Elevation of CD40/CD40L Inflammatory Pathway Molecules in Carotid Plaques from Moderate-and-Severe Obstructive Sleep Apnea Patients
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients and Tissue Samples
2.2. Sleep Study
2.3. Immunohistochemistry
2.4. Evaluation of Immunohistochemistry
2.5. Statistical Analysis
3. Results
3.1. Study Participants
3.2. Immunohistochemistry
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zychowski, K.E.; Sanchez, B.; Pedrosa, R.P.; Lorenzi-Filho, G.; Drager, L.F.; Polotsky, V.Y.; Campen, M.J. Serum from obstructive sleep apnea patients induces inflammatory responses in coronary artery endothelial cells. Atherosclerosis 2016, 254, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Franklin, K.A.; Lindberg, E. Obstructive sleep apnea is a common disorder in the population—A review on the epidemiology of sleep apnea. J. Thorac. Dis. 2015, 7, 1311–1322. [Google Scholar]
- Geovanini, G.R.; Wang, R.; Weng, J.; Jenny, N.S.; Shea, S.; Allison, M.; Libby, P.; Redline, S. Association between Obstructive Sleep Apnea and Cardiovascular Risk Factors: Variation by Age, Sex, and Race. The Multi-Ethnic Study of Atherosclerosis. Ann. Am. Thorac. Soc. 2018, 15, 970–977. [Google Scholar] [CrossRef] [PubMed]
- Drager, L.F.; Polotsky, V.Y.; Lorenzi-Filho, G. Obstructive Sleep Apnea: An Emerging Risk Factor for Atherosclerosis. Chest 2011, 140, 534–542. [Google Scholar] [CrossRef]
- Virmani, R.; Kolodgie, F.D.; Burke, A.P.; Finn, A.V.; Gold, H.K.; Tulenko, T.N.; Wrenn, S.P.; Narula, J. Atherosclerotic plaque progression and vulnerability to rupture: Angiogenesis as a source of intraplaque hemorrhage. Arterioscler. Thromb. Vasc. Biol. 2005, 25, 2054–2061. [Google Scholar] [CrossRef]
- Kheirandish-Gozal, L.; Gozal, D. Obstructive Sleep Apnea and Inflammation: Proof of Concept Based on Two Illustrative Cytokines. Int. J. Mol. Sci. 2019, 20, 459. [Google Scholar] [CrossRef]
- Bosmans, L.A.; Bosch, L.; Kusters, P.J.; Lutgens, E.; Seijkens, T.T. The CD40-CD40L Dyad as Immunotherapeutic Target in Cardiovascular Disease. J. Cardiovasc. Transl. Res. 2021, 14, 13–22. [Google Scholar] [CrossRef]
