Evaluating Lumbar Intervertebral Disc Degeneration on a Compositional Level Using Chemical Exchange Saturation Transfer: Preliminary Results in Patients with Adolescent Idiopathic Scoliosis
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Population
2.2. MR Imaging
2.3. MR Image Analysis
2.4. Statistical Analysis
3. Results
3.1. Study Population
3.2. Morphologic Grading of IVD Degeneration
3.3. GagCEST Values: Healthy Controls vs. AIS Patients
3.4. GagCEST Values: Affected vs. Unaffected IVDs
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Konieczny, M.R.; Senyurt, H.; Krauspe, R. Epidemiology of adolescent idiopathic scoliosis. J. Child. Orthop. 2013, 7, 3–9. [Google Scholar] [CrossRef]
- Cheng, J.C.; Castelein, R.M.; Chu, W.C.; Danielsson, A.J.; Dobbs, M.B.; Grivas, T.B.; Gurnett, C.A.; Luk, K.D.; Moreau, A.; Newton, P.O.; et al. Adolescent idiopathic scoliosis. Nat. Rev. Dis. Primer 2015, 1, 15030. [Google Scholar] [CrossRef]
- Horne, J.P.; Flannery, R.; Usman, S. Adolescent Idiopathic Scoliosis: Diagnosis and Management. Am. Fam. Physician 2014, 89, 6. [Google Scholar]
- Urban, J.P.G.; Winlove, C.P. Pathophysiology of the intervertebral disc and the challenges for MRI. J. Magn. Reson. Imaging 2007, 25, 419–432. [Google Scholar] [CrossRef]
- Bertram, H.; Steck, E.; Zimmermann, G.; Chen, B.; Carstens, C.; Nerlich, A.; Richter, W. Accelerated intervertebral disc degeneration in scoliosis versus physiological ageing develops against a background of enhanced anabolic gene expression. Biochem. Biophys. Res. Commun. 2006, 342, 963–972. [Google Scholar] [CrossRef]
- Griffith, J.F.; Wang, Y.-X.J.; Antonio, G.E.; Choi, K.C.; Yu, A.; Ahuja, A.T.; Leung, P.C. Modified Pfirrmann Grading System for Lumbar Intervertebral Disc Degeneration. Spine 2007, 32, E708. [Google Scholar] [CrossRef] [PubMed]
- Lenke, L.G.; Betz, R.R.; Harms, J.; Bridwell, K.H.; Clements, D.H.; Lowe, T.G.; Blanke, K. Adolescent idiopathic scoliosis: A new classification to determine extent of spinal arthrodesis. J. Bone Jt. Surg. Am. 2001, 83, 1169–1181. [Google Scholar] [CrossRef]
