Detection of COVID-19 Virus on Surfaces Using Photonics: Challenges and Perspectives
Abstract
1. Introduction
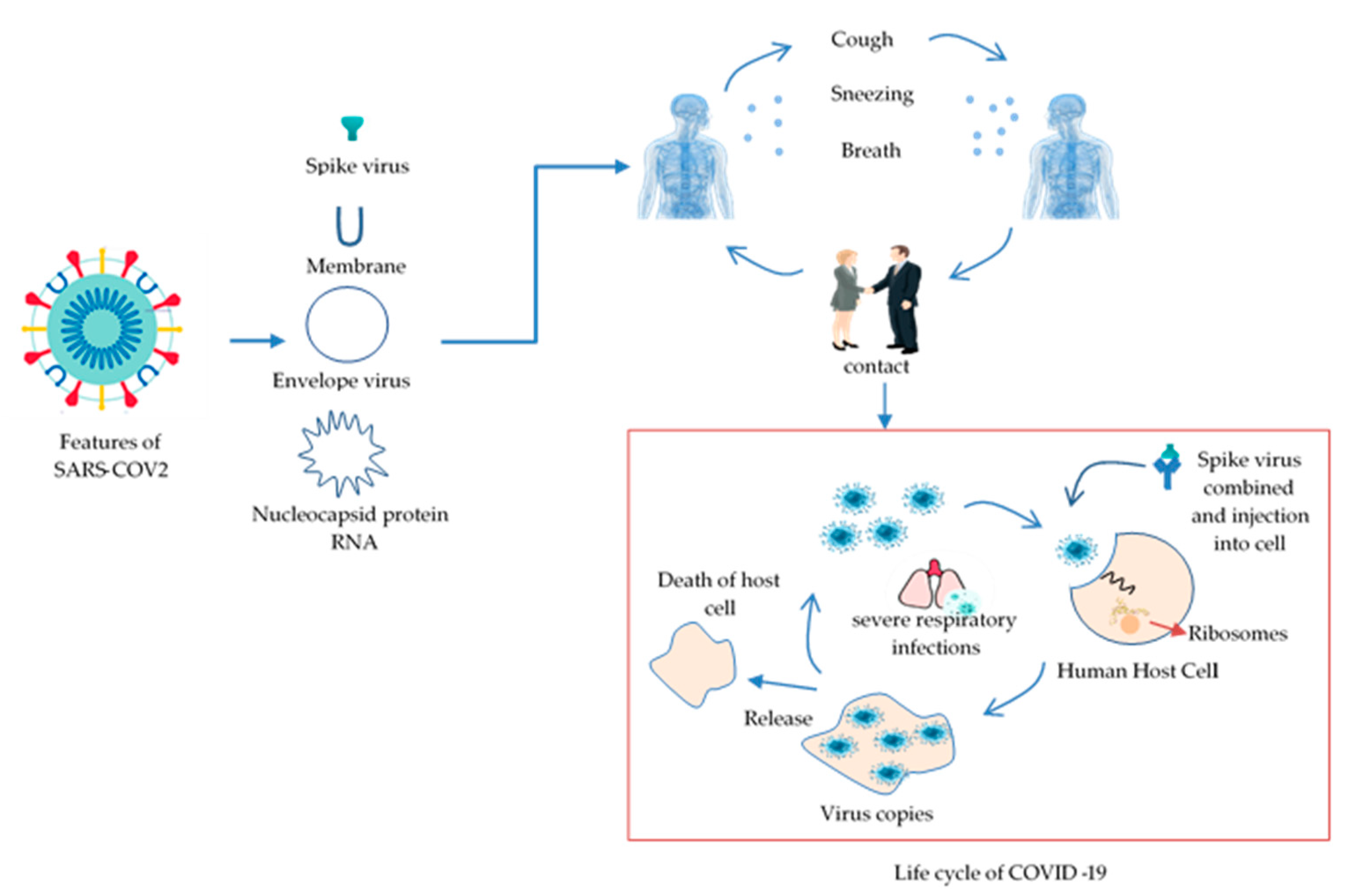
1.1. Emergence and Features of COVID-19
1.2. Background
2. COVID-19 Identification via Photonics
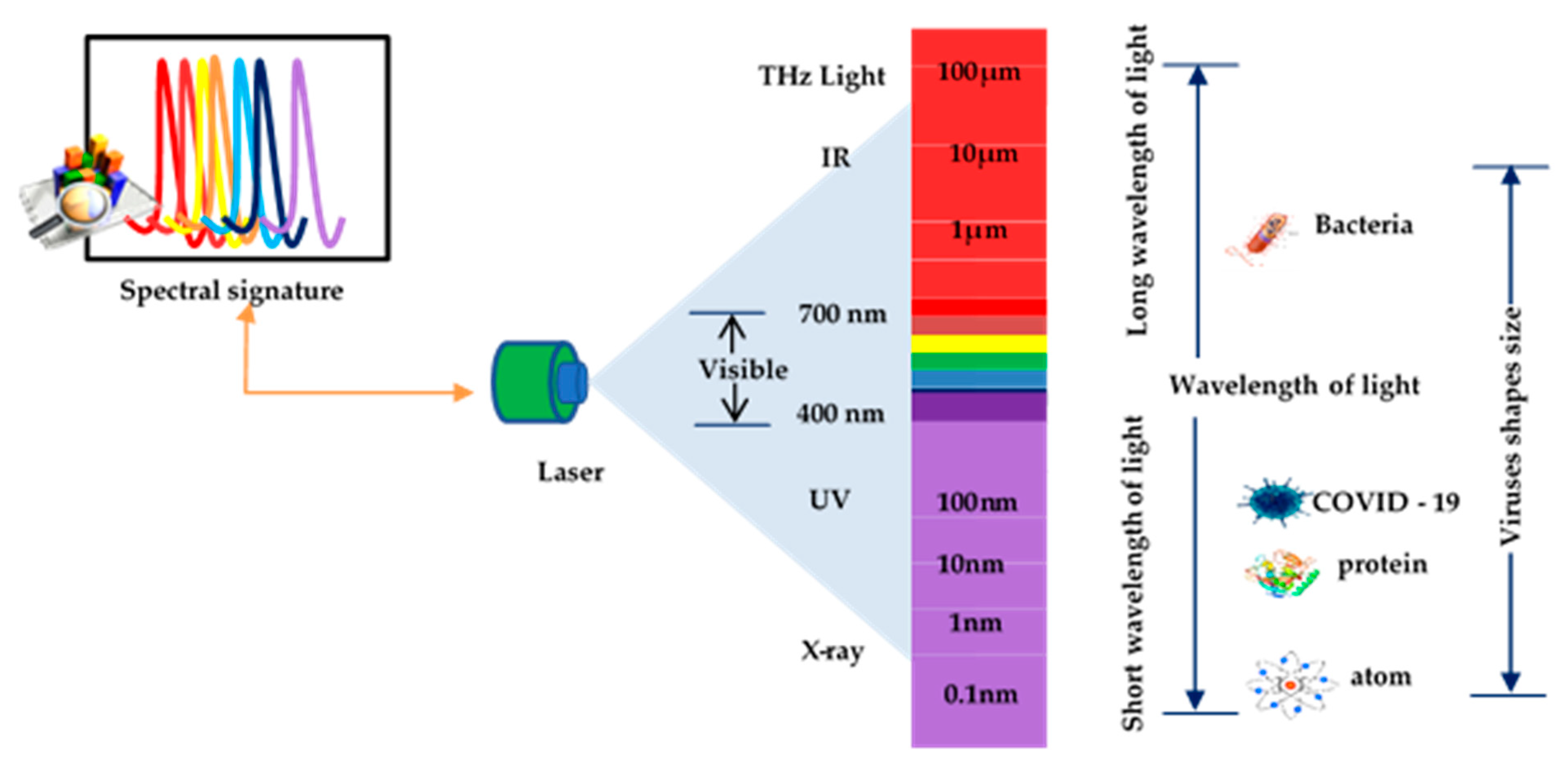
2.1. Laser Spectrum with Molecules
2.2. Enhanced Detection of Viruses by Laser
3. Detection of COVID-19 Using Light Technologies
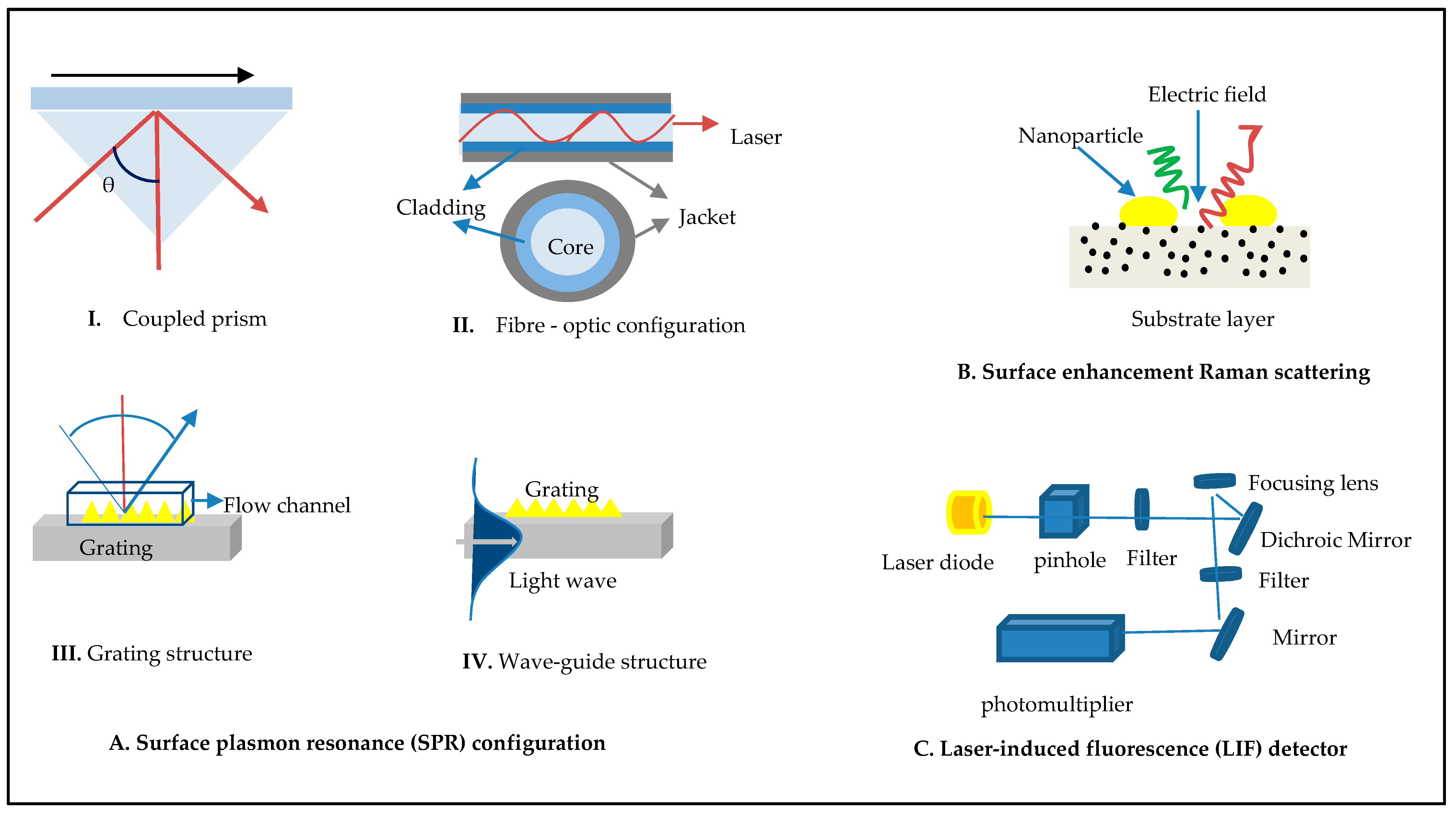
3.1. Fluorescence Methods
3.2. Surface-Enhanced Raman Scattering
3.3. Surface Plasmon Resonance (SPR)
3.4. Raman Scattering with SPR Integrated
4. Analysis Outcome of Literature Review
5. Opportunities and Limitations
- ♦
- Reduced performance of sensitivity and accuracy;
- ♦
- Time-consuming preparation and purification of samples;
- ♦
- The devices’ complex process;
- ♦
- A need for highly skilled professional staff.
