Health-Related Quality of Life (HRQoL) in Sarcoidosis: Diagnosis, Management, and Health Outcomes
Abstract
1. Introduction
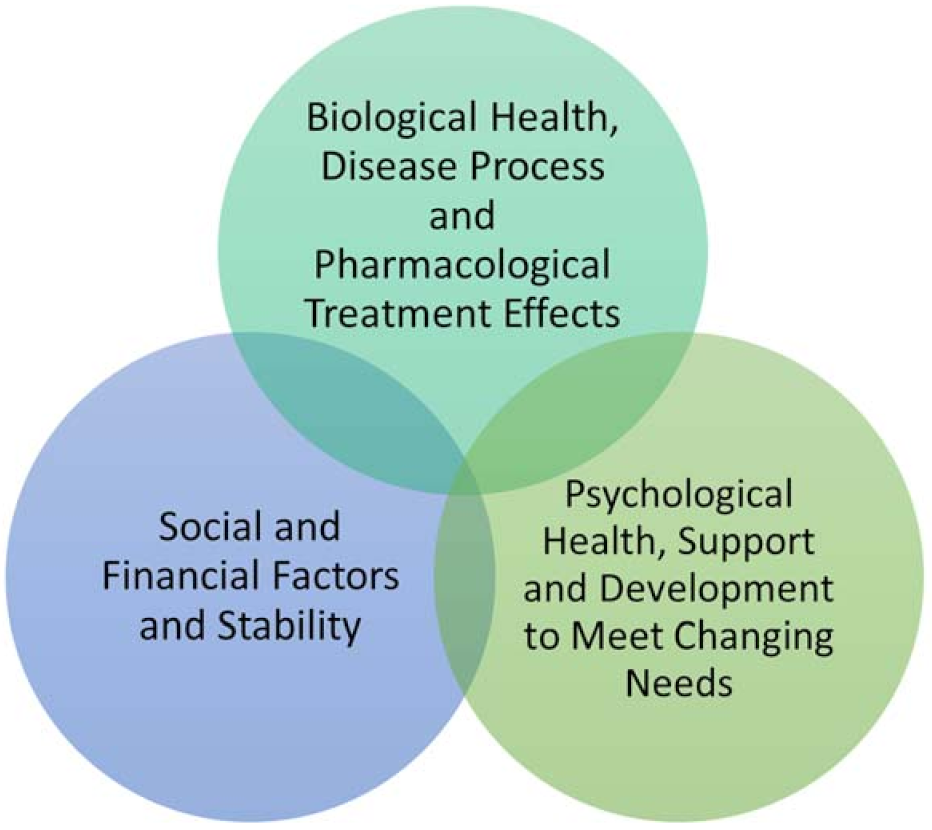
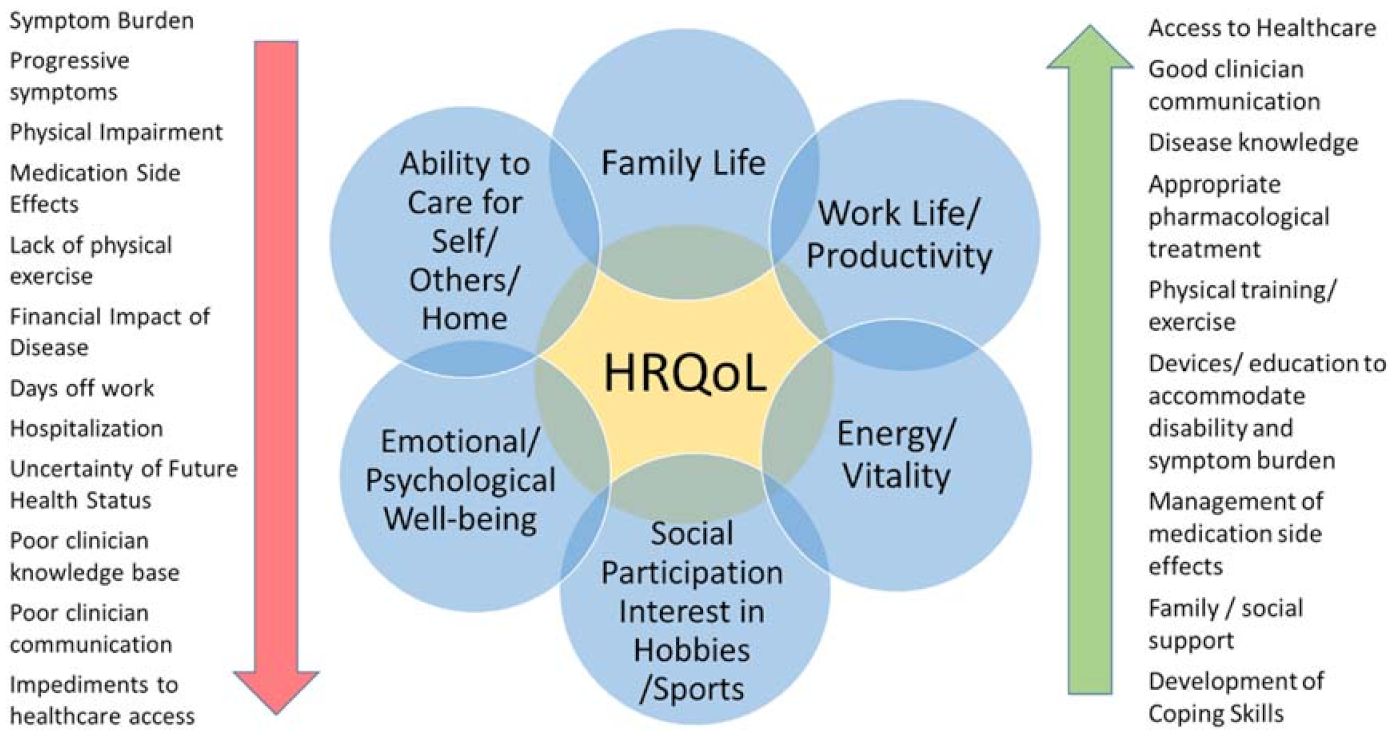
2. Health-Related Quality of Life (HRQoL)
- Personal factors include a patient’s intrinsic potential for adaptability and coping behavior, length of time living with a condition, increasing familiarity with self-management strategies for symptoms and impairment;
- Environmental factors include the extent of family support, financial resources, as well as assistive aids, devices, or techniques that improve physical, mental, or emotional function. This includes access to care and expressly, in the case of sarcoidosis, access to clinicians with sufficient knowledge of sarcoidosis [8];
- Symptom burden includes disease-related symptoms and impairment, medication side effects [11], and the psychological impact of living with the health condition.
2.1. Symptom and Impairment Burden
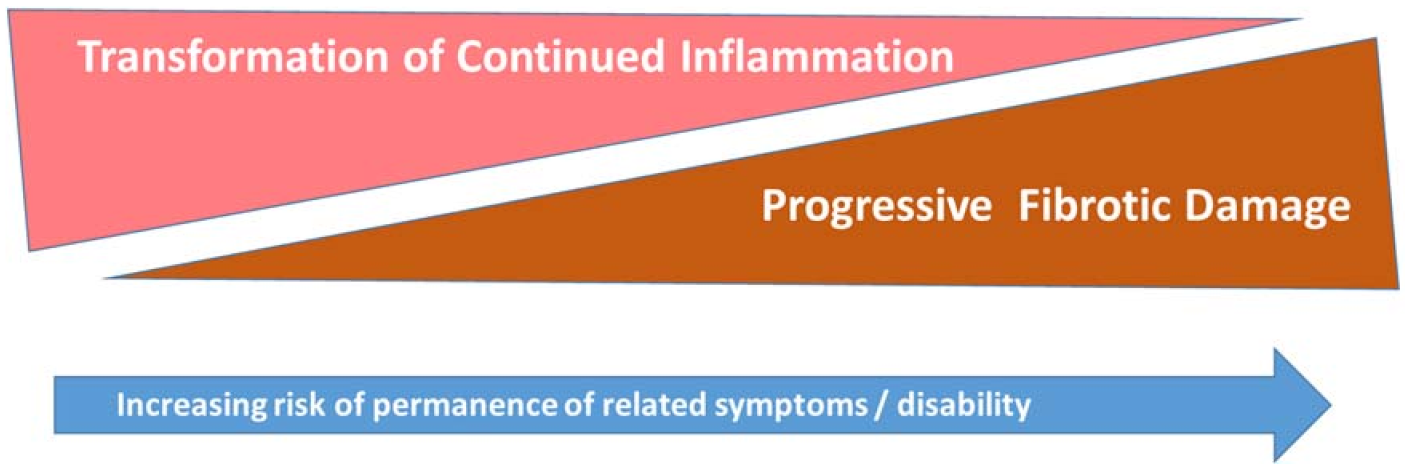
- Sarcoidosis disease activity clinically suggests granulomatous inflammation impacting health status and, therefore, impairment is potentially reversible with quiescence of disease activity, either through pharmacological treatment or disease self-remission.
