan, R.; Gajalakshmi, V.; et al. Oral cancer in southern India: The influence of smoking, drinking, paan-chewing and oral hygiene. Int. J. Cancer 2002, 98, 440–445. [Google Scholar] [CrossRef]
- Gupta, B.; Bray, F.; Kumar, N.; Johnson, N.W. Associations between oral hygiene habits, diet, tobacco and alcohol and risk of oral cancer: A case-control study from India. Cancer Epidemiol. 2017, 51, 7–14. [Google Scholar] [CrossRef]
- Wang, K.; Lu, W.; Tu, Q.; Ge, Y.; He, J.; Zhou, Y.; Gou, Y.; Van Nostrand, J.D.; Qin, Y.; Li, J.; et al. Preliminary analysis of salivary microbiome and their potential roles in oral lichen planus. Sci. Rep. 2016, 6, 22943. [Google Scholar] [CrossRef]
- Yete, S.; D’Souza, W.; Saranath, D. High-Risk Human Papillomavirus in Oral Cancer: Clinical Implications. Oncology 2018, 94, 133–141. [Google Scholar] [CrossRef]
- Reibel, J.; Gale, N.; Hille, J.; Hunt, J.L.; Lingen, M.; Muller, S.; Sloan, P.; Tilakaratne, W.M.; Westra, W.H.; Willams, M.D. Oral potentially malignant disorders and oral epithelial dysplasia. In WHO Classification of Head and Neck Tumours; El-Naggar, A.K., Chan, J.K.C., Grandis, J.R., Takata, T., Slootweg, P.J., Eds.; IARC: Lyon, France, 2017; Volume 4, pp. 112–115. [Google Scholar]
- Ganesh, D.; Sreenivasan, P.; Ohman, J.; Wallstrom, M.; Braz-Silva, P.H.; Giglio, D.; Kjeller, G.; Hasseus, B. Potentially Malignant Oral Disorders and Cancer Transformation. Anticancer Res. 2018, 38, 3223–3229. [Google Scholar] [CrossRef] [Green Version]
- Mehrotra, R.; Gupta, D.K. Exciting new advances in oral cancer diagnosis: Avenues to early detection. Head Neck Oncol. 2011, 3, 33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Speight, P.M.; Epstein, J.; Kujan, O.; Lingen, M.W.; Nagao, T.; Ranganathan, K.; Vargas, P. Screening for oral cancer—A perspective from the Global Oral Cancer Forum. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2017, 123, 680–687. [Google Scholar] [CrossRef] [PubMed]
- Tilakaratne, W.M.; Jayasooriya, P.R.; Jayasuriya, N.S.; De Silva, R.K. Oral epithelial dysplasia: Causes, quantification, prognosis, and management challenges. Periodontol. 2000 2019, 80, 126–147. [Google Scholar] [CrossRef] [PubMed]
- Ranganathan, K.; Kavitha, L. Oral epithelial dysplasia: Classifications and clinical relevance in risk assessment of oral potentially malignant disorders. J. Oral Maxillofac. Pathol. 2019, 23, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Chierici, G.; Silverman, S., Jr.; Forsythe, B. A tumor registry study of oral squamous carcinoma. J. Oral. Med. 1968, 23, 91–98. [Google Scholar] [PubMed]
- Wright, A.; Shear, M. Epithelial dysplasia immediately adjacent to oral squamous cell carcinomas. J. Oral Pathol. 1985, 14, 559–564. [Google Scholar] [CrossRef]
- Mehanna, H.M.; Rattay, T.; Smith, J.; McConkey, C.C. Treatment and follow-up of oral dysplasia—A systematic review and meta-analysis. Head Neck 2009, 31, 1600–1609. [Google Scholar] [CrossRef]
- Fischer, D.J.; Epstein, J.B.; Morton, T.H.; Schwartz, S.M. Interobserver reliability in the histopathologic diagnosis of oral pre-malignant and malignant lesions. J. Oral Pathol. Med. 2004, 33, 65–70. [Google Scholar] [CrossRef]
- Seoane, J.; Varela-Centelles, P.; Ramirez, J.R.; Romero, M.A.; De La Cruz, A. Artefacts produced by suture traction during incisional biopsy of oral lesions. Clin. Otolaryngol. Allied Sci. 2002, 27, 549–553. [Google Scholar] [CrossRef]