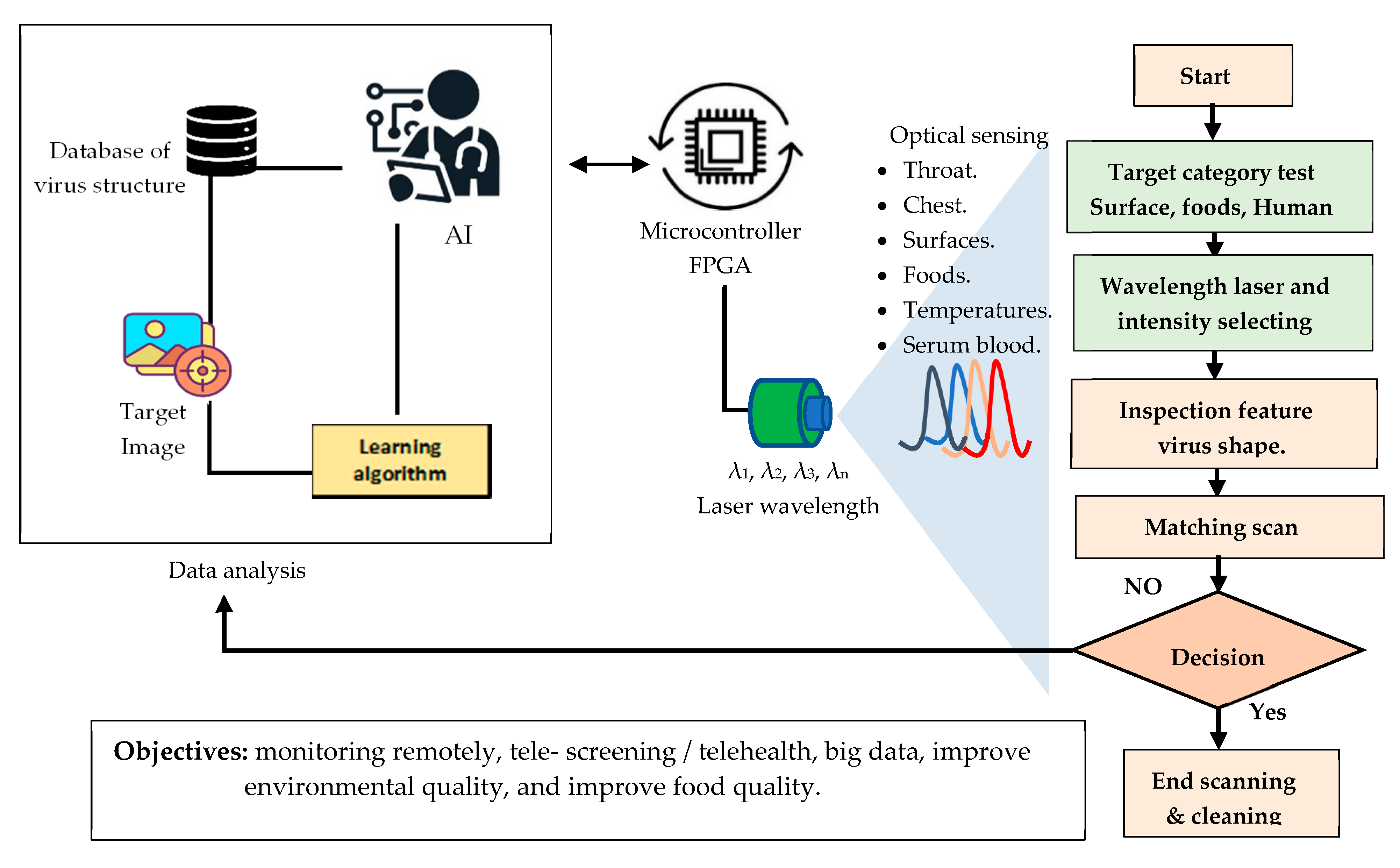
6. Tracking COVID-19 Virus in the Environment
7. Conclusions and Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Ethics and Permission to Participate
Abbreviations
| COVID-19 | Coronavirus disease |
| SARS-CoV-2 | Coronavirus severe acute respiratory syndrome 2 |
| LSPCF | Localized surface plasmon coupled fluorescence |
| SERS | Surface-enhanced Raman scattering |
| SPR | Surface plasmon resonance |
| LSPR | Localized surface plasmon resonance |
| P-FAB | Plasmonic fiber-optic absorbance biosensor |
| EWA | Evanescent wave absorbance |
| pg | Picogram |
| ng | Nanogram |
| LIF-LiDAR | Laser-induced fluorescence-light detection and ranging |
| PCR | Polymerase chain reaction |
| WHO | World Health Organization |
| SARS | Severe acute respiratory syndrome |
| MERS | Middle East respiratory syndrome |
| CDC | Centers for Disease Control and Prevention |
| RU | Response unit |
References
- Dong, E.; Du, H.; Gardner, L. COVID-19 Dashboard by the Center for Systems Science and Engineering (CSSE) at Johns Hopkins University (JHU). Lancet Inf. Dis. 2020, 19, 533–534. [Google Scholar] [CrossRef]
- Caliendo, A.M.; Gilbert, D.N.; Ginocchio, C.C.; Hanson, K.E.; May, L.; Quinn, T.C.; Tenover, F.C.; Alland, D.; Blaschke, A.J.; Bonomo, R.A.; et al. Better Tests, Better Care: Improved Diagnostics for Infectious Diseases. Clin. Infect. Dis. 2013, 57, S139–S170. [Google Scholar] [CrossRef]
- Rajapaksha, P.; Elbourne, A.J.; Gangadoo, S.; Brown, R.; Cozzolino, D.; Chapman, J. A review of methods for the detection of pathogenic microorganisms. Analyst 2019, 144, 396–411. [Google Scholar] [CrossRef]
- Chan, J.F.W.; Yuan, S.; Kok, K.H.; To, K.K.W.; Chu, H.; Yang, J.; Xing, F.; Liu, J.; Yip, C.C.Y.; Poon, R.W.S.; et al. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: A study of a family cluster. Lancet 2020, 395, 514–523. [Google Scholar] [CrossRef]
- Zipfel, W.; Williams, R.M.; Webb, W.W. Nonlinear magic: Multiphoton microscopy in the biosciences. Nat. Biotechnol. 2003, 21, 1369–1377. [Google Scholar] [CrossRef]
- Kovach, A.; Chen, D.; He, J.; Choi, H.; Dogan, A.H.; Ghasemkhani, M.; Taheri, H.; Armani, A.M. Emerging material systems for integrated optical Kerr frequency combs. Adv. Opt. Photonics 2020, 12, 135–222. [Google Scholar] [CrossRef]
- Lukose, J.; Chidangil, S.; George, S.D. Optical technologies for the detection of viruses like COVID-19: Progress and prospects. Biosens. Bioelectron. 2021, 178, 113004. [Google Scholar] [CrossRef] [PubMed]
- Abushagur, A.; Arsad, N.; Bakar, A. Cantilever Beam with a Single Fiber Bragg Grating to Measure Temperature and Transversal Force Simultaneously. Sensors 2021, 21, 2002. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Laboratory Testing for Coronavirus Disease 2019 (COVID-19) in Suspected Human Cases; WHO: Geneva, Switzerland, 2020; pp. 1–7. [Google Scholar]
- Gates, B. Responding to Covid-19—A Once-in-a-Century Pandemic? N. Engl. J. Med. 2020, 382, 1677–1679. [Google Scholar] [CrossRef]
- Mahmood, Z.; Alrefai, H.; Hetta, H.F.; Kader, H.A.; Munawar, N.; Rahman, S.A.; Elshaer, S.; Batiha, G.E.-S.; Muhammad, K. Investigating Virological, Immunological, and Pathological Avenues to Identify Potential Targets for Developing COVID-19 Treatment and Prevention Strategies. Vaccines 2020, 8, 443. [Google Scholar] [CrossRef] [PubMed]
- Saylan, Y.; Erdem, Ö.; Ünal, S.; Denizli, A. An Alternative Medical Diagnosis Method: Biosensors for Virus Detection. Biosensors 2019, 9, 65. [Google Scholar] [CrossRef]
- Cui, J.; Li, F.; Shi, Z.-L. Origin and evolution of pathogenic coronaviruses. Nat. Rev. Microbiol. 2019, 17, 181–192. [Google Scholar] [CrossRef]
- Frías, I.A.M.; Avelino, K.; Silva, R.R.; Andrade, C.A.S.; Oliveira, M.D.L. Trends in Biosensors for HPV: Identification and Diagnosis. J. Sensors 2015, 2015, 1–16. [Google Scholar] [CrossRef]
- Kumar, A.; Saxena, A.K.; Lee, G.G.C.; Kashyap, A.; Jyothsna, G. Novel Coronavirus In-Silico Vaccine Design and Drug Discovery; Springer: Berlin/Heidelberg, Germany, 2019. [Google Scholar]
- Oberfeld, B.; Achanta, A.; Carpenter, K.; Chen, P.; Gilette, N.M.; Langat, P.; Said, J.T.; Schiff, A.E.; Zhou, A.S.; Barczak, A.K.; et al. SnapShot: COVID-19. Cell 2020, 181, 954. [Google Scholar] [CrossRef]
- Zhu, N.; Zhang, D.; Wang, W.; Li, X.; Yang, B.; Song, J.; Zhao, X.; Huang, B.; Shi, W.; Lu, R.; et al. A Novel Coronavirus from Patients with Pneumonia in China, 2019. N. Engl. J. Med. 2020, 382, 727–733. [Google Scholar] [CrossRef]
