Ultrasound Imaging versus Radiographs in Differentiating Periapical Lesions: A Systematic Review
Abstract
1. Introduction
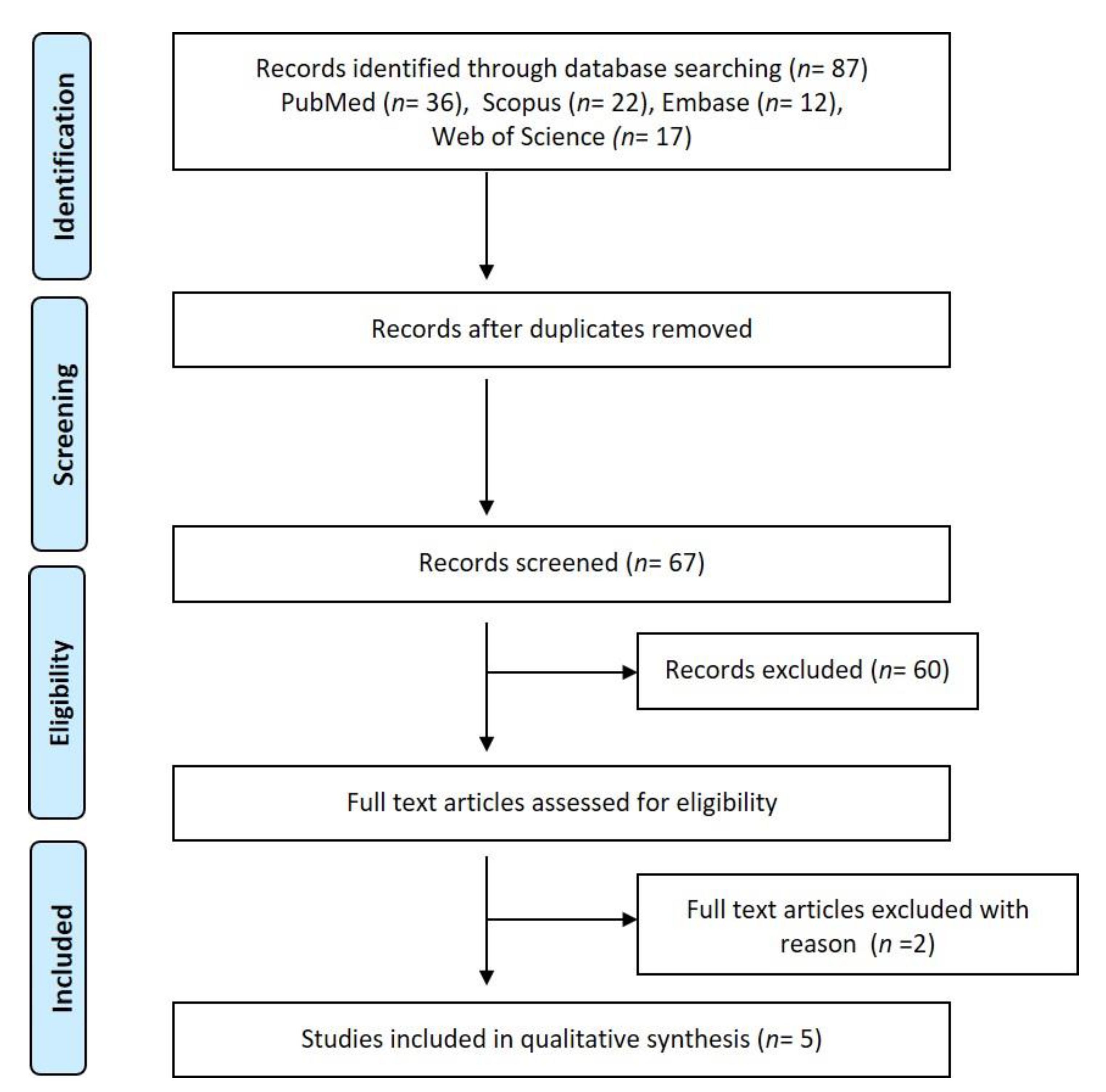
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ramachandran Nair, P.N. Light and electron microscopic studies of root canal flora and periapical lesions. J. Endod. 1987, 13, 29–39. [Google Scholar] [CrossRef]
- Nair, P.N.R. Pathogenesis of apical periodontitis and the causes of endodontic failures. Crit. Rev. Oral Biol. Med. 2004, 15, 348–381. [Google Scholar] [CrossRef] [PubMed]
- Ricucci, D.; Siqueira, J.F. Fate of the Tissue in Lateral Canals and Apical Ramifications in Response to Pathologic Conditions and Treatment Procedures. J. Endod. 2010, 36, 1–15. [Google Scholar] [CrossRef]
- Persoon, I.F.; Özok, A.R. Definitions and Epidemiology of Endodontic Infections. Curr. Oral Heal. Reports 2017, 4, 278–285. [Google Scholar] [CrossRef] [PubMed]
- Smadi, L. Apical periodontitis and endodontic treatment in patients with type II diabetes mellitus: Comparative cross-sectional survey. J. Contemp. Dent. Pract. 2017, 18, 358–362. [Google Scholar] [CrossRef]
- Liljestrand, J.M.; Mäntylä, P.; Paju, S.; Buhlin, K.; Kopra, K.A.E.; Persson, G.R.; Hernandez, M.; Nieminen, M.S.; Sinisalo, J.; Tjäderhane, L.; et al. Association of endodontic lesions with coronary artery disease. J. Dent. Res. 2016, 95, 1358–1365. [Google Scholar] [CrossRef] [PubMed]
- Lõpez-Lõpez, J.; Castellanos-Cosano, L.; Estrugo-Devesa, A.; Gõmez-Vaquero, C.; Velasco-Ortega, E.; Segura-Egea, J.J. Radiolucent periapical lesions and bone mineral density in post-menopausal women. Gerodontology 2015, 32, 195–201. [Google Scholar] [CrossRef]
- Lalonde, E.R.; Luebke, R.G. The frequency and distribution of periapical cysts and granulomas. An evaluation of 800 specimens. Oral Surg. Oral Med. Oral Pathol. 1968, 25, 861–868. [Google Scholar] [CrossRef]
- Bhaskar, S.N. Oral surgery-oral pathology conference no. 17, Walter Reed Army Medical Center. Periapical lesions-Types, incidence, and clinical features. Oral Surg. Oral Med. Oral Pathol. 1966, 21, 657–671. [Google Scholar] [CrossRef]
- Nair, P.N.R.; Pajarola, G.; Schroeder, H.E. Types and incidence of human periapical lesions obtained with extracted teeth. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 1996, 81, 93–102. [Google Scholar] [CrossRef]
- Schulz, M.; von Arx, T.; Altermatt, H.J.; Bosshardt, D. Histology of Periapical Lesions Obtained During Apical Surgery. J. Endod. 2009, 35, 634–642. [Google Scholar] [CrossRef]
- Jakovljevic, A.; Nikolic, N.; Jacimovic, J.; Pavlovic, O.; Milicic, B.; Beljic-Ivanovic, K.; Miletic, M.; Andric, M.; Milasin, J. Prevalence of Apical Periodontitis and Conventional Nonsurgical Root Canal Treatment in General Adult Population: An Updated Systematic Review and Meta-analysis of Cross-sectional Studies Published between 2012 and 2020. J. Endod. 2020, 46, 1371–1386.e8. [Google Scholar] [CrossRef] [PubMed]