- Sarcoidosis damage is the damage and scarring left in the wake of prior destructive sarcoidosis inflammation and granulomatous activity; this tissue damage is not irreversible.
- A combination or an overlap of active disease and irreversible damage; this chronic progressive phenotype suggests reversibility of impairment depends on extent of damage versus treatable inflammatory disease.
- Pre-existing and co-existing comorbidity can confound symptom interpretations and always deserve differential diagnostic attention, e.g., pre-existing hyperthyroidism with presentation with tachycardia in otherwise controlled sarcoidosis, or recent lymphoma mimicking sarcoidosis-like symptoms, even with concomitant granulomatous involvement.
- Non-sarcoidosis related, e.g., coronary artery involvement, etc.
2.2. Participation
2.2.1. Work Life
2.2.2. Family
2.2.3. Social Life
3. Patient-Centeredness
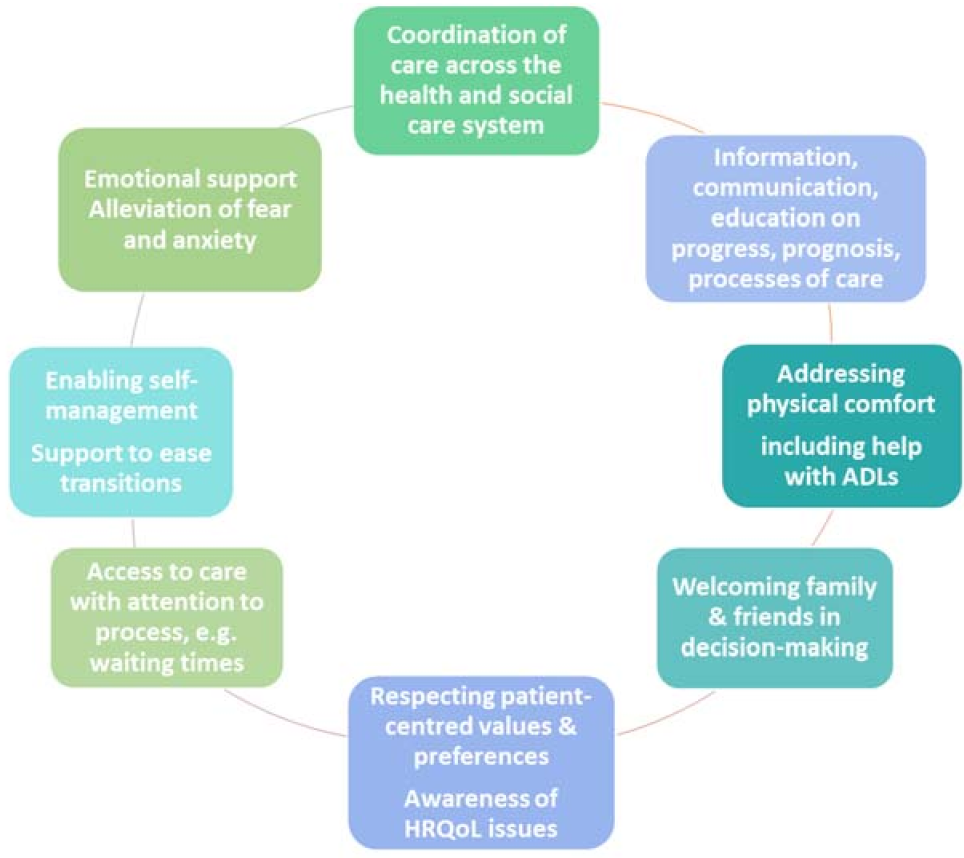
- Respect for patients’ values, preferences, and expressed needs;
- Coordination and integration of care;
- Information, communication, and education;
- Physical comfort;
- Emotional support and alleviation of fear and anxiety;
- Involvement of family and friends;
- Continuity and transition;
- Access to care.
3.1. Patient-Centered Environments
3.2. Communication
3.2.1. Trauma-Informed Patient Communication
- Safety;
- Trustworthiness and transparency;
- Peer support;
- Collaboration and mutuality;
- Empowerment and choice;
- Cultural, historical, and gender considerations.
3.2.2. Shared Decision-Making
+ risk of serious organ dysfunction versus low or no progression or chance of remission
+ disease monitoring versus active treatment
+ anticipated benefit of treatment versus risk of toxicity
= preliminary decision
- Cutaneous sarcoidosis, as an example, could lead to a provider-patient mismatch in terms of disease impact and treatment. In a case of non-facial cutaneous involvement with no other organ involvement necessitating treatment, discussion allows the patient to convey the presence and/or burden of physical symptoms (e.g., pruritus, burning, etc.), and also any psychological impact these symptoms and the cosmetic appearance might have. After the clinician explains treatment options with their benefit vs risk implications (e.g., hydroxychloroquine, topical steroids, etc.), if all symptom factors are none to minimal, the patient may decide to delay treatment in favor of observation; while the patient may opt for treatment if the cosmetic appearance or physical symptom burden is noticeable to them, thus outweighing medication-related side effects or costs.
- In a case of lupus pernio, aggressive treatment is often necessary to achieve adequate disease control (e.g., use of biologic therapy). As patients with this phenotype are often quite impacted by the disease, and typically have extra-cutaneous disease requiring treatment, generally the patient and clinician are aligned in the necessity of treating active disease. However, once active disease has been treated, the clinician may see a lack of active granulomatous inflammation as a satisfactory end result, whereas residual damage in the form of dyspigmentation and scarring may be quite troubling to patients cosmetically and psychosocially. Without discussion, the clinician may fail to recognize that the scarring is causing the patient significant distress (e.g., depressive symptoms, self-esteem). With discussion, the clinician learns of the patient’s distress, and is now in a position to provide support via communicating recognition of the patient’s distress, psychological support, and considering possible cosmetic interventions (Box 1).
3.3. Family as an Extension of the Patient
3.4. Patient Advocacy Organizations
4. Patient-Centered and HRQoL Instruments
- Patient-reported outcome measures (PROMs) that capture changes in symptoms, health status perception, or HRQoL;
- Patient-reported experience measures (PREMs) that identify patient-experienced strengths and weakness of healthcare delivery systems in order to improve the system and thereby, patient experience and HRQoL;
- Patient engagement (or activation) measures (PEMs/PAMs) that assess areas such as disease or medication knowledge, health systems familiarity, or lifestyle awareness, so that patients might receive education or counseling to strengthen self-management skills, and thereby enhance HRQoL;
- Patient preference measures supporting patient decision-making or provide data for value-based health economic choices;
- Clinical checklists that enhance patient outcomes, through decreasing complications and supporting patient-centered care, all of which increase HRQoL.
4.1. Overview of Assessments in Sarcoidosis
4.2. Patient-Centered Checklists
4.3. Operationalizing Instruments
- (a)
- As a detection tool to disclose the need for medical or other intervention in regards to patient health or environment, e.g., depression screening, severity scores, symptom scales, clinician checklists.