- Yang, M.; Li, H.; Sun, J.; Zhao, Y.; Tang, D. Focus on Characteristics of COVID-19 with the Special Reference to the Impact of COVID-19 on the Urogenital System. Curr. Urol. 2020, 14, 79–84. [Google Scholar] [CrossRef] [PubMed]
- Mousavizadeh, L.; Ghasemi, S. Genotype and phenotype of COVID-19: Their roles in pathogenesis. J. Microbiol. Immunol. Infect. 2021, 54, 159–163. [Google Scholar] [CrossRef] [PubMed]
- Taha, B.; Al Mashhadany, Y.; Mokhtar, M.H.; Bin Zan, M.D.; Arsad, N. An Analysis Review of Detection Coronavirus Disease 2019 (COVID-19) Based on Biosensor Application. Sensors 2020, 20, 6764. [Google Scholar] [CrossRef]
- Feng, W.; Newbigging, A.M.; Le, C.; Pang, B.; Peng, H.; Cao, Y.; Wu, J.; Abbas, G.; Song, J.; Wang, D.-B.; et al. Molecular Diagnosis of COVID-19: Challenges and Research Needs. Anal. Chem. 2020, 92, 10196–10209. [Google Scholar] [CrossRef]
- Passaro, V.M.N.; De Tullio, C.; Troia, B.; La Notte, M.; Giannoccaro, G.; De Leonardis, F. Recent Advances in Integrated Photonic Sensors. Sensors 2012, 12, 15558–15598. [Google Scholar] [CrossRef]
- Antiochia, R. Nanobiosensors as new diagnostic tools for SARS, MERS and COVID-19: From past to perspectives. Microchim. Acta 2020, 187, 1–13. [Google Scholar] [CrossRef] [PubMed]
- He, C.; Fang, J.; Zhang, Y.; Yang, Y.; Yu, J.; Zhang, J.; Guan, H.; Qiu, W.; Wu, P.; Dong, J.; et al. High performance all-fiber temperature sensor based on coreless side-polished fiber wrapped with polydimethylsiloxane. Opt. Express 2018, 26, 9686–9699. [Google Scholar] [CrossRef] [PubMed]
- Ab Rahman, W.B.W.; Azeman, N.H.; Kamaruddin, N.H.; Menon, P.S.; Shabaneh, A.A.; Mahdi, M.A.; Mokhtar, M.H.H.; Arsad, N.; Bakar, A.A.A. Label-Free Detection of Dissolved Carbon Dioxide Utilizing Multimode Tapered Optical Fiber Coated Zinc Oxide Nanorice. IEEE Access 2018, 7, 4538–4545. [Google Scholar] [CrossRef]
- Azeman, N.H.; Arsad, N.; A Bakar, A.A. Polysaccharides as the Sensing Material for Metal Ion Detection-Based Optical Sensor Applications. Sensors 2020, 20, 3924. [Google Scholar] [CrossRef] [PubMed]
- Sepúlveda, B.; del Rio, J.S.; Moreno, M.; Blanco, F.J.; Mayora, K.; Dominguez, C.; Lechuga, L. Optical biosensor microsystems based on the integration of highly sensitive Mach–Zehnder interferometer devices. J. Opt. A Pure Appl. Opt. 2006, 8, S561–S566. [Google Scholar] [CrossRef]
- del Rio, J.S.; Carrascosa, L.G.; Blanco, F.J.; Moreno, M.; Berganzo, J.; Calle, A.; Domínguez, C.; Lechuga, L.M. Lab-on-a-chip platforms based on highly sensitive nanophotonic Si biosensors for single nucleotide DNA testing. Integr. Optoelectron. Devices 2007 2007, 6477, 64771B. [Google Scholar] [CrossRef]
- Ahmed, M.U.; Saaem, I.; Wu, P.C.; Brown, A.S. Personalized diagnostics and biosensors: A review of the biology and technology needed for personalized medicine. Crit. Rev. Biotechnol. 2014, 34, 180–196. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-T.; Lee, Y.-C.; Lai, Y.-H.; Lim, J.-C.; Huang, N.-T.; Lin, C.-T.; Huang, J.-J. Review of Integrated Optical Biosensors for Point-Of-Care Applications. Biosensors 2020, 10, 209. [Google Scholar] [CrossRef]
- Hercher, M.; Mueller, W.; Shapiro, H.M. Detection and discrimination of individual viruses by flow cytometry. J. Histochem. Cytochem. 1979, 27, 350–352. [Google Scholar] [CrossRef]
- Buteau, S.; Simard, J.-R.; Lahaie, P.; Roy, G.; Mathieu, P.; Déry, B.; Ho, J.; McFee, J. Bioaerosol Standoff Monitoring Using Intensified Range-Gated Laser-Induced Fluorescence Spectroscopy. Adv. Environ. Monit. 2008. [Google Scholar] [CrossRef]
- Ho, C.-S.; Jean, N.; Hogan, C.A.; Blackmon, L.; Jeffrey, S.S.; Holodniy, M.; Banaei, N.; Saleh, A.A.E.; Ermon, S.; Dionne, J. Rapid identification of pathogenic bacteria using Raman spectroscopy and deep learning. Nat. Commun. 2019, 10, 1–8. [Google Scholar] [CrossRef]
- Goyal, A.K.; Spencer, M.; Kelly, M.; Costa, J.; DiLiberto, M.; Meyer, E.; Jeys, T. Active infrared multispectral imaging of chemicals on surfaces. In Proceedings of the SPIE Defense, Security, and Sensing, Orlando, FL, USA, 25–29 April 2011; p. 80180. [Google Scholar]
- Kotidis, P.; Deutsch, E.R.; Goyal, A. Standoff detection of chemical and biological threats using miniature widely tunable QCLs. Micro Nanotechnol. Sens. Syst. Appl. 2015, 9467, 94672. [Google Scholar] [CrossRef]