- Johnson, J.L. Metalloproteinases in atherosclerosis. Eur. J. Pharmacol. 2017, 816, 93–106. [Google Scholar] [CrossRef]
- Bianconi, V.; Sahebkar, A.; Atkin, S.L.; Pirro, M. The regulation and importance of monocyte chemoattractant protein-1. Curr. Opin. Hematol. 2018, 25, 44–51. [Google Scholar] [CrossRef]
- Koenig, W.; Khuseyinova, N. Biomarkers of Atherosclerotic Plaque Instability and Rupture. Arter. Thromb. Vasc. Biol. 2007, 27, 15–26. [Google Scholar] [CrossRef]
- Zhang, B.; Wu, T.; Chen, M.; Zhou, Y.; Yi, D.; Guo, R. The CD40/CD40L system: A new therapeutic target for disease. Immunol. Lett. 2013, 153, 58–61. [Google Scholar] [CrossRef]
- Pamukcu, B.; Lip, G.Y.H.; Snezhitskiy, V.; Shantsila, E. The CD40-CD40L system in cardiovascular disease. Ann. Med. 2011, 43, 331–340. [Google Scholar] [CrossRef] [PubMed]
- von Känel, R.; Dimsdale, J.E. Hemostatic alterations in patients with obstructive sleep apnea and the implications for cardiovascular disease. Chest 2003, 124, 1956–1967. [Google Scholar] [CrossRef]
- Gabryelska, A.; Łukasik, Z.M.; Makowska, J.S.; Białasiewicz, P. Obstructive Sleep Apnea: From Intermittent Hypoxia to Cardiovascular Complications via Blood Platelets. Front. Neurol. 2018, 9, 635. [Google Scholar] [CrossRef] [PubMed]
- Djupesland, P.G.; Haight, J.S.J. Nitric Oxide (NO) and Obstructive Sleep Apnea (OSA). Sleep Breath. 2003, 7, 053–062. [Google Scholar] [CrossRef]
- Viktorinova, A. Potential clinical utility of macrophage colony-stimulating factor, monocyte chemotactic protein-1 and myeloperoxidase in predicting atherosclerotic plaque instability. Discov. Med. 2020, 28, 237–245. [Google Scholar]
- Saba, L.; Mallarini, G. A comparison between NASCET and ECST methods in the study of carotids: Evaluation using Multi-Detector-Row CT angiography. Eur. J. Radiol. 2010, 76, 42–47. [Google Scholar] [CrossRef]