- Daher, M.T.; Melo, N.C.; Nascimento, V.N.; Felisbino, P., Jr.; Araújo, B.C.R.; Daher, S.; Rabahi, M.F. What Is the Best Distal Level of Arthrodesis in Lumbar Fusion in Patients with Adolescent Idiopathic Scoliosis: L3 or L4? Coluna/Columna 2019, 18, 200–204. [Google Scholar] [CrossRef]
- Akazawa, T.; Kotani, T.; Sakuma, T.; Minami, S.; Orita, S.; Fujimoto, K.; Shiga, Y.; Takaso, M.; Inoue, G.; Miyagi, M.; et al. Spinal fusion on adolescent idiopathic scoliosis patients with the level of L4 or lower can increase lumbar disc degeneration with sagittal imbalance 35 years after surgery. Spine Surg. Relat. Res. 2017, 1, 72–77. [Google Scholar] [CrossRef]
- Jones, M.; Badreddine, I.; Mehta, J.; Ede, M.N.; Gardner, A.; Spilsbury, J.; Marks, D. The Rate of Disc Degeneration on MRI in Preoperative Adolescent Idiopathic Scoliosis. Spine J. 2017, 17, S332. [Google Scholar] [CrossRef]
- An, H.S.; Anderson, P.A.; Haughton, V.M.; Iatridis, J.C.; Kang, J.D.; Lotz, J.C.; Natarajan, R.N.; Oegema, T.R.J.; Roughley, P.; Setton, L.A.; et al. Introduction: Disc Degeneration: Summary. Spine 2004, 29, 2677–2678. [Google Scholar] [CrossRef]
- Pfirrmann, C.W.; Metzdorf, A.; Zanetti, M.; Hodler, J.; Boos, N. Magnetic resonance classification of lumbar intervertebral disc degeneration. Spine 2001, 26, 1873–1878. [Google Scholar] [CrossRef] [PubMed]
- Urban, J.P.G.; McMullin, J.F. Swelling pressure of the inervertebral disc: Influence of proteoglycan and collagen contents. Biorheology 1985, 22, 145–157. [Google Scholar] [CrossRef]
- Chen, C.; Huang, M.; Han, Z.; Shao, L.; Xie, Y.; Wu, J.; Zhang, Y.; Xin, H.; Ren, A.; Guo, Y.; et al. Quantitative T2 Magnetic Resonance Imaging Compared to Morphological Grading of the Early Cervical Intervertebral Disc Degeneration: An Evaluation Approach in Asymptomatic Young Adults. PLoS ONE 2014, 9. [Google Scholar] [CrossRef]
- Gulani, V.; Calamante, F.; Shellock, F.G.; Kanal, E.; Reeder, S.B. International Society for Magnetic Resonance in Medicine Gadolinium deposition in the brain: Summary of evidence and recommendations. Lancet Neurol. 2017, 16, 564–570. [Google Scholar] [CrossRef]
- Ling, W.; Regatte, R.R.; Navon, G.; Jerschow, A. Assessment of glycosaminoglycan concentration in vivo by chemical exchange-dependent saturation transfer (gagCEST). Proc. Natl. Acad. Sci. USA 2008, 105, 2266–2270. [Google Scholar] [CrossRef] [PubMed]
