Comprehensive Echocardiography of Left Atrium and Left Ventricle Using Modern Techniques Helps in Better Revealing Atrial Fibrillation in Patients with Hypertrophic Cardiomyopathy
Abstract
:1. Introduction
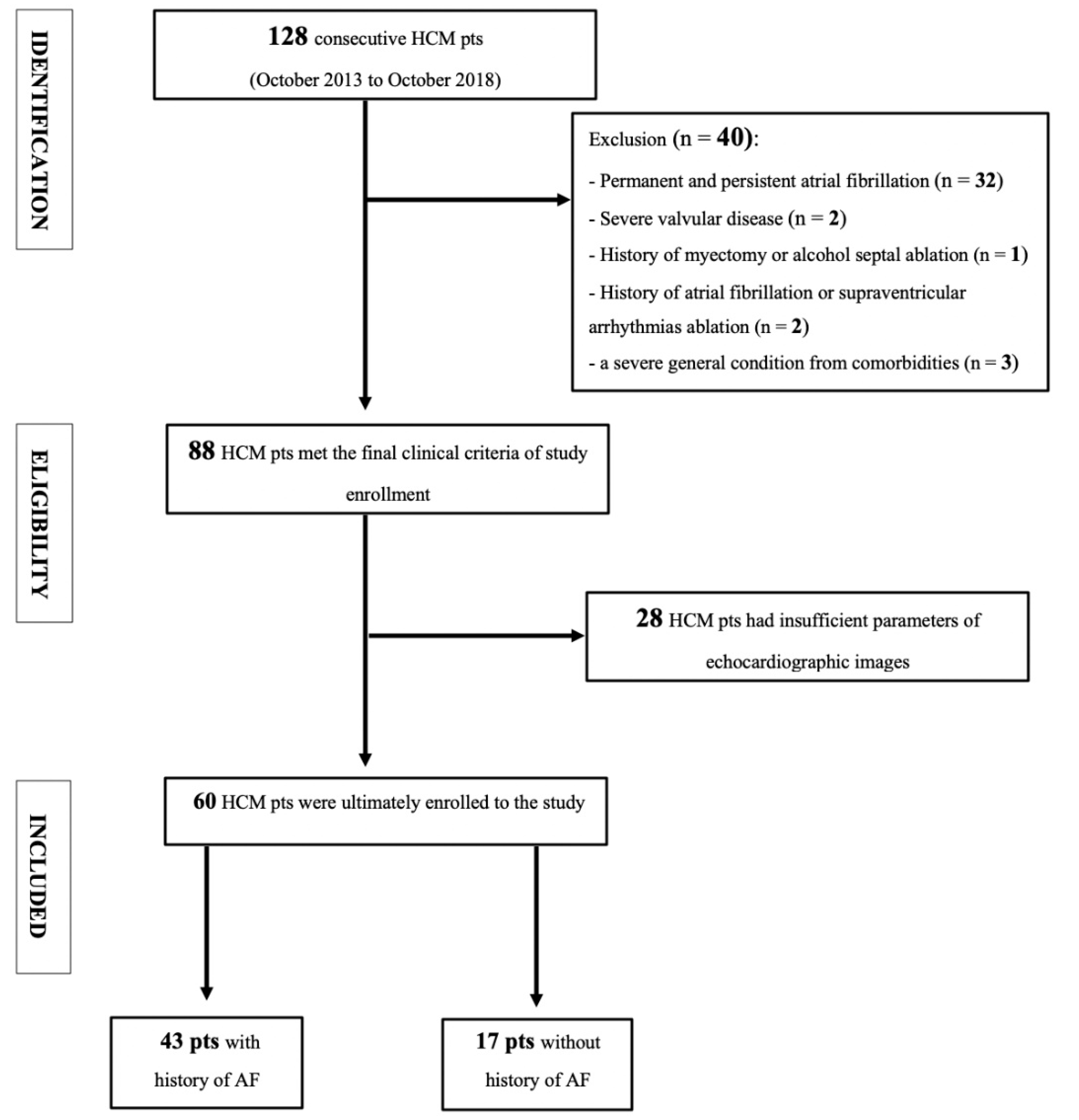
2. Materials and Methods
2.1. Study Population
2.2. Echocardiography
2.2.1. Standard Echocardiography Parameters
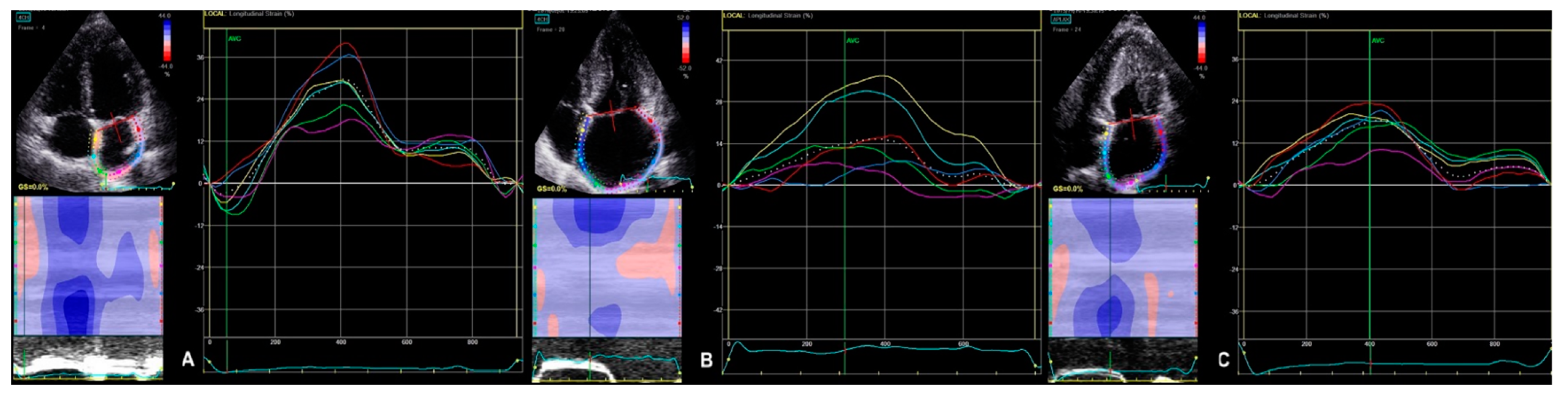
2.2.2. 2D STE Parameters
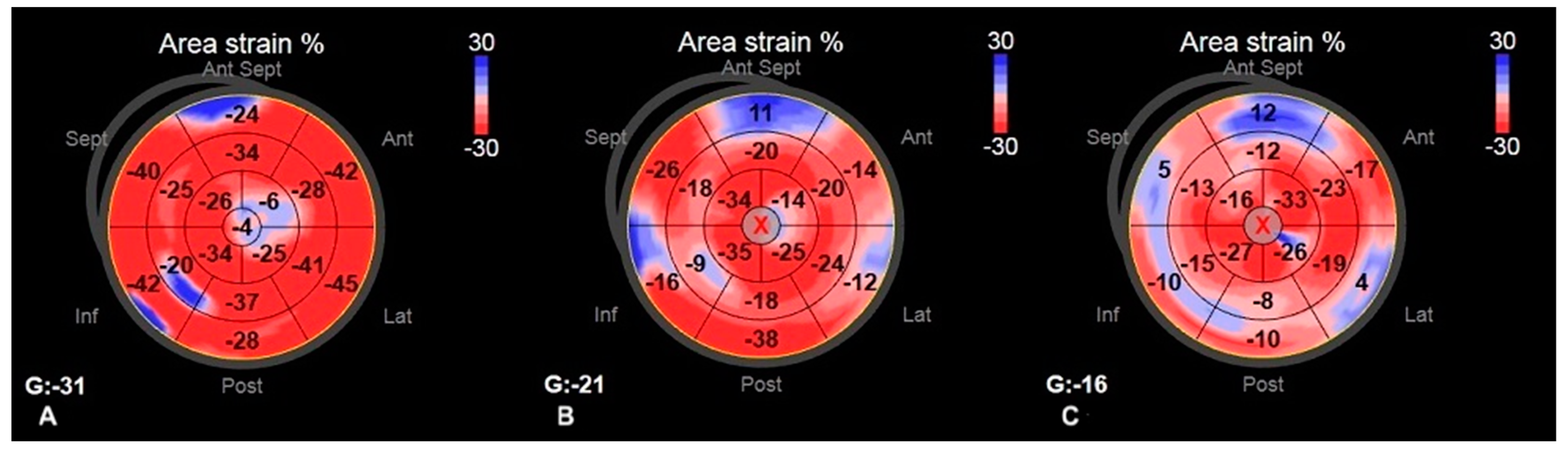
2.2.3. 3D STE Parameters
2.3. Statistics
3. Results
Study Population
4. Discussion
4.1. Clinical Implications
4.2. Study Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Maron, B.J.; Gardin, J.M.; Flack, J.M.; Gidding, S.S.; Kurosaki, T.T.; Bild, D.E. Prevalence of Hypertrophic Cardiomyopathy in a General Population of Young Adults. Circulation 1995, 92, 785–789. [Google Scholar] [CrossRef]
- Zou, Y.; Song, L.; Wang, Z.; Ma, A.; Liu, T.; Gu, H.; Lu, S.; Wu, P.; Zhang, Y.; Shen, L.; et al. Prevalence of idiopathic hypertrophic cardiomyopathy in China: A population-based echocardiographic analysis of 8080 adults. Am. J. Med. 2004, 116, 14–18. [Google Scholar] [CrossRef]
- Philipson, D.J.; Rader, F.; Siegel, R.J. Risk factors for atrial fibrillation in hypertrophic cardiomyopathy. Eur. J. Prev. Cardiol. 2019, 28, 658–665. [Google Scholar] [CrossRef]
- Olivotto, I.; Cecchi, F.; Casey, S.A.; Dolara, A.; Traverse, J.H.; Maron, B.J. Impact of Atrial Fibrillation on the Clinical Course of Hypertrophic Cardiomyopathy. Circulation 2001, 104, 2517–2524. [Google Scholar] [CrossRef] [Green Version]
- Debonnaire, P.; Joyce, E.; Hiemstra, Y.; Mertens, B.J.; Atsma, D.E.; Schalij, M.J.; Bax, J.J.; Delgado, V.; Marsan, N.A. Left Atrial Size and Function in Hypertrophic Cardiomyopathy Patients and Risk of New-Onset Atrial Fibrillation. Circ. Arrhythmia Electrophysiol. 2017, 10, e004052. [Google Scholar] [CrossRef]
- Maron, B.J.; Olivotto, I.; Bellone, P.; Conte, M.R.; Cecchi, F.; Flygenring, B.P.; Casey, S.A.; Gohman, T.E.; Bongioanni, S.; Spirito, P. Clinical profile of stroke in 900 patients with hypertrophic cardiomyopathy. J. Am. Coll. Cardiol. 2002, 39, 301–307. [Google Scholar] [CrossRef] [Green Version]
- Authors/Task Force Members; Elliott, P.M.; Anastasakis, A.; Borger, M.A.; Borggrefe, M.; Cecchi, F.; Charron, P.; Hagege, A.A.; Lafont, A.; Limongelli, G.; et al. 2014 ESC Guidelines on diagnosis and management of hypertrophic cardiomyopathy. Eur. Heart J. 2014, 35, 2733–2779. [Google Scholar] [CrossRef] [Green Version]