- Gundappa, M.; Ng, S.Y.; Whaites, E.J. Comparison of ultrasound, digital and conventional radiography in differentiating periapical lesions. Dentomaxillofacial Radiol. 2006, 35, 326–333. [Google Scholar] [CrossRef] [PubMed]
- Eggesbø, H.B. Radiological imaging of inflammatory lesions in the nasal cavity and paranasal sinuses. Eur. Radiol. 2006, 16, 872–888. [Google Scholar] [CrossRef]
- Fernandes, M.; Ataide, I. Nonsurgical management of periapical lesions. J. Conserv. Dent. 2010, 13, 240. [Google Scholar] [CrossRef]
- Lin, L.M.; Ricucci, D.; Lin, J.; Rosenberg, P.A. Nonsurgical Root Canal Therapy of Large Cyst-like Inflammatory Periapical Lesions and Inflammatory Apical Cysts. J. Endod. 2009, 35, 607–615. [Google Scholar] [CrossRef] [PubMed]
- Ng, Y.L.; Mann, V.; Gulabivala, K. A prospective study of the factors affecting outcomes of non-surgical root canal treatment: Part 2: Tooth survival. Int. Endod. J. 2011, 44, 610–625. [Google Scholar] [CrossRef]
- Yee, K.; Bhagavatula, P.; Stover, S.; Eichmiller, F.; Hashimoto, L.; MacDonald, S.; Barkley, G. Survival Rates of Teeth with Primary Endodontic Treatment after Core/Post and Crown Placement. J. Endod. 2018, 44, 220–225. [Google Scholar] [CrossRef] [PubMed]
- Natkin, E.; Oswald, R.J.; Carnes, L.I. The relationship of lesion size to diagnosis, incidence, and treatment of periapical cysts and granulomas. Oral Surg. Oral Med. Oral Pathol. 1984, 57, 82–94. [Google Scholar] [CrossRef]
- Guo, J.; Simon, J.H.; Sedghizadeh, P.; Soliman, O.N.; Chapman, T.; Enciso, R. Evaluation of the reliability and accuracy of using cone-beam computed tomography for diagnosing periapical cysts from granulomas. J. Endod. 2013, 39, 1485–1490. [Google Scholar] [CrossRef] [PubMed]
- Kruse, C.; Spin-Neto, R.; Wenzel, A.; Kirkevang, L.L. Cone beam computed tomography and periapical lesions: A systematic review analysing studies on diagnostic efficacy by a hierarchical model. Int. Endod. J. 2015, 48, 815–828. [Google Scholar] [CrossRef]
- Pitcher, B.; Alaqla, A.; Noujeim, M.; Wealleans, J.A.; Kotsakis, G.; Chrepa, V. Binary Decision Trees for Preoperative Periapical Cyst Screening Using Cone-beam Computed Tomography. J. Endod. 2017, 43, 383–388. [Google Scholar] [CrossRef] [PubMed]
- Lalonde, E.R. A new rationale for the management of periapical granulomas and cysts: An evaluation of histopathological and radiographic findings. J. Am. Dent. Assoc. 1970, 80, 1056–1059. [Google Scholar] [CrossRef] [PubMed]
- Morse, D.R.; Patnik, J.W.; Schacterle, G.R. Electrophoretic differentiation of radicular cysts and granulomas. Oral Surg. Oral Med. Oral Pathol. 1973, 35, 249–264. [Google Scholar] [CrossRef]
- Çalişkan, M.K. Prognosis of large cyst-like periapical lesions following nonsurgical root canal treatment: A clinical review. Int. Endod. J. 2004, 37, 408–416. [Google Scholar] [CrossRef]