- (b)
- As a tracking tool of symptoms, to mark improvement in patient-designated priorities [77], and other patient-reported measures to be trended over time alongside other scores, e.g., to gauge efficacy of treatment, traditional markers, and historical patient events, e.g., hospitalizations, exacerbations, antibiotic/steroid use; thus, enabling patients and clinicians to identify trends leading up to and to prevent recurrent complications [34,83,84].
- (c)
- As a patient–clinician discussion tool, whereby results provide opportunities to initiate discussions on potential reasons for score changes by the patient followed by the clinician who can then offer additional perspective or clarify any misunderstandings. Further, the psychological, emotional, physical, and intellectual exhaustion and burnout incurred by dedicated clinicians [79,80,81,82], can be offset, in addition to checklists, by the framework that PROMs and other tools provide to support difficult discussions regarding milestone health changes, e.g., need for lung transplantation assessment.
5. Common Causes of Symptom Burden in Sarcoidosis
5.1. Psychological Distress
5.2. Cognition
5.3. Pain
5.4. Fatigue
5.4.1. Physical Fatigue
5.4.2. Other Types and Causes of Fatigue
5.4.3. Assessment and Management Considerations of Fatigue
5.5. Issues of Sleep Quality in Sarcoidosis
5.6. Cardio-Respiratory Symptoms: Breathlessness and Cough
5.7. Exercise Intolerance and Muscle Impairment
6. Medication-Related and Complication-Related HRQoL
6.1. Medication-Related HRQoL
6.1.1. Adverse Outcomes
6.1.2. Enhancing Treatment Tolerability
6.2. Complication-Related HRQoL
- Prescribe sufficient medication, only up until the time for the next toxicity screening test to avoid prolonged use of medication without toxicity check.
- Monitoring and logging prednisone and NSAID dosages and duration to support proactive tapering or transitioning to more sustainable medication options.
- Counseling patients on vitamin D regulation in sarcoidosis to prevent hypervitaminosis.
- Mitigate the risk of serious infection by:
- ○
- Ensuring vaccinations are up to date;
- ○
- Considering antibiotic prophylaxis;
- ○
- Counseling on the best practices, in terms of prevention, e.g., handwashing, masking, etc.
- Counseling on medication-related red flags for complications and preventive measures for each medication.
- Counseling on sunscreen use and sun exposure with certain DMARDs and biologic use [152].
- Supporting exercise as medicine and a stress reduction strategy are expanded upon below.
7. HRQoL Self-Management Strategies for Patients and Family Members
7.1. Stress Reduction to Enhance HRQoL
7.2. Exercise and Physical Activity to Enhance HRQoL
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Baughman, R.P.; Barriuso, R.; Beyer, K.; Boyd, J.; Hochreiter, J.; Knoet, C.; Martone, F.; Quadder, B.; Richardson, J.; Spitzer, G.; et al. Sarcoidosis: Patient Treatment Priorities. ERJ Open Res. 2018, 4, 00141–02018. [Google Scholar] [CrossRef] [PubMed]
- Drent, M.; Lower, E.E.; De Vries, J. Sarcoidosis-Associated Fatigue. Eur. Respir. J. 2012, 40, 255–263. [Google Scholar] [CrossRef]
- Saketkoo, L.A.; Mittoo, S.; Huscher, D.; Khanna, D.; Dellaripa, P.F.; Distler, O.; Flaherty, K.R.; Frankel, S.; Oddis, C.V.; Denton, C.P.; et al. Connective Tissue Disease Related Interstitial Lung Diseases and Idiopathic Pulmonary Fibrosis: Provisional Core Sets of Domains and Instruments for Use in Clinical Trials. Thorax 2014, 69, 436–444. [Google Scholar] [CrossRef]
- Drent, M.; Strookappe, B.; Hoitsma, E.; De Vries, J. Consequences of Sarcoidosis. Clin. Chest Med. 2015, 36, 727–737. [Google Scholar] [CrossRef]
- De Vries, J.; Lower, E.; Drent, M. Quality of Life in Sarcoidosis: Assessment and Management. Semin. Respir. Crit. Care Med. 2010, 31, 485–493. [Google Scholar] [CrossRef]
- Voortman, M.; Hendriks, C.M.R.; Lodder, P.; Drent, M.; De Vries, J. Quality of Life of Couples Living with Sarcoidosis. Respiration 2019, 98, 373–382. [Google Scholar] [CrossRef] [PubMed]
- Borrell-Carrió, F.; Suchman, A.L.; Epstein, R.M. The Biopsychosocial Model 25 Years Later: Principles, Practice, and Scientific Inquiry. Ann. Fam. Med. 2004, 2, 576–582. [Google Scholar] [CrossRef]
- Saketkoo, L.A.; Jensen, K.; Nikoletou, D.; Newton, J.J.; Rivera, F.J.; Howie, M.; Reese, R.K.; Goodman, M.; Hart, P.B.; Bembry, W.; et al. Sarcoidosis Illuminations on Living During COVID-19: Patient Experiences of Diagnosis, Management and Survival Before and During the Pandemic. 2021; under review. [Google Scholar]
- van Helmondt, S.J.; Polish, L.B.; Judson, M.A.; Grutters, J.C. Patient Perspectives in Sarcoidosis. Curr. Opin. Pulm. Med. 2019, 25, 478–483. [Google Scholar] [CrossRef]
- Saketkoo, L.A.; Escorpizo, R.; Keen, K.J.; Fligelstone, K.; Distler, O.; EUSTAR. International Classification of Functioning, Disability and Health Core Set Construction in Systemic Sclerosis and Other Rheumatic Diseases: A EUSTAR Initiative. Rheumatology 2012, 51, 2170–2176. [Google Scholar] [CrossRef]
- Judson, M.A.; Chaudhry, H.; Louis, A.; Lee, K.; Yucel, R. The Effect of Corticosteroids on Quality of Life in a Sarcoidosis Clinic: The Results of a Propensity Analysis. Respir. Med. 2015, 109, 526–531. [Google Scholar] [CrossRef] [PubMed]
- Blok, I.M.; van Riel, A.C.M.J.; Schuuring, M.J.; Duffels, M.G.; Vis, J.C.; van Dijk, A.P.J.; Hoendermis, E.S.; Mulder, B.J.M.; Bouma, B.J. Decrease in Quality of Life Predicts Mortality in Adult Patients with Pulmonary Arterial Hypertension Due to Congenital Heart Disease. Neth. Heart J. 2015, 23, 278–284. [Google Scholar] [CrossRef]
- Jaeger, V.K.; Distler, O.; Maurer, B.; Czirják, L.; Lóránd, V.; Valentini, G.; Vettori, S.; Del Galdo, F.; Abignano, G.; Denton, C.; et al. Functional Disability and Its Predictors in Systemic Sclerosis: A Study from the DeSScipher Project within the EUSTAR Group. Rheumatol. Oxf. Engl. 2018, 57, 441–450. [Google Scholar] [CrossRef]
- Liang, J.W.; Cheung, Y.K.; Willey, J.Z.; Moon, Y.P.; Sacco, R.L.; Elkind, M.S.V.; Dhamoon, M.S. Quality of Life Independently Predicts Long-Term Mortality but Not Vascular Events: The Northern Manhattan Study. Qual. Life Res. Int. J. Qual. Life Asp. Treat. Care Rehabil. 2017, 26, 2219–2228. [Google Scholar] [CrossRef]
- Kanwal, F.; Gralnek, I.M.; Hays, R.D.; Zeringue, A.; Durazo, F.; Han, S.B.; Saab, S.; Bolus, R.; Spiegel, B.M.R. Health-Related Quality of Life Predicts Mortality in Patients with Advanced Chronic Liver Disease. Clin. Gastroenterol. Hepatol. Off. Clin. Pract. J. Am. Gastroenterol. Assoc. 2009, 7, 793–799. [Google Scholar] [CrossRef]
- Berg, S.K.; Rasmussen, T.B.; Mols, R.E.; Thorup, C.B.; Borregaard, B.; Christensen, A.V.; Cromhout, P.F.; Ekholm, O.; Juel, K.; Thrysoee, L. Both Mental and Physical Health Predicts One Year Mortality and Readmissions in Patients with Implantable Cardioverter Defibrillators: Findings from the National DenHeart Study. Eur. J. Cardiovasc. Nurs. 2019, 18, 96–105. [Google Scholar] [CrossRef]