- Guttmann, O.P.; Pavlou, M.; O’Mahony, C.; Monserrat, L.; Anastasakis, A.; Rapezzi, C.; Biagini, E.; Gimeno, J.R.; Limongelli, G.; Garcia-Pavia, P.; et al. Predictors of atrial fibrillation in hypertrophic cardiomyopathy. Heart 2016, 103, 672–678. [Google Scholar] [CrossRef]
- Losi, M.-A.; Betocchi, S.; Aversa, M.; Lombardi, R.; Miranda, M.; D’Alessandro, G.; Cacace, A.; Tocchetti, C.G.; Barbati, G.; Chiariello, M. Determinants of atrial fibrillation development in patients with hypertrophic cardiomyopathy. Am. J. Cardiol. 2004, 94, 895–900. [Google Scholar] [CrossRef]
- Tani, T.; Tanabe, K.; Ono, M.; Yamaguchi, K.; Okada, M.; Sumida, T.; Konda, T.; Fujii, Y.; Kawai, J.; Yagi, T.; et al. Left atrial volume and the risk of paroxysmal atrial fibrillation in patients with hypertrophic cardiomyopathy. J. Am. Soc. Echocardiogr. 2004, 17, 644–648. [Google Scholar] [CrossRef]
- Yang, H.; Woo, A.; Monakier, D.; Jamorski, M.; Fedwick, K.; Wigle, E.D.; Rakowski, H. Enlarged Left Atrial Volume in Hypertrophic Cardiomyopathy: A Marker for Disease Severity. J. Am. Soc. Echocardiogr. 2005, 18, 1074–1082. [Google Scholar] [CrossRef]
- Vaidya, K.; Semsarian, C.; Chan, K.H. Atrial Fibrillation in Hypertrophic Cardiomyopathy. Heart Lung Circ. 2017, 26, 975–982. [Google Scholar] [CrossRef]
- Miyazawa, K.; Lip, G.Y. Atrial fibrillation and hypertrophic cardiomyopathy: Co-existing conditions with additive risks. Hell. J. Cardiol. 2017, 58, 340–341. [Google Scholar] [CrossRef]
- Siontis, K.C.; Geske, J.B.; Ong, K.; Nishimura, R.A.; Ommen, S.R.; Gersh, B.J. Atrial Fibrillation in Hypertrophic Cardiomyopathy: Prevalence, Clinical Correlations, and Mortality in a Large High-Risk Population. J. Am. Heart Assoc. 2014, 3, e001002. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vasquez, N.; Ostrander, B.T.; Lu, D.-Y.; Ventoulis, I.; Haileselassie, B.; Goyal, S.; Greenland, G.; Vakrou, S.; Olgin, J.E.; Abraham, T.P.; et al. Low Left Atrial Strain Is Associated with Adverse Outcomes in Hypertrophic Cardiomyopathy Patients. J. Am. Soc. Echocardiogr. 2019, 32, 593–603.e1. [Google Scholar] [CrossRef] [PubMed]
- Fujimoto, K.; Inoue, K.; Saito, M.; Higashi, H.; Kono, T.; Uetani, T.; Aono, J.; Nagai, T.; Nishimura, K.; Suzuki, J.; et al. Incremental value of left atrial active function measured by speckle tracking echocardiography in patients with hypertrophic cardiomyopathy. Echocardiography 2018, 35, 1138–1148. [Google Scholar] [CrossRef] [PubMed]
- Zegkos, T.; Parcharidou, D.; Ntelios, D.; Efthimiadis, G.; Karvounis, H. The Prognostic Implications of Two-Dimensional Speckle Tracking Echocardiography in Hypertrophic Cardiomyopathy. Cardiol. Rev. 2018, 26, 130–136. [Google Scholar] [CrossRef]