- Alcantara, B.A.R.; de Carli, M.L.; Beijo, L.A.; Pereira, A.A.C.; Hanemann, J.A.C. Correlation between inflammatory infiltrate and epithelial lining in 214 cases of periapical cysts. Braz. Oral Res. 2013, 27, 490–495. [Google Scholar] [CrossRef][Green Version]
- Lizio, G.; Salizzoni, E.; Coe, M.; Gatto, M.R.; Asioli, S.; Balbi, T.; Pelliccioni, G.A. Differential diagnosis between a granuloma and radicular cyst: Effectiveness of magnetic resonance imaging. Int. Endod. J. 2018, 51, 1077–1087. [Google Scholar] [CrossRef]
- Musu, D.; Rossi-Fedele, G.; Campisi, G.; Cotti, E. Ultrasonography in the diagnosis of bone lesions of the jaws: A systematic review. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2016, 122, e19–e29. [Google Scholar] [CrossRef]
- Juerchott, A.; Pfefferle, T.; Flechtenmacher, C.; Mente, J.; Bendszus, M.; Heiland, S.; Hilgenfeld, T. Differentiation of periapical granulomas and cysts by using dental MRI: A pilot study. Int. J. Oral Sci. 2018, 10, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Reda, R.; Zanza, A.; Mazzoni, A.; Cicconetti, A.; Testarelli, L.; Di Nardo, D. An Update of the Possible Applications of Magnetic Resonance Imaging (MRI) in Dentistry: A Literature Review. J. Imaging 2021, 7, 75. [Google Scholar] [CrossRef]
- Assaf, A.T.; Zrnc, T.A.; Remus, C.C.; Schönfeld, M.; Habermann, C.R.; Riecke, B.; Friedrich, R.E.; Fiehler, J.; Heiland, M.; Sedlacik, J. Evaluation of four different optimized magnetic-resonance-imaging sequences for visualization of dental and maxillo-mandibular structures at 3 T. J. Cranio-Maxillofac. Surg. 2014, 42, 1356–1363. [Google Scholar] [CrossRef] [PubMed]
- Drăgan, O.C.; Fărcăşanu, A.Ş.; Câmpian, R.S.; Turcu, R.V.F. Human tooth and root canal morphology reconstruction using magnetic resonance imaging. Clujul Med. 2016, 89, 137–142. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Choi, H. High-efficiency high-voltage class F amplifier for high-frequency wireless ultrasound systems. PLoS ONE 2021, 16. [Google Scholar] [CrossRef]
- Cotti, E.; Campisi, G.; Garau, V.; Puddu, G. A new technique for the study of periapical bone lesions: Ultrasound real time imaging. Int. Endod. J. 2002, 35, 148–152. [Google Scholar] [CrossRef]
- Cotti, E.; Campisi, G.; Ambu, R.; Dettori, C. Ultrasound real-time imaging in the differential diagnosis of periapical lesions. Int. Endod. J. 2003, 36, 556–563. [Google Scholar] [CrossRef]
- Auer, L.M.; van Velthoven, V. Intraoperative ultrasound (US) imaging. Comparison of pathomorphological findings in US and CT. Acta Neurochir. (Wien.) 1990, 104, 84–95. [Google Scholar] [CrossRef]
- Shamseer, L.; Moher, D.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, L.A.; Altman, D.G.; Booth, A.; et al. Preferred reporting items for systematic review and meta-analysis protocols (prisma-p) 2015: Elaboration and explanation. BMJ 2015, 349. [Google Scholar] [CrossRef] [PubMed]