- Deutsch, E.R.; Kotidis, P.; Zhu, N.; Goyal, A.K.; Ye, J.; Mazurenko, A.; Norman, M.; Zafiriou, K.; Baier, M.; Connors, R. Active and passive infrared spectroscopy for the detection of environmental threats. Adv. Environ. Chem. Biol. Sens. Technol. XI 2014, 9106, 91060A. [Google Scholar] [CrossRef]
- Kukushkin, V.; Ivanov, N.M.; Novoseltseva, A.A.; Gambaryan, A.S.; Yaminsky, I.V.; Kopylov, A.M.; Zavyalova, E.G. Highly sensitive detection of influenza virus with SERS aptasensor. PLoS ONE 2019, 14, e0216247. [Google Scholar] [CrossRef]
- Pilot, R. SERS detection of food contaminants by means of portable Raman instruments. J. Raman Spectrosc. 2018, 49, 954–981. [Google Scholar] [CrossRef]
- Minagawa, Y.; Ueno, H.; Tabata, K.V.; Noji, H. Mobile imaging platform for digital influenza virus counting. Lab Chip 2019, 19, 2678–2687. [Google Scholar] [CrossRef]
- Desai, S.; Mishra, S.V.; Joshi, A.; Sarkar, D.; Hole, A.; Mishra, R.; Dutt, S.; Chilakapati, M.K.; Gupta, S.; Dutt, A. Raman spectroscopy-based detection of RNA viruses in saliva: A preliminary report. J. Biophotonics 2020, 13, 1–5. [Google Scholar] [CrossRef]
- Tsen, K.T.; Tsen, S.-W.D.; Chang, C.-L.; Hung, C.-F.; Wu, T.-C.; Kiang, J.G. Inactivation of viruses by coherent excitations with a low power visible femtosecond laser. Virol. J. 2007, 4, 50. [Google Scholar] [CrossRef]
- Tsen, K.-T.; Tsen, S.-W.D.; Fu, Q.; Lindsay, S.M.; Kibler, K.; Jacobs, B.; Wu, T.C.; Karanam, B.; Jagu, S.; Roden, R.B.S.; et al. Photonic approach to the selective inactivation of viruses with a near-infrared subpicosecond fiber laser. J. Biomed. Opt. 2009, 14, 64042. [Google Scholar] [CrossRef] [PubMed]
- Seo, G.; Lee, G.; Kim, M.J.; Baek, S.-H.; Choi, M.; Ku, K.B.; Lee, C.-S.; Jun, S.; Park, D.; Kim, H.G.; et al. Rapid Detection of COVID-19 Causative Virus (SARS-CoV-2) in Human Nasopharyngeal Swab Specimens Using Field-Effect Transistor-Based Biosensor. ACS Nano 2020, 14, 5135–5142. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Yun, S.-H. The potential of optofluidic biolasers. Nat. Methods 2014, 11, 141–147. [Google Scholar] [CrossRef]
- Hales, J.E.; Matmon, G.; Dalby, P.A.; Ward, J.M.; Aeppli, G. Virus lasers for biological detection. Nat. Commun. 2019, 10, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Deckert, V.; Deckert-Gaudig, T.; Cialla-May, D.; Popp, J.; Zell, R.; Deinhard-Emmer, S.; Sokolov, A.V.; Yi, Z.; Scully, M.O. Laser spectroscopic technique for direct identification of a single virus I: FASTER CARS. Proc. Natl. Acad. Sci. USA 2020, 117, 27820–27824. [Google Scholar] [CrossRef] [PubMed]
- Němeček, D.; Thomas, G.J., Jr. Raman Spectroscopy in Virus Structure Analysis. In Digital Encyclopedia of Applied Physics; Wiley: Hoboken, NJ, USA, 2009. [Google Scholar]
- Fischer, E.P.; Fischer, M.C.; Grass, D.; Henrion, I.; Warren, W.S.; Westman, E. Low-cost measurement of face mask efficacy for filtering expelled droplets during speech. Sci. Adv. 2020, 6, eabd3083. [Google Scholar] [CrossRef] [PubMed]
- Tabish, T.A.; Narayan, R.J.; Edirisinghe, M. Rapid and label-free detection of COVID-19 using coherent anti-Stokes Raman scattering microscopy. MRS Commun. 2020, 10, 566–572. [Google Scholar] [CrossRef]
- Kakkar, T.; Keijzer, C.; Rodier, M.; Bukharova, T.; Taliansky, M.; Love, A.J.; Milner, J.J.; Karimullah, A.S.; Barron, L.D.; Gadegaard, N.; et al. Superchiral near fields detect virus structure. Light. Sci. Appl. 2020, 9, 1–10. [Google Scholar] [CrossRef]
- Al-Kinani, M.A.; Haider, A.J.; Al-Musawi, S. Design and Synthesis of Nanoencapsulation with a New Formulation ofFe @Au-CS-CU-FANPs by Pulsed Laser Ablation in Liquid (PLAL) Method in Breast Cancer Therapy: In Vitro and In Vivo. Plasmonics 2021. [Google Scholar] [CrossRef]
- Bajaj, S.; Dey, D.; Bhukar, R.; Kumar, M.; Banerjee, M. Non-Enveloped Virus Entry: Structural Determinants and Mechanism of Functioning of a Viral Lytic Peptide. J. Mol. Biol. 2016, 428, 3540–3556. [Google Scholar] [CrossRef]
- Katzir, A. Medical Lasers. Lasers Opt. Fibers Med. 1993, 26, 15–58. [Google Scholar] [CrossRef]
- Huber; Frost Light scattering by small particles. J. Water Supply Res. Technol. 2008, 47, 87–94. [CrossRef]
- Li, J.-F.; Li, C.-Y.; Aroca, R.F. Plasmon-enhanced fluorescence spectroscopy. Chem. Soc. Rev. 2017, 46, 3962–3979. [Google Scholar] [CrossRef] [PubMed]
- Fothergill, S.M.; Joyce, C.; Xie, F. Metal enhanced fluorescence biosensing: From ultra-violet towards second near-infrared window. Nanoscale 2018, 10, 20914–20929. [Google Scholar] [CrossRef] [PubMed]