- Epstein, J.B.; Zhang, L.; Rosin, M. Advances in the diagnosis of oral premalignant and malignant lesions. J. Can. Dent. Assoc. 2002, 68, 617–621. [Google Scholar] [PubMed]
- Vijayakumar, V.; Reghunathan, D.; Edacheriyan, B.; Mukundan, A. Role of Toluidine Blue Staining in Suspicious Lesions of Oral Cavity and Oropharynx. Indian J. Otolaryngol. Head Neck Surg. 2019, 71, 142–146. [Google Scholar] [CrossRef] [PubMed]
- Warnakulasuriya, K.A.; Johnson, N.W. Sensitivity and specificity of OraScan (R) toluidine blue mouthrinse in the detection of oral cancer and precancer. J. Oral Pathol. Med. 1996, 25, 97–103. [Google Scholar] [CrossRef]
- Petruzzi, M.; Lucchese, A.; Baldoni, E.; Grassi, F.R.; Serpico, R. Use of Lugol’s iodine in oral cancer diagnosis: An overview. Oral Oncol. 2010, 46, 811–813. [Google Scholar] [CrossRef]
- Masthan, K.M.; Babu, N.A.; Dash, K.C.; Elumalai, M. Advanced diagnostic aids in oral cancer. Asian Pac. J. Cancer Prev. 2012, 13, 3573–3576. [Google Scholar] [CrossRef] [Green Version]
- Satoskar, S.D.A. Diagnostic aids in early oral cancer detection-a review. J. Indian Acad. Oral Med. Radiol. 2006, 18, 82–89. [Google Scholar]
- Mercadante, V.; Paderni, C.; Campisi, G. Novel non-invasive adjunctive techniques for early oral cancer diagnosis and oral lesions examination. Curr. Pharm. Des. 2012, 18, 5442–5451. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perez-Sayans, M.; Somoza-Martin, J.M.; Barros-Angueira, F.; Reboiras-Lopez, M.D.; Gandara-Vila, P.; Gandara Rey, J.M.; Garcia-Garcia, A. Exfoliative cytology for diagnosing oral cancer. Biotech. Histochem. 2010, 85, 177–187. [Google Scholar] [CrossRef]
- Driemel, O.; Kosmehl, H.; Rosenhahn, J.; Berndt, A.; Reichert, T.E.; Zardi, L.; Dahse, R. Expression analysis of extracellular matrix components in brush biopsies of oral lesions. Anticancer Res. 2007, 27, 1565–1570. [Google Scholar] [PubMed]
- Sandler, H.C. Veterans Administration cooperative study of oral exfoliative cytology. Acta Cytol. 1963, 7, 180–182. [Google Scholar]
- Ogden, G.R. The future role for oral exfoliative cytology—Bleak or bright? Oral Oncol. 1997, 33, 2–4. [Google Scholar] [CrossRef]
- Dabelsteen, E.; Roed-Petersen, B.; Smith, C.J.; Pindborg, J.J. The limitations of exfoliative cytology for the detection of epithelial atypia in oral leukoplakias. Br. J. Cancer 1971, 25, 21–24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Folsom, T.C.; White, C.P.; Bromer, L.; Canby, H.F.; Garrington, G.E. Oral exfoliative study. Review of the literature and report of a three-year study. Oral Surg. Oral Med. Oral Pathol. 1972, 33, 61–74. [Google Scholar] [CrossRef]
- Diniz-Freitas, M.; Garcia-Garcia, A.; Crespo-Abelleira, A.; Martins-Carneiro, J.L.; Gandara-Rey, J.M. Applications of exfoliative cytology in the diagnosis of oral cancer. Med. Oral 2004, 9, 355–361. [Google Scholar] [PubMed]
- Lingen, M.W.; Kalmar, J.R.; Karrison, T.; Speight, P.M. Critical evaluation of diagnostic aids for the detection of oral cancer. Oral Oncol. 2008, 44, 10–22. [Google Scholar] [CrossRef] [Green Version]
- Acha, A.; Ruesga, M.T.; Rodriguez, M.J.; Martinez de Pancorbo, M.A.; Aguirre, J.M. Applications of the oral scraped (exfoliative) cytology in oral cancer and precancer. Med. Oral Patol. Oral Cir. Bucal. 2005, 10, 95–102. [Google Scholar] [PubMed]