- Huang, X.; Yue, Y.; Wang, Y.; Deng, Y.; Liu, L.; Di, Y.; Sun, S.; Chen, D.; Fan, L.; Cao, J. Assessment of left ventricular systolic and diastolic abnormalities in patients with hypertrophic cardiomyopathy using real-time three-dimensional echocardiography and two-dimensional speckle tracking imaging. Cardiovasc. Ultrasound 2018, 16, 23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vergé, M.-P.; Cochet, H.; Reynaud, A.; Morlon, L.; Peyrou, J.; Vincent, C.; Rooryck, C.; Ritter, P.; Lafitte, S.; Reant, P. Characterization of hypertrophic cardiomyopathy according to global, regional, and multi-layer longitudinal strain analysis, and prediction of sudden cardiac death. Int. J. Cardiovasc. Imaging 2018, 34, 1091–1098. [Google Scholar] [CrossRef] [PubMed]
- Correia, E.; Rodrigues, B.; Santos, L.F.; Moreira, D.; Gama, P.; Cabral, C.; Santos, O. Longitudinal Left Ventricular Strain in Hypertrophic Cardiomyopathy: Correlation with Nonsustained Ventricular Tachycardia. Echocardiography 2011, 28, 709–714. [Google Scholar] [CrossRef]
- Saito, M.; Okayama, H.; Yoshii, T.; Higashi, H.; Morioka, H.; Hiasa, G.; Sumimoto, T.; Inaba, S.; Nishimura, K.; Inoue, K.; et al. Clinical significance of global two-dimensional strain as a surrogate parameter of myocardial fibrosis and cardiac events in patients with hypertrophic cardiomyopathy. Eur. Heart J. Cardiovasc. Imaging 2012, 13, 617–623. [Google Scholar] [CrossRef] [PubMed]
- Debonnaire, P.; Thijssen, J.; Leong, D.P.; Joyce, E.; Katsanos, S.; Hoogslag, G.E.; Schalij, M.J.; Atsma, D.E.; Bax, J.J.; Delgado, V.; et al. Global longitudinal strain and left atrial volume index improve prediction of appropriate implantable cardioverter defibrillator therapy in hypertrophic cardiomyopathy patients. Int. J. Cardiovasc. Imaging 2014, 30, 549–558. [Google Scholar] [CrossRef] [PubMed]
- Hartlage, G.R.; Kim, J.H.; Strickland, P.T.; Cheng, A.C.; Ghasemzadeh, N.; Pernetz, M.A.; Clements, S.D.; Williams, B.R. The prognostic value of standardized reference values for speckle-tracking global longitudinal strain in hypertrophic cardiomyopathy. Int. J. Cardiovasc. Imaging 2015, 31, 557–565. [Google Scholar] [CrossRef]
- Reant, P.; Mirabel, M.; Lloyd, G.; Peyrou, J.; Lopez-Ayala, J.M.; Dickie, S.; Bulluck, H.; Captur, G.; Rosmini, S.; Guttmann, O.; et al. Global longitudinal strain is associated with heart failure outcomes in hypertrophic cardiomyopathy. Heart 2016, 102, 741–747. [Google Scholar] [CrossRef]