- Zhang, Z.; Sowho, M.; Otvos, T.; Sperandio, L.S.; East, R.J.; Sgambati, F.; Schwartz, A.; Schneider, H. A comparison of automated and manual sleep staging and respiratory event recognition in a portable sleep diagnostic device with in-lab sleep study. J. Clin. Sleep Med. 2020, 16, 563–573. [Google Scholar] [CrossRef]
- Ramos-Vara, J.A. Principles and Methods of Immunohistochemistry. Methods Mol. Biol. 2017, 1641, 115–128. [Google Scholar] [CrossRef]
- Magaki, S.; Hojat, S.A.; Wei, B.; So, A.; Yong, W.H. An Introduction to the Performance of Immunohistochemistry. Methods Mol. Biol. 2019, 1897, 289–298. [Google Scholar] [CrossRef]
- Dempsey, J.A.; Veasey, S.C.; Morgan, B.J.; O’Donnell, C.P. Pathophysiology of Sleep Apnea. Physiol. Rev. 2010, 90, 47–112. [Google Scholar] [CrossRef]
- Ohga, E.; Tomita, T.; Wada, H.; Yamamoto, H.; Nagase, T.; Ouchi, Y. Effects of obstructive sleep apnea on circulating ICAM-1, IL-8, and MCP-1. J. Appl. Physiol. 2003, 94, 179–184. [Google Scholar] [CrossRef]
- Kim, J.; Lee, C.H.; Park, C.S.; Kim, B.G.; Kim, S.W.; Cho, J.H. Plasma Levels of MCP-1 and Adiponectin in Obstructive Sleep Apnea Syndrome. Arch. Otolaryngol.-Head Neck Surg. 2010, 136, 896–899. [Google Scholar] [CrossRef][Green Version]
- Chuang, L.-P.; Chen, N.-H.; Lin, Y.; Ko, W.-S.; Pang, J.-H.S. Increased MCP-1 gene expression in monocytes of severe OSA patients and under intermittent hypoxia. Sleep Breath. 2015, 20, 425–433. [Google Scholar] [CrossRef] [PubMed]
- Perrini, S.; Cignarelli, A.; Quaranta, V.N.; Falcone, V.A.; Kounaki, S.; Porro, S.; Ciavarella, A.; Ficarella, R.; Barbaro, M.; Genchi, V.A.; et al. Correction of intermittent hypoxia reduces inflammation in obese subjects with obstructive sleep apnea. JCI Insight 2017, 2. [Google Scholar] [CrossRef] [PubMed]
- Kheirandish-Gozal, L.; Gileles-Hillel, A.; Alonso-Álvarez, M.L.; Peris, E.; Bhattacharjee, R.; Terán-Santos, J.; Duran-Cantolla, J.; Gozal, D. Effects of adenotonsillectomy on plasma inflammatory biomarkers in obese children with obstructive sleep apnea: A community-based study. Int. J. Obes. 2015, 39, 1094–1100. [Google Scholar] [CrossRef]