- Baekelandt, B.M.G.; Hjermstad, M.J.; Nordby, T.; Fagerland, M.W.; Kure, E.H.; Heiberg, T.; Buanes, T.; Labori, K.J. Preoperative Cognitive Function Predicts Survival in Patients with Resectable Pancreatic Ductal Adenocarcinoma. HPB 2016, 18, 247–254. [Google Scholar] [CrossRef]
- Irwin, K.E.; Greer, J.A.; Khatib, J.; Temel, J.S.; Pirl, W.F. Early Palliative Care and Metastatic Non-Small Cell Lung Cancer: Potential Mechanisms of Prolonged Survival. Chron. Respir. Dis. 2013, 10, 35–47. [Google Scholar] [CrossRef] [PubMed]
- Giese-Davis, J.; Collie, K.; Rancourt, K.M.S.; Neri, E.; Kraemer, H.C.; Spiegel, D. Decrease in Depression Symptoms Is Associated with Longer Survival in Patients with Metastatic Breast Cancer: A Secondary Analysis. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2011, 29, 413–420. [Google Scholar] [CrossRef]
- Bakitas, M.; Lyons, K.D.; Hegel, M.T.; Balan, S.; Brokaw, F.C.; Seville, J.; Hull, J.G.; Li, Z.; Tosteson, T.D.; Byock, I.R.; et al. Effects of a Palliative Care Intervention on Clinical Outcomes in Patients With Advanced Cancer: The Project ENABLE II Randomized Controlled Trial. JAMA 2009, 302, 741. [Google Scholar] [CrossRef]
- Ratjen, I.; Schafmayer, C.; Enderle, J.; di Giuseppe, R.; Waniek, S.; Koch, M.; Burmeister, G.; Nöthlings, U.; Hampe, J.; Schlesinger, S.; et al. Health-Related Quality of Life in Long-Term Survivors of Colorectal Cancer and Its Association with All-Cause Mortality: A German Cohort Study. BMC Cancer 2018, 18, 1156. [Google Scholar] [CrossRef] [PubMed]
- Michielsen, H.J.; Peros-Golubicic, T.; Drent, M.; De Vries, J. Relationship between Symptoms and Quality of Life in a Sarcoidosis Population. Respiration 2007, 74, 401–405. [Google Scholar] [CrossRef]
- Voortman, M.; Hendriks, C.M.R.; Elfferich, M.D.P.; Bonella, F.; Møller, J.; De Vries, J.; Costabel, U.; Drent, M. The Burden of Sarcoidosis Symptoms from a Patient Perspective. Lung 2019, 197, 155–161. [Google Scholar] [CrossRef]
- Drent, M.; Proesmans, V.L.J.; Elfferich, M.D.P.; Jessurun, N.T.; de Jong, S.M.G.; Ebner, N.M.; Lewis, E.D.O.; Bast, A. Ranking Self-Reported Gastrointestinal Side Effects of Pharmacotherapy in Sarcoidosis. Lung 2020, 198, 395–403. [Google Scholar] [CrossRef] [PubMed]
- Hendriks, C.M.R.; Saketkoo, L.A.; Elfferich, M.D.P.; De Vries, J.; Wijnen, P.A.H.M.; Drent, M. Sarcoidosis and Work Participation: The Need to Develop a Disease-Specific Core Set for Assessment of Work Ability. Lung 2019, 197, 407–413. [Google Scholar] [CrossRef]
- Arkema, E.V.; Eklund, A.; Grunewald, J.; Bruze, G. Work Ability before and after Sarcoidosis Diagnosis in Sweden. Respir. Med. 2018, 144S, S7–S12. [Google Scholar] [CrossRef]
- Gerke, A.K.; Judson, M.A.; Cozier, Y.C.; Culver, D.A.; Koth, L.L. Disease Burden and Variability in Sarcoidosis. Ann. Am. Thorac. Soc. 2017, 14 (Suppl. S6), S421–S428. [Google Scholar] [CrossRef] [PubMed]
- Rice, J.B.; White, A.; Lopez, A.; Conway, A.; Wagh, A.; Nelson, W.W.; Philbin, M.; Wan, G.J. Economic Burden of Sarcoidosis in a Commercially-Insured Population in the United States. J. Med. Econ. 2017, 20, 1048–1055. [Google Scholar] [CrossRef]
- Kawalec, P.P.; Malinowski, K.P. The Indirect Costs of Systemic Autoimmune Diseases, Systemic Lupus Erythematosus, Systemic Sclerosis and Sarcoidosis: A Summary of 2012 Real-Life Data from the Social Insurance Institution in Poland. Expert Rev. Pharmacoecon. Outcomes Res. 2015, 15, 667–673. [Google Scholar] [CrossRef]
- Sharp, M.; Eakin, M.N.; Drent, M. Socioeconomic Determinants and Disparities in Sarcoidosis. Curr. Opin. Pulm. Med. 2020, 26, 568–573. [Google Scholar] [CrossRef] [PubMed]
- Hena, K.M. Sarcoidosis Epidemiology: Race Matters. Front. Immunol. 2020, 11, 537382. [Google Scholar] [CrossRef] [PubMed]
- Harper, L.J.; Gerke, A.K.; Wang, X.-F.; Ribeiro Neto, M.L.; Baughman, R.P.; Beyer, K.; Drent, M.; Judson, M.A.; Maier, L.A.; Serchuck, L.; et al. Income and Other Contributors to Poor Outcomes in U.S. Patients with Sarcoidosis. Am. J. Respir. Crit. Care Med. 2020, 201, 955–964. [Google Scholar] [CrossRef] [PubMed]
- Markham, S.E.; Markham, I.S. Biometeorological Effects on Worker Absenteeism. Int. J. Biometeorol. 2005, 49, 317–324. [Google Scholar] [CrossRef] [PubMed]
- Russell, A.-M.; Adamali, H.; Molyneaux, P.L.; Lukey, P.T.; Marshall, R.P.; Renzoni, E.A.; Wells, A.U.; Maher, T.M. Daily Home Spirometry: An Effective Tool for Detecting Progression in Idiopathic Pulmonary Fibrosis. Am. J. Respir. Crit. Care Med. 2016, 194, 989–997. [Google Scholar] [CrossRef]
- Tuckson, R.V.; Edmunds, M.; Hodgkins, M.L. Telehealth. N. Engl. J. Med. 2017, 377, 1585–1592. [Google Scholar] [CrossRef]
- Hassanin, A.M.; Ismail, N.N.; El Guindi, A.; Sowailam, H.A. The Emotional Burden of Chronic Skin Disease Dominates Physical Factors among Women, Adversely Affecting Quality of Life and Sexual Function. J. Psychosom. Res. 2018, 115, 53–57. [Google Scholar] [CrossRef]
- Saketkoo, L.A.; Mittoo, S.; Frankel, S.; LeSage, D.; Sarver, C.; Phillips, K.; Strand, V.; Matteson, E.L. OMERACT Connective Tissue Disease–Interstitial Lung Diseases Working Group; OMERACT Connective Tissue Disease-Interstitial Lung Diseases Working Group. Reconciling Healthcare Professional and Patient Perspectives in the Development of Disease Activity and Response Criteria in Connective Tissue Disease-Related Interstitial Lung Diseases. J. Rheumatol. 2014, 41, 792–798. [Google Scholar] [CrossRef]
- Mittoo, S.; Frankel, S.; LeSage, D.; Strand, V.; Shah, A.A.; Christopher-Stine, L.; Danoff, S.; Hummers, L.K.; Swigris, J.J.; Huscher, D.; et al. Patient Perspectives in OMERACT Provide an Anchor for Future Metric Development and Improved Approaches to Healthcare Delivery in Connective Tissue Disease Related Interstitial Lung Disease (CTD-ILD). Curr. Respir. Med. Rev. 2015, 11, 175–183. [Google Scholar] [CrossRef]
- Moor, C.C.; van Manen, M.J.G.; van Hagen, P.M.; Miedema, J.R.; van den Toorn, L.M.; Gür-Demirel, Y.; Berendse, A.P.C.; van Laar, J.A.M.; Wijsenbeek, M.S. Needs, Perceptions and Education in Sarcoidosis: A Live Interactive Survey of Patients and Partners. Lung 2018, 196, 569–575. [Google Scholar] [CrossRef]
- Hendriks, C.; Drent, M.; De Kleijn, W.; Elfferich, M.; Wijnen, P.; De Vries, J. Everyday Cognitive Failure and Depressive Symptoms Predict Fatigue in Sarcoidosis: A Prospective Follow-up Study. Respir. Med. 2018, 138S, S24–S30. [Google Scholar] [CrossRef]
- Wuyts, W.A.; Peccatori, F.A.; Russell, A.-M. Patient-Centred Management in Idiopathic Pulmonary Fibrosis: Similar Themes in Three Communication Models. Eur. Respir. Rev. Off. J. Eur. Respir. Soc. 2014, 23, 231–238. [Google Scholar] [CrossRef]
- Gierisch, J.M.; Hughes, J.M.; Williams, J.W.; Gordon, A.M.; Goldstein, K.M. Qualitative Exploration of Engaging Patients as Advisors in a Program of Evidence Synthesis: Cobuilding the Science to Enhance Impact. Med. Care 2019, 57 (Suppl. S3), S246–S252. [Google Scholar] [CrossRef]
- Principles of Person Centred Care. Available online: https://www.picker.org/about-us/picker-principles-of-person-centred-care/ (accessed on 29 April 2021).