- Sciubba, J.J. Improving detection of precancerous and cancerous oral lesions. Computer-assisted analysis of the oral brush biopsy. U.S. Collaborative OralCDx Study Group. J. Am. Dent. Assoc. 1999, 130, 1445–1457. [Google Scholar] [CrossRef] [Green Version]
- Mehrotra, R.; Mishra, S.; Singh, M.; Singh, M. The efficacy of oral brush biopsy with computer-assisted analysis in identifying precancerous and cancerous lesions. Head Neck Oncol. 2011, 3, 39. [Google Scholar] [CrossRef] [Green Version]
- Svirsky, J.A.; Burns, J.C.; Carpenter, W.M.; Cohen, D.M.; Bhattacharyya, I.; Fantasia, J.E.; Lederman, D.A.; Lynch, D.P.; Sciubba, J.J.; Zunt, S.L. Comparison of computer-assisted brush biopsy results with follow up scalpel biopsy and histology. Gen. Dent. 2002, 50, 500–503. [Google Scholar] [PubMed]
- Rick, G.M. Oral brush biopsy: The problem of false positives. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2003, 96, 252. [Google Scholar] [CrossRef]
- Greenberg, M.S. The “brush” controversy. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2002, 93, 217–218. [Google Scholar] [CrossRef] [PubMed]
- Messadi, D.V. Diagnostic aids for detection of oral precancerous conditions. Int. J. Oral. Sci. 2013, 5, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Balasubramaniam, A.M.; Sriraman, R.; Sindhuja, P.; Mohideen, K.; Parameswar, R.A.; Muhamed Haris, K.T. Autofluorescence based diagnostic techniques for oral cancer. J. Pharm. Bioallied. Sci. 2015, 7, S374–S377. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Gleysteen, J.; Teraphongphom, N.T.; Li, Y.; Rosenthal, E. In-vivo optical imaging in head and neck oncology: Basic principles, clinical applications and future directions. Int. J. Oral Sci. 2018, 10, 10. [Google Scholar] [CrossRef] [Green Version]
- Shashidara, R.; Sreeshyla, H.S.; Sudheendra, U.S. Chemiluminescence: A diagnostic adjunct in oral precancer and cancer: A review. J. Cancer Res. Ther. 2014, 10, 487–491. [Google Scholar] [CrossRef] [PubMed]
- Awan, K.H.; Morgan, P.R.; Warnakulasuriya, S. Evaluation of an autofluorescence based imaging system (VELscope) in the detection of oral potentially malignant disorders and benign keratoses. Oral Oncol. 2011, 47, 274–277. [Google Scholar] [CrossRef] [PubMed]
- Farah, C.S.; McIntosh, L.; Georgiou, A.; McCullough, M.J. Efficacy of tissue autofluorescence imaging (VELScope) in the visualization of oral mucosal lesions. Head Neck 2012, 34, 856–862. [Google Scholar] [CrossRef] [PubMed]
- Ram, S.; Siar, C.H. Chemiluminescence as a diagnostic aid in the detection of oral cancer and potentially malignant epithelial lesions. Int. J. Oral Maxillofac. Surg. 2005, 34, 521–527. [Google Scholar] [CrossRef] [Green Version]
- Epstein, J.B.; Gorsky, M.; Lonky, S.; Silverman, S., Jr.; Epstein, J.D.; Bride, M. The efficacy of oral lumenoscopy (ViziLite) in visualizing oral mucosal lesions. Spec. Care Dentist. 2006, 26, 171–174. [Google Scholar] [CrossRef]
- Kerr, A.R.; Sirois, D.A.; Epstein, J.B. Clinical evaluation of chemiluminescent lighting: An adjunct for oral mucosal examinations. J. Clin. Dent. 2006, 17, 59–63. [Google Scholar]