- Vuralkan, E.; Mutlu, M.; Firat, I.H.; Akaydin, S.; Sagit, M.; Akin, I.; Miser, E.; Ardic, S. Changes in serum levels of MDA and MMP-9 after UPF in patients with OSAS. Eur. Arch. Oto-Rhino-Laryngol. 2014, 271, 1329–1334. [Google Scholar] [CrossRef] [PubMed]
- Tazaki, T.; Minoguchi, K.; Yokoe, T.; Samson, K.T.R.; Minoguchi, H.; Tanaka, A.; Watanabe, Y.; Adachi, M. Increased Levels and Activity of Matrix Metalloproteinase-9 in Obstructive Sleep Apnea Syndrome. Am. J. Respir. Crit. Care Med. 2004, 170, 1354–1359. [Google Scholar] [CrossRef] [PubMed]
- Hopps, E.; Canino, B.; Montana, M.; Calandrino, V.; Urso, C.; Presti, R.L.; Caimi, G. Gelatinases and their tissue inhibitors in a group of subjects with obstructive sleep apnea syndrome. Clin. Hemorheol. Microcirc. 2016, 62, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Chuang, L.-P.; Chen, N.-H.; Lin, S.-W.; Chang, Y.-L.; Chao, I.-J.; Pang, J.-H.S. Increased matrix metalloproteinases-9 after sleep in plasma and in monocytes of obstructive sleep apnea patients. Life Sci. 2013, 93, 220–225. [Google Scholar] [CrossRef]
- Feng, X.; Liu, B.; Wang, J.; Xiao, X. Research on the serum levels of matrix metalloproteinase-9 and free fatty acids in OSAHS cases. J. Clin. Otorhinolaryngol. Head Neck Surg. 2011, 25, 109–113. [Google Scholar]
- Wang, S.; Li, S.; Wang, B.; Liu, J.; Tang, Q. Matrix Metalloproteinase-9 Is a Predictive Factor for Systematic Hypertension and Heart Dysfunction in Patients with Obstructive Sleep Apnea Syndrome. BioMed Res. Int. 2018, 2018, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Kaditis, A.G.; Alexopoulos, E.I.; Karathanasi, A.; Ntamagka, G.; Oikonomidi, S.; Kiropoulos, T.S.; Zintzaras, E.; Gourgoulianis, K. Adiposity and low-grade systemic inflammation modulate matrix metalloproteinase-9 levels in Greek children with sleep apnea. Pediatr. Pulmonol. 2010, 45, 693–699. [Google Scholar] [CrossRef] [PubMed]