- Coats, H.L. African American Elders’ Psychological-Social-Spiritual Cultural Experiences across Serious Illness: An Integrative Literature Review through a Palliative Care Lens. Ann. Palliat. Med. 2017, 6, 253–269. [Google Scholar] [CrossRef]
- Nicholas, S.O.; Giang, A.T.; Yap, P.L.K. The Effectiveness of Horticultural Therapy on Older Adults: A Systematic Review. J. Am. Med. Dir. Assoc. 2019, 20, 1351-e1–1351-e11. [Google Scholar] [CrossRef] [PubMed]
- Freeman, M.; Ayers, C.; Peterson, C.; Kansagara, D. Aromatherapy and Essential Oils: A Map of the Evidence; VA Evidence-Based Synthesis Program Reports; Department of Veterans Affairs (US): Washington, DC, USA, 2019. [Google Scholar]
- Engineer, A.; Ida, A.; Sternberg, E.M. Healing Spaces: Designing Physical Environments to Optimize Health, Wellbeing, and Performance. Int. J. Environ. Res. Public. Health 2020, 17, 1155. [Google Scholar] [CrossRef]
- Eagle, A. Interiors: Distinctive Design. Creating the [Patient Centered] Room. Health Facil. Manag. 2007, 20, 40–44. [Google Scholar]
- Tavee, J. The Last Day of Suffering: Five Steps to Health and Happiness. 2011. Available online: https://www.amazon.com/Last-Day-Suffering-Health-Happiness/dp/0615542751 (accessed on 7 June 2021).
- Tavee, J. Bloom: Stories to Heal the Mind. 2015. Available online: https://www.amazon.com/Bloom-stories-Jinny-Tavee-M-D/dp/0692421238 (accessed on 7 June 2021).
- Tavee, J. The Regret Vaccine: Stories for a Healthy Mind and a Happy Life. 2020. Available online: https://www.amazon.com/Regret-Vaccine-stories-healthy-happy/dp/0578795833 (accessed on 7 June 2021).
- Nixon, J.; Gray, L.; Turner, J.; Bernard, A.; Scaife, J.; Cartmill, B. Communicating Actively Responding Empathically (CARE): Comparison of Communication Training Workshops for Health Professionals Working in Cancer Care. J. Cancer Educ. Off. J. Am. Assoc. Cancer Educ. 2020, 35, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Fauchon, C.; Faillenot, I.; Perrin, A.M.; Borg, C.; Pichot, V.; Chouchou, F.; Garcia-Larrea, L.; Peyron, R. Does an Observer’s Empathy Influence My Pain? Effect of Perceived Empathetic or Unempathetic Support on a Pain Test. Eur. J. Neurosci. 2017, 46, 2629–2637. [Google Scholar] [CrossRef] [PubMed]
- Caring for Patients Who Have Experienced Trauma: ACOG Committee Opinion, Number 825. Obstet. Gynecol. 2021, 137, e94–e99. [CrossRef] [PubMed]
- Preventing Sexual Violence |Violence Prevention|Injury Center|CDC. Available online: https://www.cdc.gov/violenceprevention/sexualviolence/fastfact.html (accessed on 29 April 2021).
- Bird, C.M.; Webb, E.K.; Schramm, A.T.; Torres, L.; Larson, C.; deRoon-Cassini, T.A. Racial Discrimination Is Associated with Acute Posttraumatic Stress Symptoms and Predicts Future Posttraumatic Stress Disorder Symptom Severity in Trauma-Exposed Black Adults in the United States. J. Trauma. Stress 2021. [Google Scholar] [CrossRef] [PubMed]
- Temkin, D.; Harper, K.; Stratford, B.; Sacks, V.; Rodriguez, Y.; Bartlett, J.D. Moving Policy Toward a Whole School, Whole Community, Whole Child Approach to Support Children Who Have Experienced Trauma. J. Sch. Health 2020, 90, 940–947. [Google Scholar] [CrossRef] [PubMed]
- Hall, S.; White, A.; Ballas, J.; Saxton, S.N.; Dempsey, A.; Saxer, K. Education in Trauma-Informed Care in Maternity Settings Can Promote Mental Health During the COVID-19 Pandemic. J. Obstet. Gynecol. Neonatal Nurs. 2021, 50, 340–351. [Google Scholar] [CrossRef]
- Infographic: 6 Guiding Principles To A Trauma-Informed Approach|CDC. Available online: https://www.cdc.gov/cpr/infographics/6_principles_trauma_info.htm (accessed on 29 April 2021).
- Elwyn, G.; Cochran, N.; Pignone, M. Shared Decision Making-The Importance of Diagnosing Preferences. JAMA Intern. Med. 2017, 177, 1239–1240. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.-Y.; Molassiotis, A.; Lloyd-Williams, M.; Yorke, J. Burden, Emotional Distress and Quality of Life among Informal Caregivers of Lung Cancer Patients: An Exploratory Study. Eur. J. Cancer Care 2018, 27, e12691. [Google Scholar] [CrossRef] [PubMed]
- Strang, S.; Osmanovic, M.; Hallberg, C.; Strang, P. Family Caregivers’ Heavy and Overloaded Burden in Advanced Chronic Obstructive Pulmonary Disease. J. Palliat. Med. 2018, 21, 1768–1772. [Google Scholar] [CrossRef]
- Russell, A.-M.; Ripamonti, E.; Vancheri, C. Qualitative European Survey of Patients with Idiopathic Pulmonary Fibrosis: Patients’ Perspectives of the Disease and Treatment. BMC Pulm. Med. 2016, 16, 10. [Google Scholar] [CrossRef] [PubMed]
- Sanfod, D.; Reynolds, P. The Psychological Distress That Idiopathic Pulmonary Fibrosis Patients And Caregivers Experience. The Invisible Injury That Adds Complexity To Management And Treatment. Respirology 2019, 24, 89. [Google Scholar] [CrossRef]
- Schulz, R.; Beach, S.R.; Friedman, E.M. Caregiving Factors as Predictors of Care Recipient Mortality. Am. J. Geriatr. Psychiatry Off. J. Am. Assoc. Geriatr. Psychiatry 2021, 29, 295–303. [Google Scholar] [CrossRef]
- Moon, H.E.; Haley, W.E.; Rote, S.M.; Sears, J.S. Caregiver Well-Being and Burden: Variations by Race/Ethnicity and Care Recipient Nativity Status. Innov. Aging 2020, 4, igaa045. [Google Scholar] [CrossRef]
- Perkins, M.; Howard, V.J.; Wadley, V.G.; Crowe, M.; Safford, M.M.; Haley, W.E.; Howard, G.; Roth, D.L. Caregiving Strain and All-Cause Mortality: Evidence from the REGARDS Study. J. Gerontol. B. Psychol. Sci. Soc. Sci. 2013, 68, 504–512. [Google Scholar] [CrossRef]
- Lindell, K.O.; Nouraie, M.; Klesen, M.J.; Klein, S.; Gibson, K.F.; Kass, D.J.; Rosenzweig, M.Q. Randomised Clinical Trial of an Early Palliative Care Intervention (SUPPORT) for Patients with Idiopathic Pulmonary Fibrosis (IPF) and Their Caregivers: Protocol and Key Design Considerations. BMJ Open Respir. Res. 2018, 5, e000272. [Google Scholar] [CrossRef]
- Lee, J.; Cagle, J.G. Measures of Financial Burden for Families Dealing with Serious Illness: A Systematic Review and Analysis. Palliat. Med. 2021, 35, 280–294. [Google Scholar] [CrossRef]
- Biden, J. The Biden Plan for Mobilizing American Talent and Heart to Create a 21st Century Caregiving and Education Workforce. Available online: https://medium.com/@JoeBiden/the-biden-plan-for-mobilizing-american-talent-and-heart-to-create-a-21st-century-caregiving-and-af5ba2a2dfeb (accessed on 29 April 2021).