- Whiting, P.F.; Rutjes, A.W.S.; Westwood, M.E.; Mallett, S.; Deeks, J.J.; Reitsma, J.B.; Leeflang, M.M.G.; Sterne, J.A.C.; Bossuyt, P.M.M. Quadas-2: A revised tool for the quality assessment of diagnostic accuracy studies. Ann. Intern. Med. 2011, 155, 529–536. [Google Scholar] [CrossRef]
- Handbook for DTA Reviews | Cochrane Screening and Diagnostic Tests. Available online: https://methods.cochrane.org/sdt/handbook-dta-reviews (accessed on 12 May 2021).
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; Altman, D.; Antes, G.; Atkins, D.; Barbour, V.; Barrowman, N.; Berlin, J.A.; et al. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef]
- Raghav, N.; Reddy, S.S.; Giridhar, A.G.; Murthy, S.; Devi, Y.; Santana, N.; Rakesh, N.; Kaushik, A. Comparison of the efficacy of conventional radiography, digital radiography, and ultrasound in diagnosing periapical lesions. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontology 2010, 110, 379–385. [Google Scholar] [CrossRef]
- Goel, S.; Nagendrareddy, S.; Raju, M.; Krishnojirao, D.R.; Rastogi, R.; Mohan, R.P.; Gupta, S. Ultrasonography with color Doppler and power Doppler in the diagnosis of periapical lesions. Indian J. Radiol. Imaging 2011, 21, 279–283. [Google Scholar] [CrossRef]
- Sandhu, S.S.; Singh, S.; Arora, S.; Sandhu, A.K.; Dhingra, R. Comparative evaluation of advanced and conventional diagnostic aids for endodontic management of periapical lesions, an in vivo study. J. Clin. Diagn. Res. 2015, 9, ZC01–ZC04. [Google Scholar] [CrossRef]
- Khambete, N.; Kumar, R. Ultrasound in differential diagnosis of periapical radiolucencies: A radiohistopathological study. J. Conserv. Dent. 2015, 18, 39–43. [Google Scholar] [CrossRef]
- Sönmez, G.; Kamburoǧlu, K.; Yilmaz, F.; Koç, C.; Bariş, E.; Tüzüner, A. Versatility of high resolution ultrasonography in the assessment of granulomas and radicular cysts: A comparative in vivo study. Dentomaxillofacial Radiol. 2019, 48. [Google Scholar] [CrossRef]
- Prince, C.; Annapurna, C.; Sivaraj, S.; Ali, I. Ultrasound imaging in the diagnosis of periapical lesions. J. Pharm. Bioallied Sci. 2012, 4, 369. [Google Scholar] [CrossRef]
- Natanasabapathy, V.; Arul, B.; Mishra, A.; Varghese, A.; Padmanaban, S.; Elango, S.; Arockiam, S. Ultrasound imaging for the differential diagnosis of periapical lesions of endodontic origin in comparison with histopathology—A systematic review and meta-analysis. Int. Endod. J. 2020, 54, 693–711. [Google Scholar] [CrossRef] [PubMed]
- Petersson, A.; Axelsson, S.; Davidson, T.; Frisk, F.; Hakeberg, M.; Kvist, T.; Norlund, A.; Mejàre, I.; Portenier, I.; Sandberg, H.; et al. Radiological diagnosis of periapical bone tissue lesions in endodontics: A systematic review. Int. Endod. J. 2012, 45, 783–801. [Google Scholar] [CrossRef] [PubMed]
- White, S.; Pharoah, M. Oral Radiology-E-Book: Principles and Interpretation; Mosby: Maryland Heights, MO, USA, 2014. [Google Scholar]