- Saar, G.; Zhang, B.; Ling, W.; Regatte, R.R.; Navon, G.; Jerschow, A. Assessment of GAG Concentration Changes in the Intervertebral Disc via CEST. Nmr Biomed. 2012, 25, 255–261. [Google Scholar] [CrossRef]
- Müller-Lutz, A.; Schleich, C.; Schmitt, B.; Topgöz, M.; Pentang, G.; Antoch, G.; Wittsack, H.-J.; Miese, F. Improvement of gagCEST imaging in the human lumbar intervertebral disc by motion correction. Skelet. Radiol. 2015, 44, 505–511. [Google Scholar] [CrossRef] [PubMed]
- Haneder, S.; Apprich, S.R.; Schmitt, B.; Michaely, H.J.; Schoenberg, S.O.; Friedrich, K.M.; Trattnig, S. Assessment of glycosaminoglycan content in intervertebral discs using chemical exchange saturation transfer at 3.0 Tesla: Preliminary results in patients with low-back pain. Eur. Radiol. 2013, 23, 861–868. [Google Scholar] [CrossRef]
- Schleich, C.; Müller-Lutz, A.; Eichner, M.; Schmitt, B.; Matuschke, F.; Bittersohl, B.; Zilkens, C.; Wittsack, H.-J.; Antoch, G.; Miese, F. Glycosaminoglycan Chemical Exchange Saturation Transfer of Lumbar Intervertebral Discs in Healthy Volunteers. Spine 2016, 41, 146–152. [Google Scholar] [CrossRef]
- Müller-Lutz, A.; Schleich, C.; Pentang, G.; Schmitt, B.; Lanzman, R.S.; Matuschke, F.; Wittsack, H.-J.; Miese, F. Age-dependency of glycosaminoglycan content in lumbar discs: A 3t gagcEST study. J. Magn. Reson. Imaging JMRI 2015, 42, 1517–1523. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Chan, Q.; Anthony, M.-P.; Cheung, K.M.C.; Samartzis, D.; Khong, P.-L. Assessment of glycosaminoglycan distribution in human lumbar intervertebral discs using chemical exchange saturation transfer at 3 T: Feasibility and initial experience. NMR Biomed. 2011, 24, 1137–1144. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Kim, H.S.; Moon, E.S.; Yoon, C.-S.; Chung, T.-S.; Song, H.-T.; Suh, J.-S.; Lee, Y.H.; Kim, S. Scoliosis Imaging: What Radiologists Should Know. RadioGraphics 2010, 30, 1823–1842. [Google Scholar] [CrossRef]
- Schleich, C.; Müller-Lutz, A.; Blum, K.; Boos, J.; Bittersohl, B.; Schmitt, B.; Gerß, J.; Matuschke, F.; Wittsack, H.-J.; Antoch, G.; et al. Facet tropism and facet joint orientation: Risk factors for the development of early biochemical alterations of lumbar intervertebral discs. Osteoarthr. Cartil. 2016, 24, 1761–1768. [Google Scholar] [CrossRef] [PubMed]