- Huber, M.A.; Bsoul, S.A.; Terezhalmy, G.T. Acetic acid wash and chemiluminescent illumination as an adjunct to conventional oral soft tissue examination for the detection of dysplasia: A pilot study. Quintessence Int. 2004, 35, 378–384. [Google Scholar]
- Awan, K.H.; Morgan, P.R.; Warnakulasuriya, S. Utility of chemiluminescence (ViziLite) in the detection of oral potentially malignant disorders and benign keratoses. J. Oral Pathol. Med. 2011, 40, 541–544. [Google Scholar] [CrossRef] [PubMed]
- Messadi, D.V.; Younai, F.S.; Liu, H.H.; Guo, G.; Wang, C.Y. The clinical effectiveness of reflectance optical spectroscopy for the in vivo diagnosis of oral lesions. Int. J. Oral Sci. 2014, 6, 162–167. [Google Scholar] [CrossRef] [PubMed]
- Mascitti, M.; Orsini, G.; Tosco, V.; Monterubbianesi, R.; Balercia, A.; Putignano, A.; Procaccini, M.; Santarelli, A. An Overview on Current Non-invasive Diagnostic Devices in Oral Oncology. Front. Physiol. 2018, 9, 1510. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lalla, Y.; Matias, M.A.; Farah, C.S. Assessment of oral mucosal lesions with autofluorescence imaging and reflectance spectroscopy. J. Am. Dent. Assoc. 2016, 147, 650–660. [Google Scholar] [CrossRef] [PubMed]
- Santosh, A.B.; Jones, T.; Harvey, J. A review on oral cancer biomarkers: Understanding the past and learning from the present. J. Cancer Res. Ther. 2016, 12, 486–492. [Google Scholar] [CrossRef] [PubMed]
- Hanson, E.K.; Lubenow, H.; Ballantyne, J. Identification of forensically relevant body fluids using a panel of differentially expressed microRNAs. Anal. Biochem. 2009, 387, 303–314. [Google Scholar] [CrossRef] [PubMed]
- Mayeux, R. Biomarkers: Potential uses and limitations. NeuroRx 2004, 1, 182–188. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, S.; Sankaranarayanan, R.; Bapat, B.; Somanathan, T.; Thomas, G.; Mathew, B.; Vinoda, J.; Ramadas, K. Cost-effectiveness of oral cancer screening: Results from a cluster randomized controlled trial in India. Bull. World Health Organ. 2009, 87, 200–206. [Google Scholar] [CrossRef]
- Hashim, D.; Genden, E.; Posner, M.; Hashibe, M.; Boffetta, P. Head and neck cancer prevention: From primary prevention to impact of clinicians on reducing burden. Ann. Oncol. 2019, 30, 744–756. [Google Scholar] [CrossRef] [Green Version]
- Ecco, G.; Imbeault, M.; Trono, D. KRAB zinc finger proteins. Development 2017, 144, 2719–2729. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, Y.; Hong, X.H.; Li, K.; Li, Y.Q.; Li, Y.Q.; He, S.W.; Zhang, P.P.; Li, J.Y.; Li, Q.; Liang, Y.L.; et al. ZNF582 hypermethylation promotes metastasis of nasopharyngeal carcinoma by regulating the transcription of adhesion molecules Nectin-3 and NRXN3. Cancer Commun. 2020, 40, 721–737. [Google Scholar] [CrossRef]
- Van der Zee, R.P.; Richel, O.; van Noesel, C.J.M.; Novianti, P.W.; Ciocanea-Teodorescu, I.; van Splunter, A.P.; Duin, S.; van den Berk, G.E.L.; Meijer, C.; Quint, W.G.V.; et al. Host Cell Deoxyribonucleic Acid Methylation Markers for the Detection of High-grade Anal Intraepithelial Neoplasia and Anal Cancer. Clin. Infect. Dis. 2019, 68, 1110–1117. [Google Scholar] [CrossRef]
- Huang, J.; Wang, G.; Tang, J.; Zhuang, W.; Wang, L.P.; Liou, Y.L.; Liu, Y.Z.; Zhou, H.H.; Zhu, Y.S. DNA Methylation Status of PAX1 and ZNF582 in Esophageal Squamous Cell Carcinoma. Int. J. Environ. Res. Public Health 2017, 14, 216. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sivakamasundari, V.; Kraus, P.; Sun, W.; Hu, X.; Lim, S.L.; Prabhakar, S.; Lufkin, T. A developmental transcriptomic analysis of Pax1 and Pax9 in embryonic intervertebral disc development. Biol. Open 2017, 6, 187–199. [Google Scholar] [CrossRef] [Green Version]