- Kea, B.; Hall, M.K.; Wang, R. Recognising bias in studies of diagnostic tests part 2: Interpreting and verifying the index test. Emerg. Med. J. 2019, 36, 501–505. [Google Scholar] [CrossRef]
- Hashem, A.; Chi, M.T.H.; Friedman, C.P. Medical errors as a result of specialization. J. Biomed. Inform. 2003, 36, 61–69. [Google Scholar] [CrossRef]
- Probst, P.; Grummich, K.; Heger, P.; Zaschke, S.; Knebel, P.; Ulrich, A.; Büchler, M.W.; Diener, M.K. Blinding in randomized controlled trials in general and abdominal surgery: Protocol for a systematic review and empirical study. Syst. Rev. 2016, 5, 1–6. [Google Scholar] [CrossRef]
- Lin, H.P.; Chen, H.M.; Yu, C.H.; Kuo, R.C.; Kuo, Y.S.; Wang, Y.P. Clinicopathological study of 252 jaw bone periapical lesions from a private pathology laboratory. J. Formos. Med. Assoc. 2010, 109, 810–818. [Google Scholar] [CrossRef][Green Version]
- Koivisto, T.; Bowles, W.R.; Rohrer, M. Frequency and distribution of radiolucent jaw lesions: A retrospective analysis of 9723 cases. J. Endod. 2012, 38, 729–732. [Google Scholar] [CrossRef] [PubMed]
- Rossi, M.; Bruno, G.; De Stefani, A.; Perri, A.; Gracco, A. Quantitative CBCT evaluation of maxillary and mandibular cortical bone thickness and density variability for orthodontic miniplate placement. Int. Orthod. 2017, 15, 610–624. [Google Scholar] [CrossRef] [PubMed]
- Porto, O.C.L.; de Freitas Silva, B.S.; Silva, J.A.; de Araújo ESTRELA, C.R.; de ALENCAR, A.H.G.; BUENO, M.d.R.; Estrela, C. CBCT assessment of bone thickness in maxillary and mandibular teeth: An anatomic study. J. Appl. Oral Sci. 2020, 28. [Google Scholar] [CrossRef] [PubMed]
- Musu, D.; Cadeddu Dessalvi, C.; Shemesh, H.; Frenda, M.G.; Mercuro, G.; Cotti, E. Ultrasound examination for the detection of simulated periapical bone lesions in bovine mandibles: An ex vivo study. Int. Endod. J. 2020, 53, 1289–1298. [Google Scholar] [CrossRef]
- Laird, W.R.E.; Walmsley, A.D. Ultrasound in dentistry. Part 1-biophysical interactions. J. Dent. 1991, 19, 14–17. [Google Scholar] [CrossRef]
- Baum, G.; Greenwood, I.; Slawski, S.; Smirnow, R. Observation of internal structures of teeth by ultrasonography. Science 1963, 139, 495–496. [Google Scholar] [CrossRef]
- Marotti, J.; Heger, S.; Tinschert, J.; Tortamano, P.; Chuembou, F.; Radermacher, K.; Wolfart, S. Recent advances of ultrasound imaging in dentistry—A review of the literature. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2013, 115, 819–832. [Google Scholar] [CrossRef] [PubMed]
- Hall, M.K.; Kea, B.; Wang, R. Recognising Bias in Studies of Diagnostic Tests Part 1: Patient Selection. Emerg. Med. J. 2019, 36, 431–434. [Google Scholar] [CrossRef]
- Kohn, M.A.; Carpenter, C.R.; Newman, T.B. Understanding the direction of bias in studies of diagnostic test accuracy. Acad. Emerg. Med. 2013, 20, 1194–1206. [Google Scholar] [CrossRef]
- Hajian-Tilaki, K. Sample size estimation in diagnostic test studies of biomedical informatics. J. Biomed. Inform. 2014, 48, 193–204. [Google Scholar] [CrossRef] [PubMed]