- Resch-Genger, U.; Grabolle, M.; Cavaliere-Jaricot, S.; Nitschke, R.; Nann, T. Quantum dots versus organic dyes as fluorescent labels. Nat. Methods 2008, 5, 763–775. [Google Scholar] [CrossRef] [PubMed]
- Fang, X.; Li, H.-Y.; Fang, P.; Pan, J.-Z.; Fang, Q. A handheld laser-induced fluorescence detector for multiple applications. Talanta 2016, 150, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; He, S.; Wang, X.; Yan, Y.; Liu, J.; Wu, S.; Liu, S.; Lei, Y.; Chen, M.; Li, L.; et al. Rapid lateral flow immunoassay for the fluorescence detection of SARS-CoV-2 RNA. Nat. Biomed. Eng. 2020, 4, 1150–1158. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.C.; Chang, Y.-F.; Chen, K.-H.; Su, L.-C.; Lee, C.-W.; Chen, C.-C.; Chen, Y.-M.A.; Chou, C. Detection of severe acute respiratory syndrome (SARS) coronavirus nucleocapsid protein in human serum using a localized surface plasmon coupled fluorescence fiber-optic biosensor. Biosens. Bioelectron. 2009, 25, 320–325. [Google Scholar] [CrossRef]
- Owoicho, O.; Olwal, C.O.; Quaye, O. Potential of laser-induced fluorescence-light detection and ranging for future stand-off virus surveillance. Microb. Biotechnol. 2021, 14, 126–135. [Google Scholar] [CrossRef]
- Bantz, K.C.; Meyer, A.F.; Wittenberg, N.J.; Im, H.; Kurtuluş, Ö.; Lee, S.H.; Lindquist, N.C.; Oh, S.-H.; Haynes, C.L. Recent progress in SERS biosensing. Phys. Chem. Chem. Phys. 2011, 13, 11551–11567. [Google Scholar] [CrossRef]
- Luo, S.-C.; Sivashanmugan, K.; Liao, J.-D.; Yao, C.-K.; Peng, H.-C. Nanofabricated SERS-active substrates for single-molecule to virus detection in vitro: A review. Biosens. Bioelectron. 2014, 61, 232–240. [Google Scholar] [CrossRef]
- Jones, R.R.; Hooper, D.C.; Zhang, L.; Wolverson, D.; Valev, V.K. Raman Techniques: Fundamentals and Frontiers. Nanoscale Res. Lett. 2019, 14, 231. [Google Scholar] [CrossRef]
- Li, M.; Cushing, S.; Wu, N. Plasmon-enhanced optical sensors: A review. Analyst 2015, 140, 386–406. [Google Scholar] [CrossRef] [PubMed]
- Jadhav, S.A.; Biji, P.; Panthalingal, M.K.; Krishna, C.M.; Rajkumar, S.; Joshi, D.S.; Sundaram, N. Development of integrated microfluidic platform coupled with Surface-enhanced Raman Spectroscopy for diagnosis of COVID-19. Med. Hypotheses 2021, 146, 110356. [Google Scholar] [CrossRef]
- Yang, Y.; Peng, Y.; Lin, C.; Long, L.; Hu, J.; He, J.; Zeng, H.; Huang, Z.; Li, Z.-Y.; Tanemura, M.; et al. Human ACE2-Functionalized Gold “Virus-Trap” Nanostructures for Accurate Capture of SARS-CoV-2 and Single-Virus SERS Detection. Nano Micro Lett. 2021, 13, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Kretschmann, E.; Raether, H. Notizen: Radiative Decay of Non Radiative Surface Plasmons Excited by Light. Z. Nat. Sect. A J. Phys. Sci. 1968, 23, 2135–2136. [Google Scholar] [CrossRef]
- Mauriz, E.; Calle, A.; Lechuga, L.; Quintana, J.; Montoya, A.; Manclús, J. Real-time detection of chlorpyrifos at part per trillion levels in ground, surface and drinking water samples by a portable surface plasmon resonance immunosensor. Anal. Chim. Acta 2006, 561, 40–47. [Google Scholar] [CrossRef]
- Wang, S.; Shan, X.; Patel, U.; Huang, X.; Lu, J.; Li, J.; Tao, N. Label-free imaging, detection, and mass measurement of single viruses by surface plasmon resonance. Proc. Natl. Acad. Sci. USA 2010, 107, 16028–16032. [Google Scholar] [CrossRef] [PubMed]
- Prabowo, B.A.; Wang, R.Y.; Secario, M.K.; Ou, P.-T.; Alom, A.; Liu, J.-J.; Liu, K.-C. Rapid detection and quantification of Enterovirus 71 by a portable surface plasmon resonance biosensor. Biosens. Bioelectron. 2017, 92, 186–191. [Google Scholar] [CrossRef]
- Piliarik, M.; Homola, J. Surface plasmon resonance (SPR) sensors: Approaching their limits? Opt. Express 2009, 17, 16505–16517. [Google Scholar] [CrossRef]
- Yesudasu, V.; Pradhan, H.S.; Pandya, R.J. Recent progress in surface plasmon resonance based sensors: A comprehensive review. Heliyon 2021, 7, e06321. [Google Scholar] [CrossRef]
- Mauriz, E.; Lechuga, L. Plasmonic Biosensors for Single-Molecule Biomedical Analysis. Biosensors 2021, 11, 123. [Google Scholar] [CrossRef]
- Roh, C.; Jo, S.K. Quantitative and sensitive detection of SARS coronavirus nucleocapsid protein using quantum dots-conjugated RNA aptamer on chip. J. Chem. Technol. Biotechnol. 2011, 86, 1475–1479. [Google Scholar] [CrossRef]