- Bonanno, A.; Riccobono, L.; Bonsignore, M.R.; Bue, A.L.; Salvaggio, A.; Insalaco, G.; Marrone, O. Relaxin in Obstructive Sleep Apnea: Relationship with Blood Pressure and Inflammatory Mediators. Respiration 2016, 91, 56–62. [Google Scholar] [CrossRef] [PubMed]
- Maeder, M.T.; Strobel, W.; Christ, M.; Todd, J.; Estis, J.; Wildi, K.; Thalmann, G.; Hilti, J.; Brutsche, M.; Twerenbold, R.; et al. Comprehensive biomarker profiling in patients with obstructive sleep apnea. Clin. Biochem. 2015, 48, 340–346. [Google Scholar] [CrossRef] [PubMed]
- Fang, X.; Chen, J.; Wang, W.; Feng, G.; Li, X.; Zhang, X.; Zhang, Y.; Zhang, J.; Xu, Z.; Tai, J.; et al. Matrix metalloproteinase 9 (MMP9) level and MMP9-1562C>T in patients with obstructive sleep apnea: A systematic review and meta-analysis of case-control studies. Sleep Med. 2020, 67, 110–119. [Google Scholar] [CrossRef]
- Franczak, A.; Bil-Lula, I.; Sawicki, G.; Fenton, M.; Ayas, N.; Skomro, R. Matrix metalloproteinases as possible biomarkers of obstructive sleep apnea severity – A systematic review. Sleep Med. Rev. 2019, 46, 9–16. [Google Scholar] [CrossRef]
- Hopps, E.; Presti, R.L.; Montana, M.; Canino, B.; Calandrino, V.; Caimi, G. Analysis of the correlations between oxidative stress, gelatinases and their tissue inhibitors in the human subjects with obstructive sleep apnea syndrome. J. Physiol. Pharmacol. Off. J. Pol. Physiol. Soc. 2015, 66, 803–810. [Google Scholar]
- Heo, S.H.; Cho, C.-H.; Kim, H.O.; Jo, Y.H.; Yoon, K.-S.; Lee, J.H.; Park, J.-C.; Park, K.C.; Ahn, T.-B.; Chung, K.C.; et al. Plaque Rupture is a Determinant of Vascular Events in Carotid Artery Atherosclerotic Disease: Involvement of Matrix Metalloproteinases 2 and 9. J. Clin. Neurol. 2011, 7, 69–76. [Google Scholar] [CrossRef]
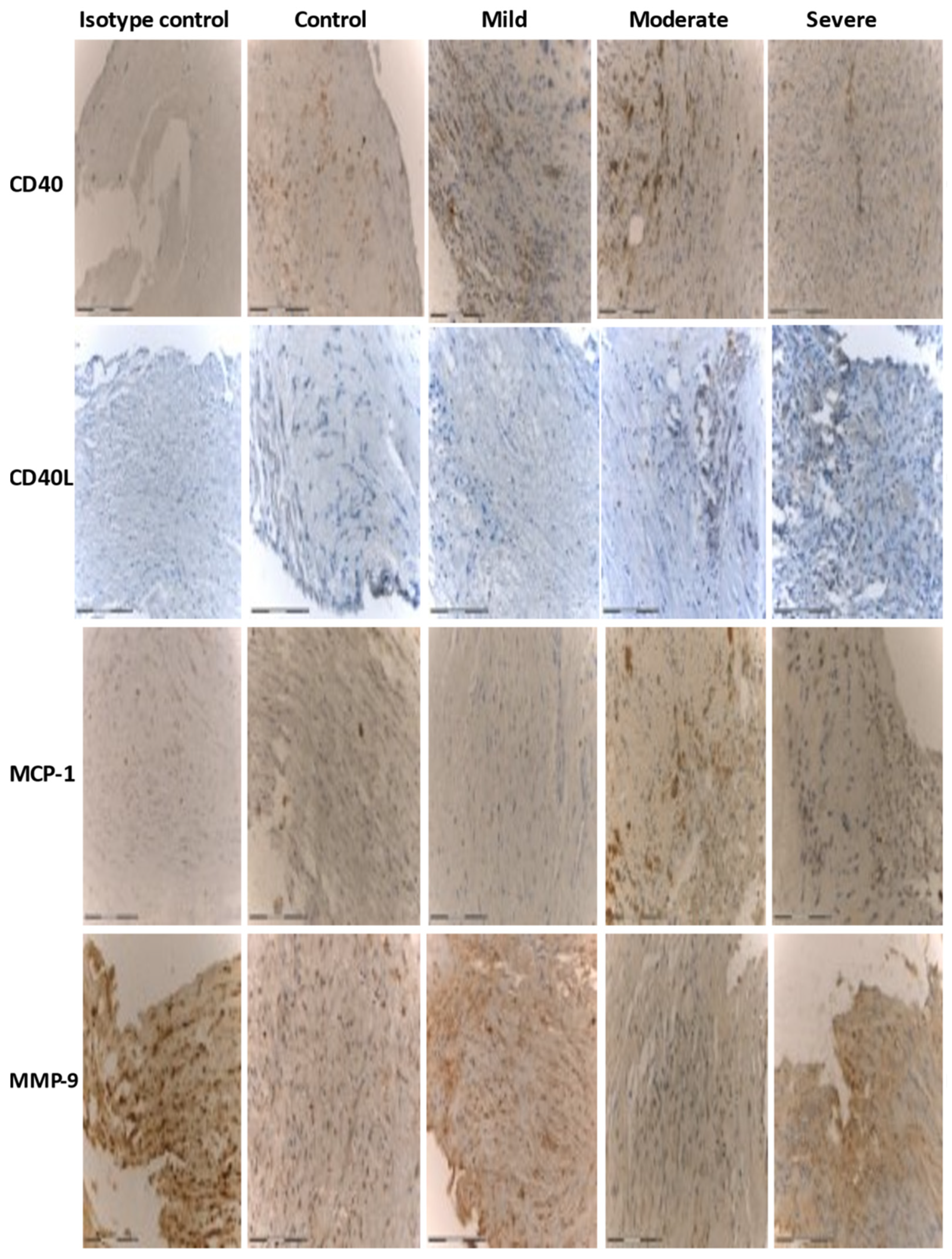
| Control and Mild | Moderate and Severe | |
|---|---|---|
| Cases, n | 27 | 19 |
| Age, years | 72.6 ± 7.65 | 73.9 ± 9.8 |
| Female/Male, n | 16/11 | 6/13 |
| BMI, kg/m2 | 26.4 ± 2.75 | 30 ± 3.6 * |
| Neck circumference, cm | 38 ± 3.9 | 40.8 ± 2.4 * |
| Waist circumference, cm | 97.7 ± 8.9 | 105.6 ± 10.1 * |