- Hung, K.; Montalvao, C.; Tanaka, R.; Kawai, T.; Bornstein, M.M. The use and performance of artificial intelligence applications in dental and maxillofacial radiology: A systematic review. Dentomaxillofacial Radiol. 2019, 49. [Google Scholar] [CrossRef] [PubMed]
- Nagi, R.; Aravinda, K.; Rakesh, N.; Gupta, R.; Pal, A.; Mann, A.K. Clinical applications and performance of intelligent systems in dental and maxillofacial radiology: A review. Imaging Sci. Dent. 2020, 50, 81–92. [Google Scholar] [CrossRef] [PubMed]
| Database | Search Details | Results |
|---|---|---|
| PubMed | (“periapical” [All Fields] OR “periapically” [All Fields] OR “periapicals” [All Fields]) AND (“lesion” [All Fields] OR “lesion s” [All Fields] OR “lesional” [All Fields] OR “lesions” [All Fields]) AND “diagnos*” [All Fields] AND (“ultrasonography” [MeSH Terms] OR “ultrasonography” [All Fields] OR (“ultrasound”[All Fields] AND “imaging”[All Fields]) OR “ultrasound imaging” [All Fields]) AND (“diagnostic imaging” [MeSH Subheading] OR (“diagnostic” [All Fields] AND “imaging” [All Fields]) OR “diagnostic imaging” [All Fields] OR “radiography” [All Fields] OR “radiography” [MeSH Terms] OR “radiographies” [All Fields] OR “radiographys” [All Fields]) | 36 |
| Scopus | TITLE-ABS-KEY (periapical AND lesion AND ultrasound AND radiography) | 22 |
| Web of Science | (periapical (ultrasound or echography) radiograph) | 17 |
| Embase | ‘periapical ultrasound radiograph’ OR (periapical AND (‘ultrasound’/exp OR ultrasound) AND (‘radiograph’/exp OR radiograph)) | 12 |
| Sno. | Author/Year, Country | Sample Size | Age | Ultrasound | Radiographs | Outcome | |||
|---|---|---|---|---|---|---|---|---|---|
| Machine | Frequency | Accuracy | Imaging Modality | Accuracy | |||||
| 1 | Gundappa et al./2006, UK | 15 | 13–40 years | LOGIQ-500 PRO (GE Medical System, USA), with color Doppler | 8–11 MHz used extra- and intra-orally | 100% | Conventional and digital IOPAs | Unable to differentiate with conventional or digital radiography | While USG underestimates the dimensions of a lesion, it is more accurate in diagnosing the nature of the lesion which is not possible with radiographs in the anterior region of the jaws |
| 2 | Raghav et al./2010, India | 21 | 15–45 years | Voluson 730 Pro Machine (GE Medical Systems) with color Doppler | 8–11 MHz used extra-orally | 95.20% | Conventional and digital IOPAs | 47.6% (conventional) and 55.6% (digital) | USG is a reliable diagnostic technique to distinguish between the nature of periapical lesions in the anterior region of the jaws compared to radiographic imaging |
| 3 | Goel et al./2011, India | 30 | 15–50 years | SonoAce 8000 Live® machine (Medison, Seoul, Korea) | 9 MHz extra- and intra-orally | 96.67% (C) and 96.67% (G) | Conventional IOPAs | 66.67% (C) and 66.67% (G) | USG is superior to intra-oral radiographs inaccurate diagnosis of the nature of periapical lesions in anterior regions of the jaws |