- Chen, H.; Gill, A.; Dove, B.K.; Emmett, S.R.; Kemp, C.F.; Ritchie, M.A.; Dee, M.; Hiscox, J.A. Mass Spectroscopic Characterization of the Coronavirus Infectious Bronchitis Virus Nucleoprotein and Elucidation of the Role of Phosphorylation in RNA Binding by Using Surface Plasmon Resonance. J. Virol. 2005, 79, 1164–1179. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Yi, Y.; Luo, X.; Xiong, N.; Liu, Y.; Li, S.; Sun, R.; Wang, Y.; Hu, B.; Chen, W.; et al. Development and clinical application of a rapid IgM-IgG combined antibody test for SARS-CoV-2 infection diagnosis. J. Med. Virol. 2020, 92, 1518–1524. [Google Scholar] [CrossRef]
- Moitra, P.; Alafeef, M.; Dighe, K.; Frieman, M.B.; Pan, D. Selective Naked-Eye Detection of SARS-CoV-2 Mediated by N Gene Targeted Antisense Oligonucleotide Capped Plasmonic Nanoparticles. ACS Nano 2020, 14, 7617–7627. [Google Scholar] [CrossRef] [PubMed]
- Tymm, C.; Zhou, J.; Tadimety, A.; Burklund, A.; Zhang, J.X.J. Scalable COVID-19 Detection Enabled by Lab-on-Chip Biosensors. Cell. Mol. Bioeng. 2020, 13, 313–329. [Google Scholar] [CrossRef] [PubMed]
- Qiu, G.; Gai, Z.; Tao, Y.; Schmitt, J.; Kullak-Ublick, G.A.; Wang, J. Dual-Functional Plasmonic Photothermal Biosensors for Highly Accurate Severe Acute Respiratory Syndrome Coronavirus 2 Detection. ACS Nano 2020, 14, 5268–5277. [Google Scholar] [CrossRef] [PubMed]
- Murugan, D.; Bhatia, H.; Sai, V.V.R.; Satija, J. P-FAB: A Fiber-Optic Biosensor Device for Rapid Detection of COVID-19. Trans. Indian Natl. Acad. Eng. 2020, 5, 211–215. [Google Scholar] [CrossRef]
- Nag, P.; Sadani, K.; Mukherji, S. Optical Fiber Sensors for Rapid Screening of COVID-19. Trans. Indian Natl. Acad. Eng. 2020, 5, 233–236. [Google Scholar] [CrossRef]
- Kim, H.; Hwang, J.; Kim, J.H.; Lee, S.; Kang, M. Sensitive Detection of Multiple Fluoresence Probes based on Surface-enhanced Raman Scattering (SERS) for MERS-CoV. In Proceedings of the 2019 IEEE 14th International Conference on Nano/Micro Engineered and Molecular Systems (NEMS), Bangkok, Thailand, 11–14 April 2019; pp. 498–501. [Google Scholar]
- Kim, E.J.; Kim, H.; Park, E.; Kim, T.; Chung, D.R.; Choi, Y.-M.; Kang, M. Paper-Based Multiplex Surface-Enhanced Raman Scattering Detection Using Polymerase Chain Reaction Probe Codification. Anal. Chem. 2021, 93, 3677–3685. [Google Scholar] [CrossRef]
- Sitjar, J.; Liao, J.-D.; Lee, H.; Tsai, H.-P.; Wang, J.-R.; Liu, P.-Y. Challenges of SERS technology as a non-nucleic acid or -antigen detection method for SARS-CoV-2 virus and its variants. Biosens. Bioelectron. 2021, 181, 113153. [Google Scholar] [CrossRef]
- Poggio, P.; Songia, P.; Vavassori, C.; Ricci, V.; Banfi, C.; Barbieri, S.S.; Garoffolo, G.; Myasoedova, V.A.; Piacentini, L.; Raucci, A.; et al. Digital PCR for high sensitivity viral detection in false-negative SARS-CoV-2 patients. Sci. Rep. 2021, 11, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Bohk-Ewald, C.; Dudel, C.; Myrskylä, M. A demographic scaling model for estimating the total number of COVID-19 infections. Int. J. Epidemiol. 2021, 49, 1963–1971. [Google Scholar] [CrossRef] [PubMed]
- Samson, R.; Navale, G.R.; Dharne, M.S. Biosensors: Frontiers in rapid detection of COVID-19. 3 Biotech 2020, 10, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Wölfel, R.; Corman, V.M.; Guggemos, W.; Seilmaier, M.; Zange, S.; Müller, M.A.; Niemeyer, D.; Jones, T.C.; Vollmar, P.; Rothe, C.; et al. Virological assessment of hospitalized patients with COVID-2019. Nat. Cell Biol. 2020, 581, 465–469. [Google Scholar] [CrossRef]
- Zou, L.; Ruan, F.; Huang, M.; Liang, L.; Huang, H.; Hong, Z.; Yu, J.; Kang, M.; Song, Y.; Xia, J.; et al. SARS-CoV-2 Viral Load in Upper Respiratory Specimens of Infected Patients. N. Engl. J. Med. 2020, 382, 1177–1179. [Google Scholar] [CrossRef]
- Iv, G.C.B.; Chen, Y.Q.; Martinez, E.; Tang, V.A.; Renner, T.M.; Langlois, M.-A.; Gulnik, S. A Novel Semiconductor-Based Flow Cytometer with Enhanced Light-Scatter Sensitivity for the Analysis of Biological Nanoparticles. Sci. Rep. 2019, 9, 1–13. [Google Scholar] [CrossRef]
- Samarrai, R.; Riccardi, A.C.; Tessema, B.; Setzen, M.; Brown, S.M. Continuation of telemedicine in otolaryngology post-COVID-19: Applications by subspecialty. Am. J. Otolaryngol. 2021, 42, 102928. [Google Scholar] [CrossRef]
- Yeh, Y.-T.; Gulino, K.; Zhang, Y.; Sabestien, A.; Chou, T.-W.; Zhou, B.; Lin, Z.; Albert, I.; Lu, H.; Swaminathan, V.; et al. A rapid and label-free platform for virus capture and identification from clinical samples. Proc. Natl. Acad. Sci. USA 2020, 117, 895–901. [Google Scholar] [CrossRef] [PubMed]