- Cheng, S.J.; Chang, C.F.; Lee, J.J.; Chen, H.M.; Wang, H.J.; Liou, Y.L.; Yen, C.; Chiang, C.P. Hypermethylated ZNF582 and PAX1 are effective biomarkers for detection of oral dysplasia and oral cancer. Oral Oncol. 2016, 62, 34–43. [Google Scholar] [CrossRef]
- Cheng, S.J.; Chang, C.F.; Ko, H.H.; Liu, Y.C.; Peng, H.H.; Wang, H.J.; Lin, H.S.; Chiang, C.P. Hypermethylated ZNF582 and PAX1 genes in oral scrapings collected from cancer-adjacent normal oral mucosal sites are associated with aggressive progression and poor prognosis of oral cancer. Oral Oncol. 2017, 75, 169–177. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.L.; Ho, S.C.; Su, Y.F.; Juan, Y.C.; Huang, C.Y.; Chao, A.S.; Hsu, Z.S.; Chang, C.F.; Fwu, C.W.; Chang, T.C. DNA methylation marker for the triage of hrHPV positive women in cervical cancer screening: Real-world evidence in Taiwan. Gynecol. Oncol. 2021. [Google Scholar] [CrossRef] [PubMed]
- Sun, R.; Juan, Y.C.; Su, Y.F.; Zhang, W.B.; Yu, Y.; Yang, H.Y.; Yu, G.Y.; Peng, X. Hypermethylated PAX1 and ZNF582 genes in the tissue sample are associated with aggressive progression of oral squamous cell carcinoma. J. Oral Pathol. Med. 2020, 49, 751–760. [Google Scholar] [CrossRef] [PubMed]
- Tsuji, T.; Katsurano, M.; Ibaragi, S.; Shima, K.; Sasaki, A.; Hu, G.F. Ornithine decarboxylase antizyme upregulates DNA-dependent protein kinase and enhances the nonhomologous end-joining repair of DNA double-strand breaks in human oral cancer cells. Biochemistry 2007, 46, 8920–8932. [Google Scholar] [CrossRef] [PubMed]
- Rom, E.; Kahana, C. Polyamines regulate the expression of ornithine decarboxylase antizyme in vitro by inducing ribosomal frame-shifting. Proc. Natl. Acad. Sci. USA 1994, 91, 3959–3963. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martin, J.L.; Gottehrer, N.; Zalesin, H.; Hoff, P.T.; Shaw, M.; Clarkson, J.H.; Haan, P.; Vartanian, M.; McLeod, T.; Swanick, S.M. Evaluation of Salivary Transcriptome Markers for the Early Detection of Oral Squamous Cell Cancer in a Prospective Blinded Trial. Compend. Contin. Educ. Dent. 2015, 36, 365–373. [Google Scholar]
- Bettuzzi, S.; Davalli, P.; Astancolle, S.; Carani, C.; Madeo, B.; Tampieri, A.; Corti, A. Tumor progression is accompanied by significant changes in the levels of expression of polyamine metabolism regulatory genes and clusterin (sulfated glycoprotein 2) in human prostate cancer specimens. Cancer Res. 2000, 60, 28–34. [Google Scholar]
- Patterson, K.I.; Brummer, T.; O’Brien, P.M.; Daly, R.J. Dual-specificity phosphatases: Critical regulators with diverse cellular targets. Biochem. J. 2009, 418, 475–489. [Google Scholar] [CrossRef] [Green Version]
- Elashoff, D.; Zhou, H.; Reiss, J.; Wang, J.; Xiao, H.; Henson, B.; Hu, S.; Arellano, M.; Sinha, U.; Le, A.; et al. Prevalidation of salivary biomarkers for oral cancer detection. Cancer Epidemiol. Biomark. Prev. 2012, 21, 664–672. [Google Scholar] [CrossRef] [Green Version]
- Genetic Testing Registry. Available online: https://www.ncbi.nlm.nih.gov/gtr/tests/561540/ (accessed on 31 May 2021).