- Haland, T.F.; Almaas, V.M.; Hasselberg, N.E.; Saberniak, J.; Leren, I.S.; Hopp, E.; Edvardsen, T.; Haugaa, K.H. Strain echocardiography is related to fibrosis and ventricular arrhythmias in hypertrophic cardiomyopathy. Eur. Heart J. Cardiovasc. Imaging 2016, 17, 613–621. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wabich, E.; Dorniak, K.; Zienciuk-Krajka, A.; Nowak, R.; Raczak, G.; Daniłowicz-Szymanowicz, L. Segmental longitudinal strain as the most accurate predictor of the patchy pattern late gadolinium enhancement in hypertrophic cardiomyopathy. J. Cardiol. 2021, 77, 475–481. [Google Scholar] [CrossRef]
- Inciardi, R.M.; Galderisi, M.; Nistri, S.; Santoro, C.; Cicoira, M.; Rossi, A. Echocardiographic advances in hypertrophic cardiomyopathy: Three-dimensional and strain imaging echocardiography. Echocardiography 2018, 35, 716–726. [Google Scholar] [CrossRef]
- Shin, M.-S.; Fukuda, S.; Song, J.-M.; Tran, H.; Oryszak, S.; Thomas, J.D.; Shiota, T. Relationship Between Left Atrial and Left Ventricular Function in Hypertrophic Cardiomyopathy: A Real-time 3-Dimensional Echocardiographic Study. J. Am. Soc. Echocardiogr. 2006, 19, 796–801. [Google Scholar] [CrossRef]
- Satriano, A.; Heydari, B.; Guron, N.; Fenwick, K.; Cheung, M.; Mikami, Y.; Merchant, N.; Lydell, C.P.; Howarth, A.G.; Fine, N.M.; et al. 3-Dimensional regional and global strain abnormalities in hypertrophic cardiomyopathy. Int. J. Cardiovasc. Imaging 2019, 35, 1913–1924. [Google Scholar] [CrossRef] [PubMed]
- Pagourelias, E.D.; Mirea, O.; Duchenne, J.; Unlu, S.; Van Cleemput, J.; Papadopoulos, C.E.; Bogaert, J.; Vassilikos, V.P.; Voigt, J.-U. Speckle tracking deformation imaging to detect regional fibrosis in hypertrophic cardiomyopathy: A comparison between 2D and 3D echo modalities. Eur. Heart J. Cardiovasc. Imaging 2020, 21, 1262–1272. [Google Scholar] [CrossRef] [PubMed]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the American society of echocardiography and the European association of cardiovascular imaging. Eur. Heart J. Cardiovasc. Imaging 2015, 16, 233–271. [Google Scholar] [CrossRef]
- Voigt, J.-U.; Pedrizzetti, G.; Lysyansky, P.; Marwick, T.H.; Houle, H.; Baumann, R.; Pedri, S.; Ito, Y.; Abe, Y.; Metz, S.; et al. Definitions for a Common Standard for 2D Speckle Tracking Echocardiography: Consensus Document of the EACVI/ASE/Industry Task Force to Standardize Deformation Imaging. J. Am. Soc. Echocardiogr. 2015, 28, 183–193. [Google Scholar] [CrossRef] [Green Version]
- Leitman, M.; Lysiansky, M.; Lysyansky, P.; Friedman, Z.; Tyomkin, V.; Fuchs, T.; Adam, D.; Krakover, R.; Vered, Z. Circumferential and Longitudinal Strain in 3 Myocardial Layers in Normal Subjects and in Patients with Regional Left Ventricular Dysfunction. J. Am. Soc. Echocardiogr. 2010, 23, 64–70. [Google Scholar] [CrossRef]