- Cox, C.E.; Donohue, J.F.; Brown, C.D.; Kataria, Y.P.; Judson, M.A. The Sarcoidosis Health Questionnaire: A New Measure of Health-Related Quality of Life. Am. J. Respir. Crit. Care Med. 2003, 168, 323–329. [Google Scholar] [CrossRef] [PubMed]
- Baughman, R.P.; Judson, M.A.; Beaumont, J.L.; Maier, L.A.; Sweiss, N.J.; Culver, D.A.; Chen, E.S.; Singh, N.; Lower, E.E.; Reeves, R.; et al. Evaluating the Minimal Clinically Important Difference of the King’s Sarcoidosis Questionnaire in a Multicenter Prospective Study. Ann. Am. Thorac. Soc. 2021, 18, 477–485. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.S.; Siegert, R.J.; Creamer, D.; Larkin, G.; Maher, T.M.; Renzoni, E.A.; Wells, A.U.; Higginson, I.J.; Birring, S.S. The Development and Validation of the King’s Sarcoidosis Questionnaire for the Assessment of Health Status. Thorax 2013, 68, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Van Manen, M.J.G.; Wapenaar, M.; Strookappe, B.; Drent, M.; Elfferich, M.; de Vries, J.; Gosker, H.R.; Birring, S.S.; Patel, A.S.; van den Toorn, L.; et al. Validation of the King’s Sarcoidosis Questionnaire (KSQ) in a Dutch Sarcoidosis Population. Sarcoidosis Vasc. Diffus. Lung Dis. Off. J. WASOG 2016, 33, 75–82. [Google Scholar]
- Judson, M.A.; Mack, M.; Beaumont, J.L.; Watt, R.; Barnathan, E.S.; Victorson, D.E. Validation and Important Differences for the Sarcoidosis Assessment Tool. A New Patient-Reported Outcome Measure. Am. J. Respir. Crit. Care Med. 2015, 191, 786–795. [Google Scholar] [CrossRef]
- de Kleijn, W.P.E.; De Vries, J.; Wijnen, P.A.H.M.; Drent, M. Minimal (Clinically) Important Differences for the Fatigue Assessment Scale in Sarcoidosis. Respir. Med. 2011, 105, 1388–1395. [Google Scholar] [CrossRef]
- Stratford, P. Assessing Disability and Change on Individual Patients: A Report of a Patient Specific Measure. Physiother. Can. 1995, 47, 258–263. [Google Scholar] [CrossRef]
- Tugwell, P.; Bombardier, C.; Buchanan, W.W.; Goldsmith, C.H.; Grace, E.; Hanna, B. The MACTAR Patient Preference Disability Questionnaire—An Individualized Functional Priority Approach for Assessing Improvement in Physical Disability in Clinical Trials in Rheumatoid Arthritis. J. Rheumatol. 1987, 14, 446–451. [Google Scholar]
- Gasperino, J. The Leapfrog Initiative for Intensive Care Unit Physician Staffing and Its Impact on Intensive Care Unit Performance: A Narrative Review. Health Policy Amst. Neth. 2011, 102, 223–228. [Google Scholar] [CrossRef]
- Gao, M.C.; Martin, P.B.; Motal, J.; Gingras, L.F.; Chai, C.; Maikoff, M.E.; Sarkisian, A.M.; Rosenthal, N.; Eiss, B.M. A Multidisciplinary Discharge Timeout Checklist Improves Patient Education and Captures Discharge Process Errors. Qual. Manag. Health Care 2018, 27, 63–68. [Google Scholar] [CrossRef]
- Patel, H.; Virapongse, A.; Baduashvili, A.; Devitt, J.; Barr, R.; Bookman, K. Implementing a COVID-19 Discharge Pathway to Improve Patient Safety. Am. J. Med. Qual. 2021, 36, 84–89. [Google Scholar] [CrossRef] [PubMed]
- Committee on Systems Approaches to Improve Patient Care by Supporting Clinician Well-Being; National Academy of Medicine; National Academies of Sciences, Engineering, and Medicine. Taking Action Against Clinician Burnout: A Systems Approach to Professional Well-Being; National Academies Press: Washington, DC, USA, 2019; p. 25521. [Google Scholar] [CrossRef]
- Moor, C.C.; Mostard, R.L.M.; Grutters, J.C.; Bresser, P.; Aerts, J.G.J.V.; Chavannes, N.H.; Wijsenbeek, M.S. Home Monitoring in Patients with Idiopathic Pulmonary Fibrosis. A Randomized Controlled Trial. Am. J. Respir. Crit. Care Med. 2020, 202, 393–401. [Google Scholar] [CrossRef] [PubMed]
- Kelly, C.; Grundy, S.; Lynes, D.; Evans, D.J.; Gudur, S.; Milan, S.J.; Spencer, S. Self-Management for Bronchiectasis. Cochrane Database Syst. Rev. 2018, 2, CD012528. [Google Scholar] [CrossRef]
- Wilsher, M.L. Psychological Stress in Sarcoidosis. Curr. Opin. Pulm. Med. 2012, 18, 524–527. [Google Scholar] [CrossRef] [PubMed]
- De Vries, J.; Drent, M. Relationship between Perceived Stress and Sarcoidosis in a Dutch Patient Population. Sarcoidosis Vasc. Diffus. Lung Dis. Off. J. WASOG 2004, 21, 57–63. [Google Scholar]
- Ireland, J.; Wilsher, M. Perceptions and Beliefs in Sarcoidosis. Sarcoidosis Vasc. Diffus. Lung Dis. Off. J. WASOG 2010, 27, 36–42. [Google Scholar]
- Goracci, A.; Fagiolini, A.; Martinucci, M.; Calossi, S.; Rossi, S.; Santomauro, T.; Mazzi, A.; Penza, F.; Fossi, A.; Bargagli, E.; et al. Quality of Life, Anxiety and Depression in Sarcoidosis. Gen. Hosp. Psychiatry 2008, 30, 441–445. [Google Scholar] [CrossRef] [PubMed]
- Chang, B.; Steimel, J.; Moller, D.R.; Baughman, R.P.; Judson, M.A.; Yeager, H.; Teirstein, A.S.; Rossman, M.D.; Rand, C.S. Depression in Sarcoidosis. Am. J. Respir. Crit. Care Med. 2001, 163, 329–334. [Google Scholar] [CrossRef]
- Elfferich, M.D.P.; De Vries, J.; Drent, M. Type D or “distressed” Personality in Sarcoidosis and Idiopathic Pulmonary Fibrosis. Sarcoidosis Vasc. Diffus. Lung Dis. Off. J. WASOG 2011, 28, 65–71. [Google Scholar]
- Sklenarova, H.; Krümpelmann, A.; Haun, M.W.; Friederich, H.-C.; Huber, J.; Thomas, M.; Winkler, E.C.; Herzog, W.; Hartmann, M. When Do We Need to Care about the Caregiver? Supportive Care Needs, Anxiety, and Depression among Informal Caregivers of Patients with Cancer and Cancer Survivors. Cancer 2015, 121, 1513–1519. [Google Scholar] [CrossRef]
- Voortman, M.; de Vries, J.; Hendriks, C.M.R.; Elfferich, M.D.P.; Wijnen, P.A.H.M.; Drent, M. Everyday Cognitive Failure in Patients Suffering from Neurosarcoidosis: Cognitive Failure in Neurosarcoidosis. Sarcoidosis Vasc. Diffus. Lung Dis. 2019, 36, 2–10. [Google Scholar] [CrossRef]