- Screaton, G.R.; Bell, M.V.; Jackson, D.G.; Cornelis, F.B.; Gerth, U.; Bell, J.I. Genomic structure of DNA encoding the lymphocyte homing receptor CD44 reveals at least 12 alternatively spliced exons. Proc. Natl. Acad. Sci. USA 1992, 89, 12160–12164. [Google Scholar] [CrossRef] [Green Version]
- Ponta, H.; Sherman, L.; Herrlich, P.A. CD44: From adhesion molecules to signalling regulators. Nat. Rev. Mol. Cell Biol. 2003, 4, 33–45. [Google Scholar] [CrossRef]
- Perez, A.; Neskey, D.M.; Wen, J.; Pereira, L.; Reategui, E.P.; Goodwin, W.J.; Carraway, K.L.; Franzmann, E.J. CD44 interacts with EGFR and promotes head and neck squamous cell carcinoma initiation and progression. Oral Oncol. 2013, 49, 306–313. [Google Scholar] [CrossRef] [Green Version]
- Emich, H.; Chapireau, D.; Hutchison, I.; Mackenzie, I. The potential of CD44 as a diagnostic and prognostic tool in oral cancer. J. Oral. Pathol. Med. 2015, 44, 393–400. [Google Scholar] [CrossRef]
- Hirvikoski, P.; Tammi, R.; Kumpulainen, E.; Virtaniemi, J.; Parkkinen, J.J.; Tammi, M.; Johansson, R.; Agren, U.; Karhunen, J.; Kosma, V.M. Irregular expression of hyaluronan and its CD44 receptor is associated with metastatic phenotype in laryngeal squamous cell carcinoma. Virchows Arch. 1999, 434, 37–44. [Google Scholar] [CrossRef]
- Ioachim, E.; Assimakopoulos, D.; Goussia, A.C.; Peschos, D.; Skevas, A.; Agnantis, N.J. Glycoprotein CD44 expression in benign, premalignant and malignant epithelial lesions of the larynx: An immunohistochemical study including correlation with Rb, p53, Ki-67 and PCNA. Histol. Histopathol. 1999, 14, 1113–1118. [Google Scholar] [CrossRef]
- Dasari, S.; Rajendra, W.; Valluru, L. Evaluation of soluble CD44 protein marker to distinguish the premalignant and malignant carcinoma cases in cervical cancer patients. Med. Oncol. 2014, 31, 139. [Google Scholar] [CrossRef] [PubMed]
- Grau, J.J.; Mesia, R.; de la Iglesia-Vicente, M.; Williams, E.S.; Taberna, M.; Caballero, M.; Larque, A.B.; de la Oliva, J.; Cordon-Cardo, C.; Domingo-Domenech, J. Enrichment of Cells with Cancer Stem Cell-Like Markers in Relapses of Chemoresistant Patients with Locally Advanced Head and Neck Squamous Cell Carcinoma. Oncology 2016, 90, 267–272. [Google Scholar] [CrossRef] [PubMed]
- Kajita, M.; Itoh, Y.; Chiba, T.; Mori, H.; Okada, A.; Kinoh, H.; Seiki, M. Membrane-type 1 matrix metalloproteinase cleaves CD44 and promotes cell migration. J. Cell Biol. 2001, 153, 893–904. [Google Scholar] [CrossRef]
- Kamarajan, P.; Shin, J.M.; Qian, X.; Matte, B.; Zhu, J.Y.; Kapila, Y.L. ADAM17-mediated CD44 cleavage promotes orasphere formation or stemness and tumorigenesis in HNSCC. Cancer Med. 2013, 2, 793–802. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Franzmann, E.J.; Donovan, M.J. Effective early detection of oral cancer using a simple and inexpensive point of care device in oral rinses. Expert Rev. Mol. Diagn. 2018, 18, 837–844. [Google Scholar] [CrossRef]
- Pereira, L.H.; Reis, I.M.; Reategui, E.P.; Gordon, C.; Saint-Victor, S.; Duncan, R.; Gomez, C.; Bayers, S.; Fisher, P.; Perez, A.; et al. Risk Stratification System for Oral Cancer Screening. Cancer Prev. Res. 2016, 9, 445–455. [Google Scholar] [CrossRef] [Green Version]