- Al Saikhan, L.; Hughes, A.D.; Chung, W.-S.; Alsharqi, M.; Nihoyannopoulos, P. Left atrial function in heart failure with mid-range ejection fraction differs from that of heart failure with preserved ejection fraction: A 2D speckle-tracking echocardiographic study. Eur. Heart J. Cardiovasc. Imaging 2018, 20, 279–290. [Google Scholar] [CrossRef] [PubMed]
- Kao, Y.-C.; Hung, M.-J. Echocardiographic Evaluation of Left Atrial Function to Discriminate Non-Valvular Atrial Fibrillation Development in Patients with Apical Hypertrophic Cardiomyopathy. Acta Cardiol. Sin. 2020, 36, 33–43. [Google Scholar] [PubMed]
- Cameli, M.; Miglioranza, M.H.; Magne, J.; Mandoli, G.E.; Benfari, G.; Ancona, R.; Sibilo, G.; Luksic, V.R.; Dejan, D.; Griseli, L.; et al. Multicentric Atrial Strain comparison between two different modalities: MASCOT HIT Study. Diagnostics 2020, 10, 946. [Google Scholar] [CrossRef]
- Badano, L.P.; Cucchini, U.; Muraru, D.; Al Nono, O.; Sarais, C.; Iliceto, S. Use of three-dimensional speckle tracking to assess left ventricular myocardial mechanics: Inter-vendor consistency and reproducibility of strain measurements. Eur. Heart J. Cardiovasc. Imaging 2012, 14, 285–293. [Google Scholar] [CrossRef] [PubMed]
- Lang, R.M.; Badano, L.P.; Tsang, W.; Adams, D.H.; Agricola, E.; Buck, T.; Faletra, F.F.; Franke, A.; Hung, J.; De Isla, L.P.; et al. EAE/ASE recommendations for image acquisition and display using three-dimensional echocardiography. Eur. Heart J. Cardiovasc. Imaging 2012, 13, 1–46. [Google Scholar] [CrossRef] [PubMed]
- Yuda, S.; Sato, Y.; Abe, K.; Kawamukai, M.; Kouzu, H.; Muranaka, A.; Kokubu, N.; Hashimoto, A.; Tsuchihashi, K.; Watanabe, N.; et al. Inter-Vendor Variability of Left Ventricular Volumes and Strains Determined by Three-Dimensional Speckle Tracking Echocardiography. Echocardiography 2014, 31, 597–604. [Google Scholar] [CrossRef] [PubMed]
- Vitarelli, A.; Gaudio, C.; Mangieri, E.; Capotosto, L.; Tanzilli, G.; Ricci, S.; Viceconte, G.N.; Placanica, A.; Placanica, G.; Ashurov, R. Bi-Atrial Function before and after Percutaneous Closure of Atrial Septum in Patients with and without Paroxysmal Atrial Fibrillation: A 2-D and 3-D Speckle Tracking Echocardiographic Study. Ultrasound Med. Biol. 2018, 44, 1198–1211. [Google Scholar] [CrossRef]
- Van Dalen, B.M.; Kauer, F.; Soliman, O.; Vletter, W.B.; Michels, M.; Cate, F.J.T.; Geleijnse, M.L. Influence of the pattern of hypertrophy on left ventricular twist in hypertrophic cardiomyopathy. Heart 2009, 95, 657–661. [Google Scholar] [CrossRef]