| pAHI, events/h | 6.3 ± 1.75 | 32.6 ± 8.2 ** |
| ODI | 2.4 ± 1.15 | 23.3 ± 6.9 ** |
| ESS score | 5.45 ± 4.8 | 8.3 ± 5.5 * |
| Control and Mild OSA (n = 27) | Moderate and Severe OSA (n = 19) | 95% CI | |||||||
|---|---|---|---|---|---|---|---|---|---|
| M | SD | M | SD | t | p | LL | UL | d Cohen | |
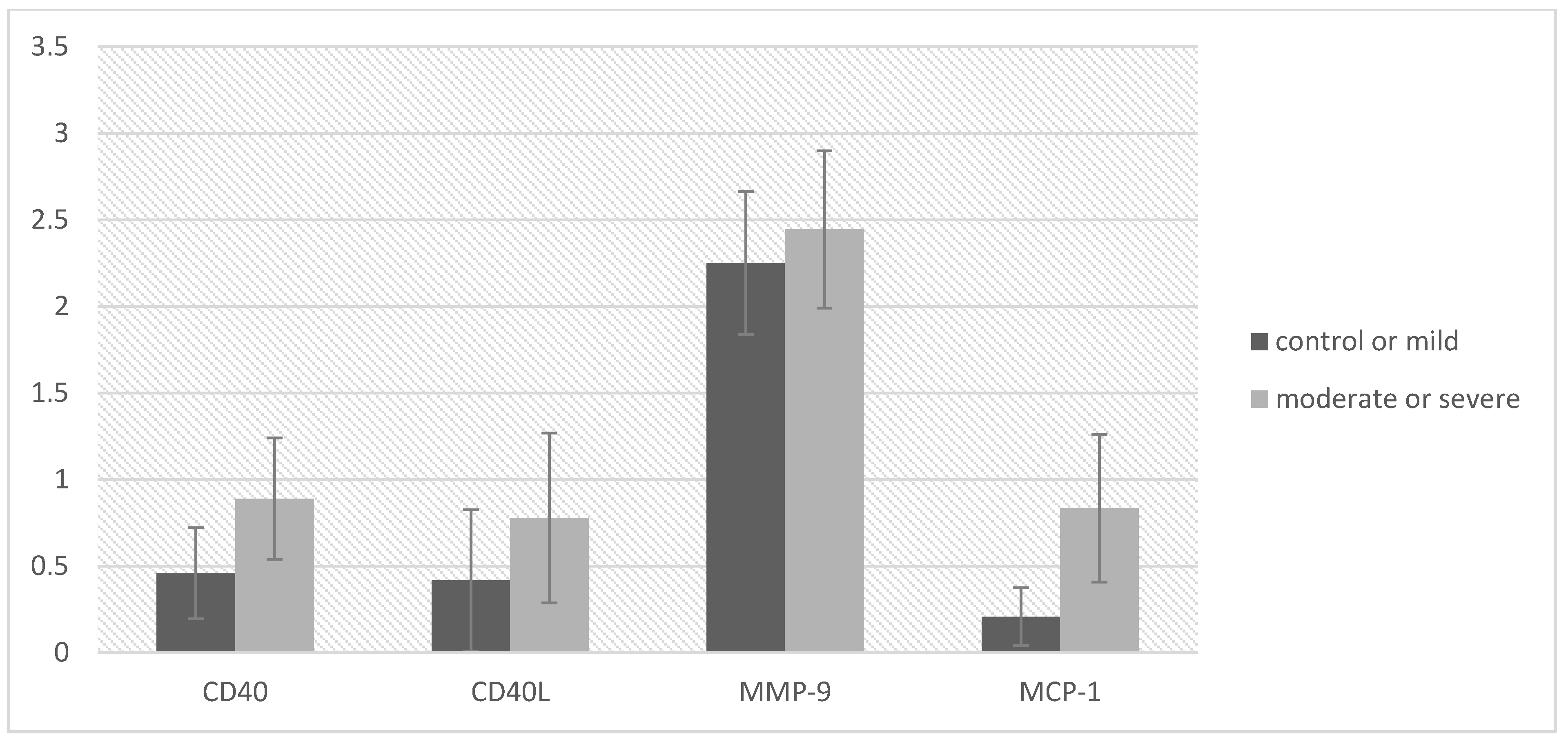
| CD40 | 0.46 | 0.66 | 0.89 | 0.76 | −1.97 | 0.056 | −0.87 | 0.01 | 0.61 |
| CD40L | 0.42 | 1.02 | 0.78 | 1.06 | −1.12 | 0.270 | −1.01 | 0.29 | 0.35 |
| MMP-9 | 2.25 | 1.03 | 2.44 | 0.98 | −0.62 | 0.541 | −0.83 | 0.44 | 0.19 |
| MCP-1 | 0.21 | 0.41 | 0.83 | 0.92 | −2.68 | 0.014 | −1.11 | −0.14 | 0.92 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Migacz, E.; Olejarz, W.; Głuszko, A.; Bednarek-Rajewska, K.; Proczka, R.; Smith, D.F.; Ishman, S.L.; Kukwa, W. Elevation of CD40/CD40L Inflammatory Pathway Molecules in Carotid Plaques from Moderate-and-Severe Obstructive Sleep Apnea Patients. Diagnostics 2021, 11, 935. https://doi.org/10.3390/diagnostics11060935
Migacz E, Olejarz W, Głuszko A, Bednarek-Rajewska K, Proczka R, Smith DF, Ishman SL, Kukwa W. Elevation of CD40/CD40L Inflammatory Pathway Molecules in Carotid Plaques from Moderate-and-Severe Obstructive Sleep Apnea Patients. Diagnostics. 2021; 11(6):935. https://doi.org/10.3390/diagnostics11060935
Chicago/Turabian StyleMigacz, Ewa, Wioletta Olejarz, Alicja Głuszko, Katarzyna Bednarek-Rajewska, Robert Proczka, David F. Smith, Stacey L. Ishman, and Wojciech Kukwa. 2021. "Elevation of CD40/CD40L Inflammatory Pathway Molecules in Carotid Plaques from Moderate-and-Severe Obstructive Sleep Apnea Patients" Diagnostics 11, no. 6: 935. https://doi.org/10.3390/diagnostics11060935
APA StyleMigacz, E., Olejarz, W., Głuszko, A., Bednarek-Rajewska, K., Proczka, R., Smith, D. F., Ishman, S. L., & Kukwa, W. (2021). Elevation of CD40/CD40L Inflammatory Pathway Molecules in Carotid Plaques from Moderate-and-Severe Obstructive Sleep Apnea Patients. Diagnostics, 11(6), 935. https://doi.org/10.3390/diagnostics11060935