- Elfferich, M.D.; Nelemans, P.J.; Ponds, R.W.; De Vries, J.; Wijnen, P.A.; Drent, M. Everyday Cognitive Failure in Sarcoidosis: The Prevalence and the Effect of Anti-TNF-α Treatment. Respiration 2010, 80, 212–219. [Google Scholar] [CrossRef]
- Voortman, M.; Stern, B.J.; Saketkoo, L.A.; Drent, M. The Burden of Neurosarcoidosis: Essential Approaches to Early Diagnosis and Treatment. Semin. Respir. Crit. Care Med. 2020, 41, 641–651. [Google Scholar] [CrossRef] [PubMed]
- Saketkoo, L.A. Wildflowers Abundant in the Garden of Systemic Sclerosis Research, While Hopeful Exotics Will One Day Bloom. Rheumatology 2018, 57, 410–413. [Google Scholar] [CrossRef] [PubMed]
- Di Franco, M.; Iannuccelli, C.; Bazzichi, L.; Atzeni, F.; Consensi, A.; Salaffi, F.; Pietropaolo, M.; Alessandri, C.; Basili, S.; Olivieri, M.; et al. Misdiagnosis in Fibromyalgia: A Multicentre Study. Clin. Exp. Rheumatol. 2011, 29 (Suppl. S69), S104–S108. [Google Scholar] [PubMed]
- Voortman, M.; Fritz, D.; Vogels, O.J.M.; Eftimov, F.; van de Beek, D.; Brouwer, M.C.; Drent, M. Small Fiber Neuropathy: A Disabling and Underrecognized Syndrome. Curr. Opin. Pulm. Med. 2017, 23, 447–457. [Google Scholar] [CrossRef]
- Voortman, M.; Beekman, E.; Drent, M.; Hoitsma, E.; De Vries, J. Determination of the Smallest Detectable Change (SDC) and the Minimal Important Difference (MID) for the Small Fiber Neuropathy Screening List (SFNSL) in Sarcoidosis. Sarcoidosis Vasc. Diffus. Lung Dis. 2018, 35, 333–341. [Google Scholar] [CrossRef]
- World Health Organisation. WHO Guidelines on Physical Activity and Sedentary Behaviour: At a Glance; World Health Organisation: Geneva, Switzerland, 2020. [Google Scholar]
- Petri, M.A.; Martin, R.S.; Scheinberg, M.A.; Furie, R.A. Assessments of Fatigue and Disease Activity in Patients with Systemic Lupus Erythematosus Enrolled in the Phase 2 Clinical Trial with Blisibimod. Lupus 2017, 26, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Minnock, P.; Kirwan, J.; Bresnihan, B. Fatigue Is a Reliable, Sensitive and Unique Outcome Measure in Rheumatoid Arthritis. Rheumatol. Oxf. Engl. 2009, 48, 1533–1536. [Google Scholar] [CrossRef] [PubMed]
- Marcellis, R.G.J.; Lenssen, A.F.; Elfferich, M.D.P.; De Vries, J.; Kassim, S.; Foerster, K.; Drent, M. Exercise Capacity, Muscle Strength and Fatigue in Sarcoidosis. Eur. Respir. J. 2011, 38, 628–634. [Google Scholar] [CrossRef] [PubMed]
- Fleischer, M.; Hinz, A.; Brähler, E.; Wirtz, H.; Bosse-Henck, A. Factors Associated with Fatigue in Sarcoidosis. Respir. Care 2014, 59, 1086–1094. [Google Scholar] [CrossRef] [PubMed]
- Gvozdenovic, B.S.; Mihailovic-Vucinic, V.; Ilic-Dudvarski, A.; Zugic, V.; Judson, M.A. Differences in Symptom Severity and Health Status Impairment between Patients with Pulmonary and Pulmonary plus Extrapulmonary Sarcoidosis. Respir. Med. 2008, 102, 1636–1642. [Google Scholar] [CrossRef] [PubMed]
- De Vries, J.; Michielsen, H.; Van Heck, G.L.; Drent, M. Measuring Fatigue in Sarcoidosis: The Fatigue Assessment Scale (FAS). Br. J. Health Psychol. 2004, 9 Pt 3, 279–291. [Google Scholar] [CrossRef]
- De Boer, S.; Wilsher, M.L. Validation of the Sarcoidosis Health Questionnaire in a Non-US Population. Respirol. Carlton Vic. 2012, 17, 519–524. [Google Scholar] [CrossRef]
- Hendriks, C.; Drent, M.; Elfferich, M.; De Vries, J. The Fatigue Assessment Scale: Quality and Availability in Sarcoidosis and Other Diseases. Curr. Opin. Pulm. Med. 2018, 24, 495–503. [Google Scholar] [CrossRef]
- Strookappe, B.; Saketkoo, L.A.; Elfferich, M.; Holland, A.; De Vries, J.; Knevel, T.; Drent, M. Physical Activity and Training in Sarcoidosis: Review and Experience-Based Recommendations. Expert Rev. Respir. Med. 2016, 10, 1057–1068. [Google Scholar] [CrossRef]
- Strookappe, B.; De Vries, J.; Elfferich, M.; Kuijpers, P.; Knevel, T.; Drent, M. Predictors of Fatigue in Sarcoidosis: The Value of Exercise Testing. Respir. Med. 2016, 116, 49–54. [Google Scholar] [CrossRef]
- Strookappe, B.; Swigris, J.; De Vries, J.; Elfferich, M.; Knevel, T.; Drent, M. Benefits of Physical Training in Sarcoidosis. Lung 2015, 193, 701–708. [Google Scholar] [CrossRef]
- Strookappe, B.; Elfferich, M.; Swigris, J.; Verschoof, A.; Verschakelen, J.; Knevel, T.; Drent, M. Benefits of Physical Training in Patients with Idiopathic or End-Stage Sarcoidosis-Related Pulmonary Fibrosis: A Pilot Study. Sarcoidosis Vasc. Diffus. Lung Dis. 2015, 32, 43–52. [Google Scholar]
- Drent, M.; Elfferich, M.; Breedveld, E.; Vries, J.D.; Strookappe, B. Benefit of Wearing an Activity Tracker in Sarcoidosis. J. Pers. Med. 2020, 10, 97. [Google Scholar] [CrossRef]
- Bosse-Henck, A.; Wirtz, H.; Hinz, A. Subjective Sleep Quality in Sarcoidosis. Sleep Med. 2015, 16, 570–576. [Google Scholar] [CrossRef] [PubMed]
- Benn, B.S.; Lehman, Z.; Kidd, S.A.; Miaskowski, C.; Sunwoo, B.Y.; Ho, M.; Sun, S.; Ramstein, J.; Gelfand, J.M.; Koth, L.L. Sleep Disturbance and Symptom Burden in Sarcoidosis. Respir. Med. 2018, 144S, S35–S40. [Google Scholar] [CrossRef]
- Hinz, A.; Geue, K.; Zenger, M.; Wirtz, H.; Bosse-Henck, A. Daytime Sleepiness in Patients Diagnosed with Sarcoidosis Compared with the General Population. Can. Respir. J. 2018, 2018, 6853948. [Google Scholar] [CrossRef]
- Lal, C.; Medarov, B.I.; Judson, M.A. Interrelationship between Sleep-Disordered Breathing and Sarcoidosis. Chest 2015, 148, 1105–1114. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.Y.; Irwin, M.R.; Krueger, J.M.; Gaddameedhi, S.; Van Dongen, H.P.A. Night Shift Schedule Alters Endogenous Regulation of Circulating Cytokines. Neurobiol. Sleep Circadian Rhythm. 2021, 10, 100063. [Google Scholar] [CrossRef]