- Serag, E.; El-Zeftawy, M. Environmental aspect and applications of nanotechnology to eliminate COVID-19 epidemiology risk. Nanotechnol. Environ. Eng. 2021, 6, 1–17. [Google Scholar] [CrossRef]
- Jones, P.R.; Rioux, M. Three-dimensional surface anthropometry: Applications to the human body. Opt. Lasers Eng. 1997, 28, 89–117. [Google Scholar] [CrossRef]
- Tokkari, N.; Verdaasdonk, R.; Liberton, N.; Wolff, J.; Heijer, M.D.; Van Der Veen, A.; Klaessens, J.H. Comparison and use of 3D scanners to improve the quantification of medical images (surface structures and volumes) during follow up of clinical (surgical) procedures. Adv. Biomed. Clin. Diagn. Surg. Guid. Syst. XV 2017, 10054, 100540. [Google Scholar] [CrossRef]
- Haleem, A.; Javaid, M. 3D scanning applications in medical field: A literature-based review. Clin. Epidemiol. Glob. Heal 2019, 7, 199–210. [Google Scholar] [CrossRef]

| Parameters of Laser Device | Description |
|---|---|
| Wavelength (λ) | The intensity of the scattered light reduces as the wavelength increases (inversely proportional). This outcome depends on the particle size, and for small particles, it is more pronounced. |
| Particle size (d) | Scattered light strength is highly dependent on the size of the particles. |
| Refractive index (n) | The scattering intensity is directly proportional to the difference in the particle’s refractive index and the medium. The smaller the difference, the lower the scattering intensity. |
| Scattering angle (θ) | The scattering intensity depends on the light angle of the incident. |
| Concentration (c) | The intensity of the scattered light is proportional to the size of the concentration. |
| Photonics Technique | Target of Virus | Material Coating | Limit of Detection | Wavelength | Diagnosis of COVID-19 | Time Duration | Ref. | |
|---|---|---|---|---|---|---|---|---|
| In Clinical | On Surfaces | |||||||
| LSPCF | SARS/nucleocapsid protein | Graphene sheet | 0.1 pg/mL | 658 nm | ✓ | ✕ | 10 min | [60] |
| Fluorescence | COVID-19 RNA | Gold | 1000 TU mL−1 | NA | ✓ | ✕ | 2–3 h | [59] |
| LIF-LiDAR | COVID-19, Zika, Ebola | NA | 9.59 × 104 PFU/cm2 | 266–550 nm | ✓ | ✕ | NA | [61] |
| SERS microfluid | COVID-19 | Au/Ag | NA | NA | ✓ | ✕ | ~few min | [66] |
| SERS | COVID-19 | Gold nanoparticles | 17.7 pM | 785 nm | ✓ | ✕ | 5 min | [67] |
| SPR | SARS/ N-protein | Quantum dots | 0.1 pg mL−1 | 345 nm | ✓ | ✕ | 1 h | [75] |
| SPR | Coronavirus/ N-protein | NA | 2.17 nM | 214 nm | ✓ | ✕ | 20 min | [76] |
| LSPR | COVID-19/ spike protein | AuNIs | 0.22 ± 0.08 pM | 532 nm | ✓ | ✕ | 800 s | [80] |
| P-FAB | COVID-19/ antibody IgM and IgG | AuNP | 106 particles/mL | 520–545 nm | ✓ | ✕ | 15 min | [81] |
| EWA-LSPR | COVID-19/ antibody IgM and IgG | Gold nanoparticle | 37 pM | LED | ✓ | ✕ | 1 h | [82] |
| SERS-LSPR | MERS | Silver nanodot | 1–106 nM | 500 to 800 nm | ✓ | ✕ | NA | [83] |
| SERS-LSPR | COVID-19 | Silver nanodot | 153.53, 230.37 pM | 526 nm 558 nm | ✓ | ✕ | More 2 h | [84] |
| Laser Diagnosis Techniques | Limitations |
|---|---|
| Fluorescence method |
|
| Surface-enhanced Raman scattering |
|
| Surface plasmon resonance |
|
| Raman scattering with SPR integrated |
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Taha, B.A.; Al Mashhadany, Y.; Bachok, N.N.; Ashrif A Bakar, A.; Hafiz Mokhtar, M.H.; Dzulkefly Bin Zan, M.S.; Arsad, N. Detection of COVID-19 Virus on Surfaces Using Photonics: Challenges and Perspectives. Diagnostics 2021, 11, 1119. https://doi.org/10.3390/diagnostics11061119
Taha BA, Al Mashhadany Y, Bachok NN, Ashrif A Bakar A, Hafiz Mokhtar MH, Dzulkefly Bin Zan MS, Arsad N. Detection of COVID-19 Virus on Surfaces Using Photonics: Challenges and Perspectives. Diagnostics. 2021; 11(6):1119. https://doi.org/10.3390/diagnostics11061119
Chicago/Turabian StyleTaha, Bakr Ahmed, Yousif Al Mashhadany, Nur Nadia Bachok, Ahmad Ashrif A Bakar, Mohd Hadri Hafiz Mokhtar, Mohd Saiful Dzulkefly Bin Zan, and Norhana Arsad. 2021. "Detection of COVID-19 Virus on Surfaces Using Photonics: Challenges and Perspectives" Diagnostics 11, no. 6: 1119. https://doi.org/10.3390/diagnostics11061119
APA StyleTaha, B. A., Al Mashhadany, Y., Bachok, N. N., Ashrif A Bakar, A., Hafiz Mokhtar, M. H., Dzulkefly Bin Zan, M. S., & Arsad, N. (2021). Detection of COVID-19 Virus on Surfaces Using Photonics: Challenges and Perspectives. Diagnostics, 11(6), 1119. https://doi.org/10.3390/diagnostics11061119