- Broome, A.M.; Ryan, D.; Eckert, R.L. S100 protein subcellular localization during epidermal differentiation and psoriasis. J. Histochem. Cytochem. 2003, 51, 675–685. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaur, J.; Matta, A.; Kak, I.; Srivastava, G.; Assi, J.; Leong, I.; Witterick, I.; Colgan, T.J.; Macmillan, C.; Siu, K.W.; et al. S100A7 overexpression is a predictive marker for high risk of malignant transformation in oral dysplasia. Int. J. Cancer 2014, 134, 1379–1388. [Google Scholar] [CrossRef]
- Chauhan, S.S.; Kaur, J.; Kumar, M.; Matta, A.; Srivastava, G.; Alyass, A.; Assi, J.; Leong, I.; MacMillan, C.; Witterick, I.; et al. Prediction of recurrence-free survival using a protein expression-based risk classifier for head and neck cancer. Oncogenesis 2015, 4, e147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hwang, J.T.; Gu, Y.R.; Shen, M.; Ralhan, R.; Walfish, P.G.; Pritzker, K.P.; Mock, D. Individualized five-year risk assessment for oral premalignant lesion progression to cancer. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2017, 123, 374–381. [Google Scholar] [CrossRef]
- Ilhan, B.; Lin, K.; Guneri, P.; Wilder-Smith, P. Improving Oral Cancer Outcomes with Imaging and Artificial Intelligence. J. Dent. Res. 2020, 99, 241–248. [Google Scholar] [CrossRef]
- Kar, A.; Wreesmann, V.B.; Shwetha, V.; Thakur, S.; Rao, V.U.S.; Arakeri, G.; Brennan, P.A. Improvement of oral cancer screening quality and reach: The promise of artificial intelligence. J. Oral Pathol. Med. 2020, 49, 727–730. [Google Scholar] [CrossRef]
- Amisha, M.P.; Pathania, M.; Rathaur, V.K. Overview of artificial intelligence in medicine. J. Family Med. Prim. Care 2019, 8, 2328–2331. [Google Scholar] [CrossRef]
- LeCun, Y.; Bengio, Y.; Hinton, G. Deep learning. Nature 2015, 521, 436–444. [Google Scholar] [CrossRef] [PubMed]
- Ilhan, B.; Guneri, P.; Wilder-Smith, P. The contribution of artificial intelligence to reducing the diagnostic delay in oral cancer. Oral Oncol. 2021, 116, 105254. [Google Scholar] [CrossRef]
- Song, B.; Sunny, S.; Uthoff, R.D.; Patrick, S.; Suresh, A.; Kolur, T.; Keerthi, G.; Anbarani, A.; Wilder-Smith, P.; Kuriakose, M.A.; et al. Automatic classification of dual-modalilty, smartphone-based oral dysplasia and malignancy images using deep learning. Biomed. Opt. Express 2018, 9, 5318–5329. [Google Scholar] [CrossRef] [PubMed]
- Uthoff, R.D.; Song, B.; Sunny, S.; Patrick, S.; Suresh, A.; Kolur, T.; Keerthi, G.; Spires, O.; Anbarani, A.; Wilder-Smith, P.; et al. Point-of-care, smartphone-based, dual-modality, dual-view, oral cancer screening device with neural network classification for low-resource communities. PLoS ONE 2018, 13, e0207493. [Google Scholar] [CrossRef] [PubMed]
- De Almeida Geraldino, R.; Rezende, L.; da-Silva, C.Q.; Almeida, J.C.F. Remote diagnosis of traumatic dental injuries using digital photographs captured via a mobile phone. Dent. Traumatol. 2017, 33, 350–357. [Google Scholar] [CrossRef] [PubMed]