- Russo, C.; Jin, Z.; Sera, F.; Lee, E.S.; Homma, S.; Rundek, T.; Elkind, M.S.; Sacco, R.L.; Di Tullio, M.R. Left Ventricular Systolic Dysfunction by Longitudinal Strain Is an Independent Predictor of Incident Atrial Fibrillation. Circ. Cardiovasc. Imaging 2015, 8, e003520. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zegkos, T.; Ntelios, D.; Parcharidou, D.; Katranas, S.; Panagiotidis, T.; Papanastasiou, C.A.; Karagiannidis, E.; Rouskas, P.; Vassilikos, V.; Karvounis, H.; et al. The predictive value of left ventricular and left atrial mechanics for atrial fibrillation and heart failure in hypertrophic cardiomyopathy: A prospective cohort study. Int. J. Cardiovasc. Imaging 2021, 1–12. [Google Scholar] [CrossRef]
- Muraru, D.; Niero, A.; Rodriguez-Zanella, H.; Cherata, D.; Badano, L. Three-dimensional speckle-tracking echocardiography: Benefits and limitations of integrating myocardial mechanics with three-dimensional imaging. Cardiovasc. Diagn. Ther. 2018, 8, 101–117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tanaka, H.; Tatsumi, K.; Matsumoto, K.; Kawai, H.; Hirata, K.-I. Emerging Role of Three-Dimensional Speckle Tracking Strain for Accurate Quantification of Left Ventricular Dyssynchrony. Echocardiography 2013, 30, E292–E295. [Google Scholar] [CrossRef]



| HCM AF+ (n = 43) | HCM AF− (n = 17) | p | |
|---|---|---|---|
| Age | 57 (45.5–63) | 56 (41–63) | 0.381 |
| SCD in family history n (%) | 7 (16) | 6 (35) | 0.163 |
| 5-year risk of SCD n (%) | 3.6 (2.6–4.9) | 5.5 (3.5–9.4) | <0.030 |
| History of non- sustained ventricular tachycardia, n (%) | 9 (21) | 7 (41) | 0.195 |
| History of syncope/ presyncope n (%) | 17 (40) | 8 (47) | 0.772 |
| Implantable cardioverter—defibrillator, n (%) | 7 (16) | 8 (47) | <0.021 |
| Concomitant diseases | |||
| Hypertension n (%) | 25 (58%) | 6 (35%) | 0.154 |
| Coronary artery disease n (%) | 3 (7%) | 0 (0%) | 0.551 |
| Myocardial infarction n (%) | 1 (2%) | 0 (0%) | 1.000 |
| Diabetes mellitus type 2 n (%) | 8 (19) | 2 (12) | 0.709 |
| Hyperlipidemia n (%) | 26 (62) | 11 (65) | 1.000 |
| Smoking n (%) | 16 (37) | 6 (35) | 1.000 |
| Medications | |||
| Beta-blockers, n (%) | 32 (48) | 16 (94) | 0.151 |
| ACE inhibitors, n (%) | 19 (44) | 7 (41) | 1.000 |
| Spironolactone n (%) | 1 (2) | 1 (6) | 0.490 |
| Calcium—blocker n (%) | 6 (14) | 2 (12) | 1.000 |
| Cordarone/Sotalol n (%) | 3 (7) | 3 (12) | 0.338 |
| Diuretics n (%) | 10 (23) | 2 (12) | 0.479 |
| Statins n (%) | 19 (44) | 8 (47) | 1.000 |