- Müller-Lutz, A.; Schleich, C.; Schmitt, B.; Antoch, G.; Matuschke, F.; Quentin, M.; Wittsack, H.-J.; Miese, F. Gender, BMI and T2 dependencies of glycosaminoglycan chemical exchange saturation transfer in intervertebral discs. Magn. Reson. Imaging 2016, 34, 271–275. [Google Scholar] [CrossRef]
- Zhang, Z. Naïve Bayes classification in R. Ann. Transl. Med. 2016, 4, 241. [Google Scholar] [CrossRef]
- Beaumont, C. Comparison of Henderson’s Method I and restricted maximum likelihood estimation of genetic parameters of reproductive traits. Poult. Sci. 1991, 70, 1462–1468. [Google Scholar] [CrossRef]
- Urban, M.R.; Fairbank, J.C.T.; Bibby, S.R.S.; Urban, J.P.G. Intervertebral Disc Composition in Neuromuscular Scoliosis: Changes in Cell Density and Glycosaminoglycan Concentration at the Curve Apex. Spine 2001, 26, 610–617. [Google Scholar] [CrossRef]
- Lyons, G.; Eisenstein, S.M.; Sweet, M.B.E. Biochemical changes in intervertebral disc degeneration. Biochim. Biophys. Acta BBA Gen. Subj. 1981, 673, 443–453. [Google Scholar] [CrossRef]
- Danielsson, A.J.; Cederlund, C.G.; Ekholm, S.; Nachemson, A.L. The prevalence of disc aging and back pain after fusion extending into the lower lumbar Spine: A matched MR study twenty-five years after surgery for adolescent idiopathic scoliosis. Acta Radiol. 2001, 42, 187–197. [Google Scholar] [CrossRef]
- Nohara, A.; Kawakami, N.; Tsuji, T.; Ohara, T.; Saito, T.; Kawakami, K. Intervertebral Disc Degeneration During Postoperative Follow-up More Than 10 Years After Corrective Surgery in Idiopathic Scoliosis: Comparison Between Patients With and Without Surgery. Spine 2018, 43, 255–261. [Google Scholar] [CrossRef]
- Connolly, P.J.; Von Schroeder, H.P.; Johnson, G.E.; Kostuik, J.P. Adolescent idiopathic scoliosis. Long-term effect of instrumentation extending to the lumbar spine. JBJS 1995, 77, 1210–1216. [Google Scholar] [CrossRef] [PubMed]
- Müller, E.B.; Nordwall, A.; Odén, A. Progression of Scoliosis in Children with Myelomeningocele. Spine 1994, 19, 147–150. [Google Scholar] [CrossRef] [PubMed]