- Skandarajah, A.; Sunny, S.P.; Gurpur, P.; Reber, C.D.; D’Ambrosio, M.V.; Raghavan, N.; James, B.L.; Ramanjinappa, R.D.; Suresh, A.; Kandasarma, U.; et al. Mobile microscopy as a screening tool for oral cancer in India: A pilot study. PLoS ONE 2017, 12, e0188440. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.Y.; Tsai, T.; Chen, H.M.; Chen, C.T.; Chiang, C.P. PLS-ANN based classification model for oral submucous fibrosis and oral carcinogenesis. Lasers Surg. Med. 2003, 32, 318–326. [Google Scholar] [CrossRef] [PubMed]
- Fu, Q.; Chen, Y.; Li, Z.; Jing, Q.; Hu, C.; Liu, H.; Bao, J.; Hong, Y.; Shi, T.; Li, K.; et al. A deep learning algorithm for detection of oral cavity squamous cell carcinoma from photographic images: A retrospective study. EClinicalMedicine 2020, 27, 100558. [Google Scholar] [CrossRef]
- Tseng, Y.J.; Wang, H.Y.; Lin, T.W.; Lu, J.J.; Hsieh, C.H.; Liao, C.T. Development of a Machine Learning Model for Survival Risk Stratification of Patients With Advanced Oral Cancer. JAMA Netw. Open 2020, 3, e2011768. [Google Scholar] [CrossRef]
- Chang, S.W.; Abdul-Kareem, S.; Merican, A.F.; Zain, R.B. Oral cancer prognosis based on clinicopathologic and genomic markers using a hybrid of feature selection and machine learning methods. BMC Bioinform. 2013, 14, 170. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Yang, J.; Wei, C.; Zhou, G.; Wu, L.; Gao, Q.; He, X.; Shi, J.; Mei, Y.; Liu, Y.; et al. A personalized computational model predicts cancer risk level of oral potentially malignant disorders and its web application for promotion of non-invasive screening. J. Oral Pathol. Med. 2020, 49, 417–426. [Google Scholar] [CrossRef] [PubMed]
- Pandya, D.; Nagarajappa, A.K.; Reddy, S.; Bhasin, M. Lab-on-a-Chip—Oral Cancer Diagnosis at Your Door Step. J. Int. Oral Health 2015, 7, 122–128. [Google Scholar]
- Daniel, G.S.T.; Thiruppathy, M.; Aswath, N.; Narayanan, S.R. Lab on a Chip: Conquer Disease at the Earliest. J. Pharm. Bioallied Sci. 2018, 10, 106–108. [Google Scholar] [CrossRef]
- Ziober, B.L.; Mauk, M.G.; Falls, E.M.; Chen, Z.; Ziober, A.F.; Bau, H.H. Lab-on-a-chip for oral cancer screening and diagnosis. Head Neck 2008, 30, 111–121. [Google Scholar] [CrossRef] [Green Version]
- Gau, V.; Wong, D. Oral fluid nanosensor test (OFNASET) with advanced electrochemical-based molecular analysis platform. Ann. N. Y. Acad. Sci. 2007, 1098, 401–410. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Su, Y.-F.; Chen, Y.-J.; Tsai, F.-T.; Li, W.-C.; Hsu, M.-L.; Wang, D.-H.; Yang, C.-C. Current Insights into Oral Cancer Diagnostics. Diagnostics 2021, 11, 1287. https://doi.org/10.3390/diagnostics11071287
Su Y-F, Chen Y-J, Tsai F-T, Li W-C, Hsu M-L, Wang D-H, Yang C-C. Current Insights into Oral Cancer Diagnostics. Diagnostics. 2021; 11(7):1287. https://doi.org/10.3390/diagnostics11071287
Chicago/Turabian StyleSu, Yee-Fun, Yi-Ju Chen, Fa-Tzu Tsai, Wan-Chun Li, Ming-Lun Hsu, Ding-Han Wang, and Cheng-Chieh Yang. 2021. "Current Insights into Oral Cancer Diagnostics" Diagnostics 11, no. 7: 1287. https://doi.org/10.3390/diagnostics11071287
APA StyleSu, Y.-F., Chen, Y.-J., Tsai, F.-T., Li, W.-C., Hsu, M.-L., Wang, D.-H., & Yang, C.-C. (2021). Current Insights into Oral Cancer Diagnostics. Diagnostics, 11(7), 1287. https://doi.org/10.3390/diagnostics11071287