| HCM All n = 60 | Healthy Volunteers n = 23 | p HCM vs. Healthy | AF+ n = 43 | AF− n = 17 | p AF+ vs. AF− | p AF+ vs. Healthy | p AF− vs. Healthy | |
|---|---|---|---|---|---|---|---|---|
| Standard echocardiography parameters | ||||||||
| LADs | 45 (42–48) | 38 (35–40) | <0.000 | 45 (42–48) | 45 (42–49) | 0.474 | <0.000 | <0.002 |
| LAVi | 53 (42–62) | 27 (23–32) | <0.000 | 58 (45–63) | 51 (41–61) | 0.162 | <0.000 | <0.000 |
| LVMi | 178 (149–215) | 69 (60–76) | <0.000 | 198 (152–218) | 155 (140–172) | 0.061 | <0.000 | <0.000 |
| E/A | 0.97 (0.77–0.04) | 1.28 (0.94–1.57) | 0.073 | 0.94 (0.76–1.04) | 1.23 (0.79–1.04) | 0.357 | 0.066 | 0.242 |
| DecT | 185 (150–263) | 182 (149–214) | 0.216 | 182 (149–268) | 208 (167–240) | 0.352 | 0.318 | 0.122 |
| Em | 0.06 (0.05–0.08) | 0.12 (0.09–0.14) | <0.000 | 0.06 (0.05–0.08) | 0.06 (0.05–0.07) | 0.347 | <0.000 | <0.000 |
| E/Em | 10.7 (9.0–14.5) | 6.8 (5.4–8.0) | <0.000 | 10 (8.5–14.9) | 11.5 (9.8–12.2) | 0.374 | <0.000 | <0.000 |
| LVEF | 64 (55–69) | 63 (61–63) | 0.098 | 53 (47–64) | 67 (61–70) | <0.010 | 0.052 | <0.008 |
| EDV | 90 (71–121) | 108 (89–126) | 0.055 | 90 (71–116) | 101 (70–125) | 0.453 | <0.049 | 0.202 |
| ESV | 35 (23–45) | 40 (33–47) | <0.037 | 31 (21–44) | 41 (27–58) | 0.069 | <0.027 | 0.421 |
| RVID | 25 (23–27) | 26 (22–29) | 0.142 | 25 (22–27) | 25 (24–26) | 0.353 | 0.128 | 0.306 |
| Speckle tracking echocardiography parameters | ||||||||
| 2D LA peak strain | 15.9 (12.3–20.0) | 28.5 (22.4–31.3) | <0.000 | 13.8 (10.6–18.4) | 16.5 (12.9–21.5) | 0.164 | <0.000 | <0.000 |
| 2D LV GLS | −15.2 (−17.5–−12.1) | −19.6 (−20.9–−17.9) | <0.000 | −12.4 (−14.2–−10.0) | −16.3 (−19.1–−13.9) | <0.003 | <0.000 | <0.001 |
| 3D LV area strain | −23.0 (−26.5–−21.0) | −26.5 (−28.8–−20.0) | 0.109 | −19.5 (−20.8– −17.5) | −25.0 (−27.0–−22.0) | <0.009 | <0.015 | 0.282 |
| 3D LV radial strain | 35.0 (29.5–42.5) | 39.5 (−29.0–−46.8) | 0.146 | 27.7 (24.8– −30.3) | 37.0 (32.0–−44.0) | <0.006 | <0.011 | 0.364 |
| 2D Twist | 21.2 (16.3–25.5) | 24 (19.8–26.3) | 0.063 | 16.75 (12.54–22.06) | 22.01 (17.25–25.45) | 0.165 | <0.036 | 0.126 |
| 2D Torsion | 2.9 (2–3.4) | 3 (2.7–3.9) | 0.059 | 2.4 (1.8–3) | 3 (2.3–4) | 0.198 | <0.036 | 0.121 |
| Parameter | AUC | Characteristics (95% CI) | Predictive Value (95% CI) | ||
|---|---|---|---|---|---|
| Sensivity (%) | Specificity (%) | Positive (%) | Negative (%) | ||
| LADs ≥ 45 mm | 63.7 | 67 | 60 | 88 | 30 |
| LAVi ≥ 57 mL/m2 | 75.9 | 79 | 67 | 91 | 42 |
| LVEF ≤ 55% | 70.4 | 89 | 60 | 89 | 60 |
| LA peak strain ≤ 22% | 73.0 | 44 | 100 | 100 | 29 |
| LV GLS ≥ −16% | 85.6 | 67 | 100 | 100 | 40 |
| LV area strain ≥ −23.5% | 84.6 | 63 | 100 | 100 | 29 |