- Lonstein, J.E.; Carlson, J.M. The prediction of curve progression in untreated idiopathic scoliosis during growth. J. Bone Jt. Surg. Am. 1984, 66, 1061–1071. [Google Scholar] [CrossRef]
- Saito, N.; Ebara, S.; Ohotsuka, K.; Kumeta, H.; Takaoka, K. Natural history of scoliosis in spastic cerebral palsy. Lancet Lond. Engl. 1998, 351, 1687–1692. [Google Scholar] [CrossRef]
- Weinstein, S.L.; Dolan, L.A.; Cheng, J.C.; Danielsson, A.; Morcuende, J.A. Adolescent idiopathic scoliosis. Lancet 2008, 371, 1527–1537. [Google Scholar] [CrossRef]
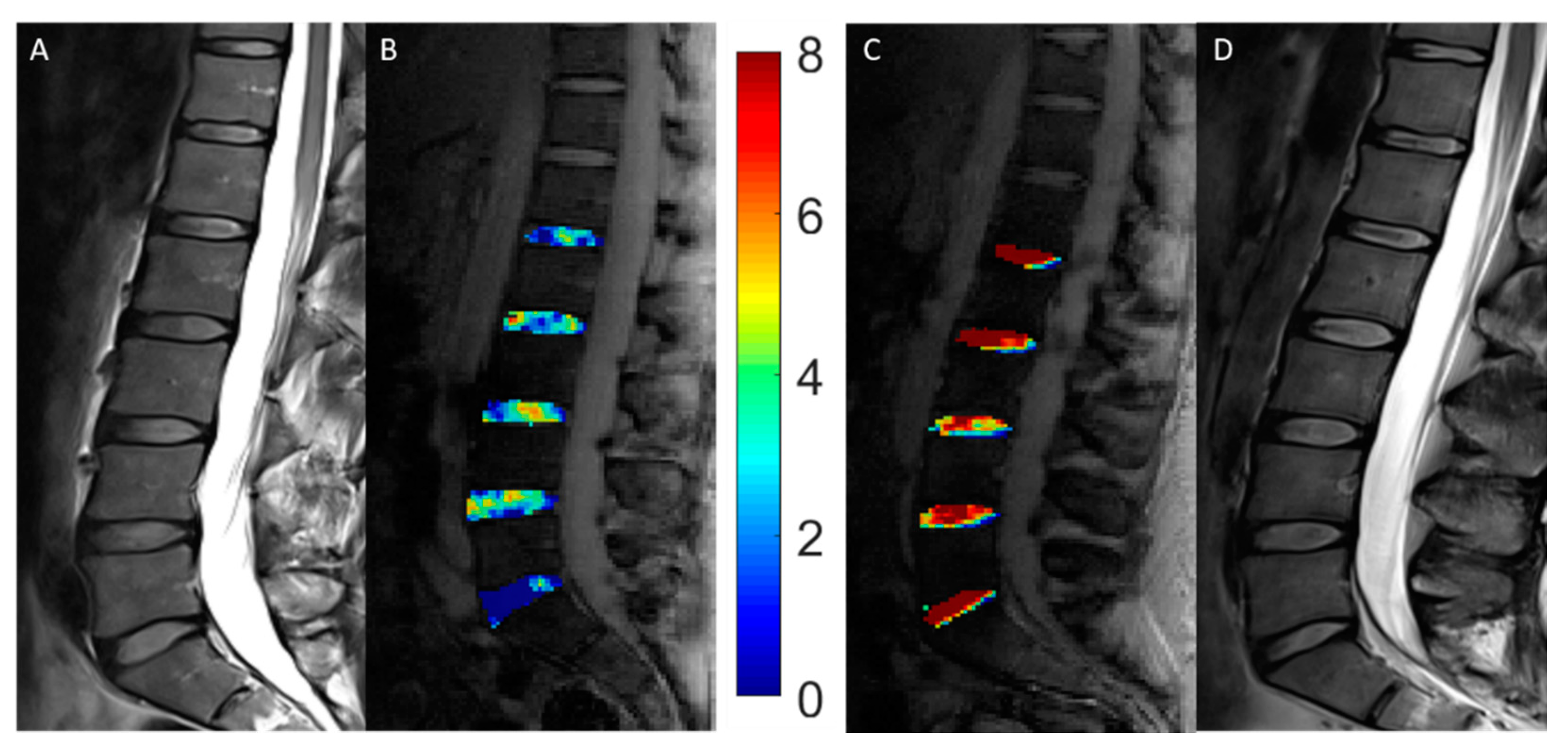
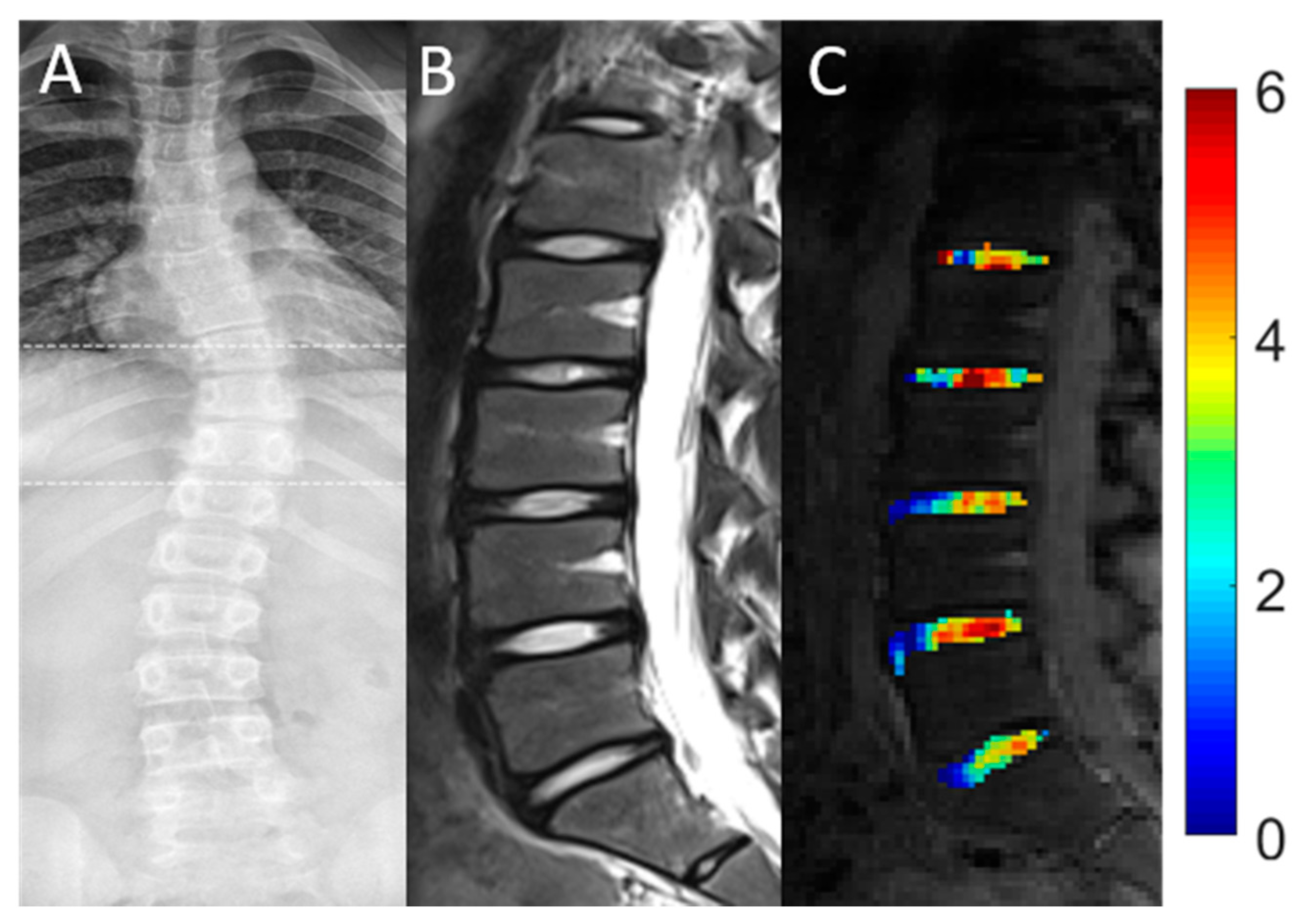
| AIS Patients | Controls | |
|---|---|---|
| Age [years] | 18.3 ± 8.2 | 25.5 ± 1.7 |
| Gender [female/male] | 7/4 | 8/8 |
| Number of involved vertebrae [n] | ||
| ● Major curve | 7.5 ± 3.2 (4–15) | n/a |
| ● Minor curve | 6 ± 0.5 (5–6) | n/a |
| Orientation of major curve [dextro-, levoscoliosis] | Dextroscoliosis n = 6 Levoscoliosis n = 5 | n/a |
| Cobb angle [°] major curve | 34.8 ± 21.5 (15–73) | n/a |
| Cobb angle [°] minor curve | 21.8 ± 10.2 (11–37) | n/a |
| Disease duration [months] | 59.2 ± 102.0 | n/a |
| Type of treatment | n/a | |
| ● Physical therapy | 4 | n/a |
| ● Bracing (conservative) | 5 | n/a |
| ● Bracing (+subsequent surgery) | 2 | n/a |
| Sequence | |||||
|---|---|---|---|---|---|
| STIR | T2w TSE | T1w TSE | gagCEST | WASSR | |
| Orientation | Sagittal | Sagittal | Sagittal | Sagittal | Sagittal |
| TE/TR [ms] | 57/3800 | 95/3500 | 9.5/650 | 5.1/10 | 5.1/10 |
| Flip Angle [°] | 150 | 160 | 150 | 10 | 10 |
| Slice Thickness [mm] | 4 | 4 | 4 | 5 | 5 |
| FoV [mm × mm] | 300 × 300 | 300 × 300 | 300 × 300 | 300 × 300 | 300 × 300 |
| Pixel Size [mm × mm] | 0.8 × 0.8 | 0.7 × 0.7 | 0.7 × 0.7 | 1.6 × 1.6 | 1.6 × 1.6 |
| Score | L1/2 | L2/3 | L3/4 | L4/5 | L5/S1 | All Segments | |
|---|---|---|---|---|---|---|---|
| gagCEST values [%] | Controls | 3.340 [2.721, 3.959] | 3.466 [2.844, 4.089] | 3.218 [2.586, 3.850] | 3.679 [2.082, 5.276] | 3.389 [2.877, 3.902] | 3.510 [3.159, 3.861] |
| AIS | 2.867 [2.005, 3.729] | 2.646 [1.831, 3.461] | 3.908 [3.079, 4.736] | 3.700 [1.581, 5.818] | 1.381 [0.661, 2.102] | 2.759 [2.320, 3.199] | |
| p-values | 0.399 | 0.139 | 0.220 | 0.988 | <0.001 | 0.005 | |
| Score | Segment | p-Value | ||||||