| 4 | Sandhu et al./2015, India | 30 | 15–50 years | Volusion-730- expert (GE Medical System, USA), with color Doppler | 6–12 MHz used extra-orally | 16/16 (G) C—not reported | Conventional and digital IOPAs | 11/16 (G) C—not reported | Radiographic imaging only provides information about the presence or absence of a periapical lesion, their nature, however, especially of mixed lesions can only be diagnosed with USG |
| 5 | Khambete and Kumar/2015, India | 10 | 19–40 years | LOGIQ-500 PRO (GE Medical System, USA), with color Doppler function | 8–11 MHz used extra-orally | 100% | Conventional IOPAs | Unable to differentiate with conventional or digital radiography | USG can be used as an adjunct for imaging and diagnosis of periapical lesions and is a valuable tool in the radiation-free non-invasive diagnosis of these lesions |
| SNo. | Author/Year, Country | Risk of Bias | Applicability Concern | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Patient Selection | Index Test 1 (USG) | Index Test 2 (Radiograph) | Reference Test | Flow and Timing | Patient Selection | Index Test 1 | Index Test 2 | Reference Test | ||
| 1 | Gundappa et al./2006, UK | H | L | U | L | L | H | L | U | L |
| 2 | Raghav et al./2010, India | H | L | L | L | L | H | L | L | L |
| 3 | Goel et al./2011, India | H | L | L | L | L | H | L | L | L |
| 4 | Sandhu et al./2015, India | H | L | L | L | L | H | L | L | L |
| 5 | Khambete and Kumar/2015, India | H | L | U | L | L | H | L | U | L |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Patil, S.; Alkahtani, A.; Bhandi, S.; Mashyakhy, M.; Alvarez, M.; Alroomy, R.; Hendi, A.; Varadarajan, S.; Reda, R.; Raj, A.T.; et al. Ultrasound Imaging versus Radiographs in Differentiating Periapical Lesions: A Systematic Review. Diagnostics 2021, 11, 1208. https://doi.org/10.3390/diagnostics11071208
Patil S, Alkahtani A, Bhandi S, Mashyakhy M, Alvarez M, Alroomy R, Hendi A, Varadarajan S, Reda R, Raj AT, et al. Ultrasound Imaging versus Radiographs in Differentiating Periapical Lesions: A Systematic Review. Diagnostics. 2021; 11(7):1208. https://doi.org/10.3390/diagnostics11071208
Chicago/Turabian StylePatil, Shankargouda, Ahmed Alkahtani, Shilpa Bhandi, Mohammed Mashyakhy, Mario Alvarez, Riyadh Alroomy, Ali Hendi, Saranya Varadarajan, Rodolfo Reda, A. Thirumal Raj, and et al. 2021. "Ultrasound Imaging versus Radiographs in Differentiating Periapical Lesions: A Systematic Review" Diagnostics 11, no. 7: 1208. https://doi.org/10.3390/diagnostics11071208
APA StylePatil, S., Alkahtani, A., Bhandi, S., Mashyakhy, M., Alvarez, M., Alroomy, R., Hendi, A., Varadarajan, S., Reda, R., Raj, A. T., & Testarelli, L. (2021). Ultrasound Imaging versus Radiographs in Differentiating Periapical Lesions: A Systematic Review. Diagnostics, 11(7), 1208. https://doi.org/10.3390/diagnostics11071208