| LV radial strain ≤ 33% | 86.4 | 71 | 100 | 100 | 33 |
| Parameter | Univariate Analysis | |
|---|---|---|
| OR (95% CI) | p | |
| LADs ≥ 45 mm | 3.00 (0.94–9.55) | 0.078 |
| LAVi ≥ 57 mL/m2 | 7.45 (1.89–29.41) | 0.004 |
| LVEF ≤ 55% | 12.25 (3.22–46.61) | 0.001 |
| LA peak strain ≤ 22% | 17.11 (0.95–308.16) | 0.005 |
| LV GLS ≥ −16% | 48.00 (2.68–859.36) | 0.001 |
| LV area strain ≥ −23.5% | 20.80 (1.08–399.06) | 0.005 |
| LV radial strain ≤ 33% | 29.00 (1.5–560.98) | 0.002 |
| LV GLS ≥ −16% and LA peak strain ≤ 22% | 76.36 (4.13–1411.36) | 0.001 |
| Parameter | Intra-Observer | Inter-Observer | ||||||
|---|---|---|---|---|---|---|---|---|
| Bias | 95% Limits of Agreement | ICC | Coefficient of Variation (%) | Bias | 95% Limits of Agreement | ICC | Coefficient of Variation (%) | |
| 2D LA peak strain | −0.8 | −7–5.5 | 0.8 * | 19.2 | −0.6 | −5.9–4.6 | 0.8 * | 15.6 |
| 2D LV GLS | 0.6 | −3.9–5.1 | 0.8 * | 14.6 | −0.4 | −5.2–4.4 | 0.8 * | 16.2 |
| 3D area strain | 0.4 | −4.9–5.6 | 0.9 * | 11.7 | 0.5 | −5.4–6.3 | 0.9 * | 12.8 |
| 3D radial strain | −1.4 | −13.8–11.1 | 0.9 * | 17.6 | −0.7 | −11.5–10.2 | 0.9 * | 15.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wabich, E.; Zienciuk-Krajka, A.; Nowak, R.; Raczak, A.; Daniłowicz-Szymanowicz, L. Comprehensive Echocardiography of Left Atrium and Left Ventricle Using Modern Techniques Helps in Better Revealing Atrial Fibrillation in Patients with Hypertrophic Cardiomyopathy. Diagnostics 2021, 11, 1288. https://doi.org/10.3390/diagnostics11071288
Wabich E, Zienciuk-Krajka A, Nowak R, Raczak A, Daniłowicz-Szymanowicz L. Comprehensive Echocardiography of Left Atrium and Left Ventricle Using Modern Techniques Helps in Better Revealing Atrial Fibrillation in Patients with Hypertrophic Cardiomyopathy. Diagnostics. 2021; 11(7):1288. https://doi.org/10.3390/diagnostics11071288
Chicago/Turabian StyleWabich, Elżbieta, Agnieszka Zienciuk-Krajka, Radosław Nowak, Alicja Raczak, and Ludmiła Daniłowicz-Szymanowicz. 2021. "Comprehensive Echocardiography of Left Atrium and Left Ventricle Using Modern Techniques Helps in Better Revealing Atrial Fibrillation in Patients with Hypertrophic Cardiomyopathy" Diagnostics 11, no. 7: 1288. https://doi.org/10.3390/diagnostics11071288
APA StyleWabich, E., Zienciuk-Krajka, A., Nowak, R., Raczak, A., & Daniłowicz-Szymanowicz, L. (2021). Comprehensive Echocardiography of Left Atrium and Left Ventricle Using Modern Techniques Helps in Better Revealing Atrial Fibrillation in Patients with Hypertrophic Cardiomyopathy. Diagnostics, 11(7), 1288. https://doi.org/10.3390/diagnostics11071288