|---|---|---|---|---|---|---|---|---|
| L1/2 | L2/3 | L3/4 | L4/5 | L5/S1 | All Segments | |||
| IVDs affected by scoliosis | Yes | 3.171 [0.129, 4.213] | 2.886 [2.155, 3.616] | 5.103 [2.686, 7.520] | 1.520 [0.377, 2.663] | n/a | 2.929 [1.479, 4.379] | 0.258 |
| No | −0.004 [−3.666, 3.659] | 2.666 [1.179, 4.153] | 3.100 [2.093, 4.107] | 5.762 [1.116, 10.408] | 2.186 [0.942, 3.429] | 2.316 [0.936, 3.697] | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wollschläger, L.M.; Nebelung, S.; Schleich, C.; Müller-Lutz, A.; Radke, K.L.; Frenken, M.; Boschheidgen, M.; Prost, M.; Antoch, G.; Konieczny, M.R.; et al. Evaluating Lumbar Intervertebral Disc Degeneration on a Compositional Level Using Chemical Exchange Saturation Transfer: Preliminary Results in Patients with Adolescent Idiopathic Scoliosis. Diagnostics 2021, 11, 934. https://doi.org/10.3390/diagnostics11060934
Wollschläger LM, Nebelung S, Schleich C, Müller-Lutz A, Radke KL, Frenken M, Boschheidgen M, Prost M, Antoch G, Konieczny MR, et al. Evaluating Lumbar Intervertebral Disc Degeneration on a Compositional Level Using Chemical Exchange Saturation Transfer: Preliminary Results in Patients with Adolescent Idiopathic Scoliosis. Diagnostics. 2021; 11(6):934. https://doi.org/10.3390/diagnostics11060934
Chicago/Turabian StyleWollschläger, Lena M., Sven Nebelung, Christoph Schleich, Anja Müller-Lutz, Karl L. Radke, Miriam Frenken, Matthias Boschheidgen, Max Prost, Gerald Antoch, Markus R. Konieczny, and et al. 2021. "Evaluating Lumbar Intervertebral Disc Degeneration on a Compositional Level Using Chemical Exchange Saturation Transfer: Preliminary Results in Patients with Adolescent Idiopathic Scoliosis" Diagnostics 11, no. 6: 934. https://doi.org/10.3390/diagnostics11060934
APA StyleWollschläger, L. M., Nebelung, S., Schleich, C., Müller-Lutz, A., Radke, K. L., Frenken, M., Boschheidgen, M., Prost, M., Antoch, G., Konieczny, M. R., & Abrar, D. B. (2021). Evaluating Lumbar Intervertebral Disc Degeneration on a Compositional Level Using Chemical Exchange Saturation Transfer: Preliminary Results in Patients with Adolescent Idiopathic Scoliosis. Diagnostics, 11(6), 934. https://doi.org/10.3390/diagnostics11060934