- Dolsen, M.R.; Crosswell, A.D.; Prather, A.A. Links Between Stress, Sleep, and Inflammation: Are There Sex Differences? Curr. Psychiatry Rep. 2019, 21, 8. [Google Scholar] [CrossRef] [PubMed]
- Moon, S.; Schmidt, M.; Smirnova, I.V.; Colgrove, Y.; Liu, W. Qigong Exercise May Reduce Serum TNF-α Levels and Improve Sleep in People with Parkinson’s Disease: A Pilot Study. Medicines 2017, 4, 23. [Google Scholar] [CrossRef]
- Verbraecken, J.; Hoitsma, E.; van der Grinten, C.P.M.; Cobben, N.A.M.; Wouters, E.F.M.; Drent, M. Sleep Disturbances Associated with Periodic Leg Movements in Chronic Sarcoidosis. Sarcoidosis Vasc. Diffus. Lung Dis. Off. J. WASOG 2004, 21, 137–146. [Google Scholar] [CrossRef]
- Mehdipoor, M.; Damirchi, A.; Razavi Tousi, S.M.T.; Babaei, P. Concurrent Vitamin D Supplementation and Exercise Training Improve Cardiac Fibrosis via TGF-β/Smad Signaling in Myocardial Infarction Model of Rats. J. Physiol. Biochem. 2021, 77, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.-Y.; Hong, Y.; Zhou, M.-C.; Huang, H.-L.; Shyu, W.-C.; Chen, J.-S.; Ting, H.; Cheng, Y.-J.; Yang, A.-L.; Lee, S.-D. Exercise Training Attenuates Cardiac Inflammation and Fibrosis in Hypertensive Ovariectomized Rats. J. Appl. Physiol. 2020, 128, 1033–1043. [Google Scholar] [CrossRef] [PubMed]
- Bay, M.L.; Pedersen, B.K. Muscle-Organ Crosstalk: Focus on Immunometabolism. Front. Physiol. 2020, 11, 567881. [Google Scholar] [CrossRef] [PubMed]
- Sveaas, S.H.; Smedslund, G.; Hagen, K.B.; Dagfinrud, H. Effect of Cardiorespiratory and Strength Exercises on Disease Activity in Patients with Inflammatory Rheumatic Diseases: A Systematic Review and Meta-Analysis. Br. J. Sports Med. 2017, 51, 1065–1072. [Google Scholar] [CrossRef] [PubMed]
- Cronin, O.; Keohane, D.M.; Molloy, M.G.; Shanahan, F. The Effect of Exercise Interventions on Inflammatory Biomarkers in Healthy, Physically Inactive Subjects: A Systematic Review. QJM Int. J. Med. 2017, 110, 629–637. [Google Scholar] [CrossRef] [PubMed]
- Ouchi, N.; Oshima, Y.; Ohashi, K.; Higuchi, A.; Ikegami, C.; Izumiya, Y.; Walsh, K. Follistatin-like 1, a Secreted Muscle Protein, Promotes Endothelial Cell Function and Revascularization in Ischemic Tissue through a Nitric-Oxide Synthase-Dependent Mechanism. J. Biol. Chem. 2008, 283, 32802–32811. [Google Scholar] [CrossRef]
- Sinha, A.; Lee, K.K.; Rafferty, G.F.; Yousaf, N.; Pavord, I.D.; Galloway, J.; Birring, S.S. Predictors of Objective Cough Frequency in Pulmonary Sarcoidosis. Eur. Respir. J. 2016, 47, 1461–1471. [Google Scholar] [CrossRef]
- Tully, T.; Birring, S.S. Cough in Sarcoidosis. Lung 2016, 194, 21–24. [Google Scholar] [CrossRef]
- Gvozdenovic, B.S.; Mihailovic-Vucinic, V.; Vukovic, M.; Stjepanovic, M.; Buha, I.; Mihailovic, S.V.; Maric, N.B. Predictors of Cough-Specific and Generic Quality of Life in Sarcoidosis Patients. Sarcoidosis Vasc. Diffus. Lung Dis. 2020, 37, 158–168. [Google Scholar] [CrossRef]
- Fraser, S.D.; Thackray-Nocera, S.; Shepherd, M.; Flockton, R.; Wright, C.; Sheedy, W.; Brindle, K.; Morice, A.H.; Kaye, P.M.; Crooks, M.G.; et al. Azithromycin for Sarcoidosis Cough: An Open-Label Exploratory Clinical Trial. ERJ Open Res. 2020, 6, 00534–02020. [Google Scholar] [CrossRef]
- Carel, H. Phenomenology of Illness, 1st ed.; Oxford University Press: Oxford, UK, 2016. [Google Scholar]
- Herigstad, M.; Faull, O.K.; Hayen, A.; Evans, E.; Hardinge, F.M.; Wiech, K.; Pattinson, K.T.S. Treating Breathlessness via the Brain: Changes in Brain Activity over a Course of Pulmonary Rehabilitation. Eur. Respir. J. 2017, 50, 1701029. [Google Scholar] [CrossRef]
- Malpass, A.; Dodd, J.; Feder, G.; Macnaughton, J.; Rose, A.; Walker, O.; Williams, T.; Carel, H. Disrupted Breath, Songlines of Breathlessness: An Interdisciplinary Response. Med. Humanit. 2019, 45, 294–303. [Google Scholar] [CrossRef]
- Bruton, A.; Lee, A.; Yardley, L.; Raftery, J.; Arden-Close, E.; Kirby, S.; Zhu, S.; Thiruvothiyur, M.; Webley, F.; Taylor, L.; et al. Physiotherapy Breathing Retraining for Asthma: A Randomised Controlled Trial. Lancet Respir. Med. 2018, 6, 19–28. [Google Scholar] [CrossRef]
- Spruit, M.A.; Thomeer, M.J.; Gosselink, R.; Troosters, T.; Kasran, A.; Debrock, A.J.T.; Demedts, M.G.; Decramer, M. Skeletal Muscle Weakness in Patients with Sarcoidosis and Its Relationship with Exercise Intolerance and Reduced Health Status. Thorax 2005, 60, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Baydur, A.; Alavy, B.; Nawathe, A.; Liu, S.; Louie, S.; Sharma, O.P. Fatigue and Plasma Cytokine Concentrations at Rest and during Exercise in Patients with Sarcoidosis. Clin. Respir. J. 2011, 5, 156–164. [Google Scholar] [CrossRef]
- Nessrine, A.; Zahra, A.F.; Taoufik, H. Musculoskeletal Involvement in Sarcoidosis. J. Bras. Pneumol. 2014, 40, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Cremers, J.P.; Van Kroonenburgh, M.J.; Mostard, R.L.; Vöö, S.A.; Wijnen, P.A.; Koek, G.H.; Drent, M. Extent of Disease Activity Assessed by 18F-FDG PET/CT in a Dutch Sarcoidosis Population. Sarcoidosis Vasc. Diffus. Lung Dis. Off. J. WASOG 2014, 31, 37–45. [Google Scholar]
- Huitema, M.P.; Grutters, J.C.; Rensing, B.J.W.M.; Reesink, H.J.; Post, M.C. Pulmonary Hypertension Complicating Pulmonary Sarcoidosis. Neth. Heart J. Mon. J. Neth. Soc. Cardiol. Neth. Heart Found. 2016, 24, 390–399. [Google Scholar] [CrossRef][Green Version]
- Baughman, R.P.; Engel, P.J.; Nathan, S. Pulmonary Hypertension in Sarcoidosis. Clin. Chest Med. 2015, 36, 703–714. [Google Scholar] [CrossRef]
- Ernste, F.C.; Chong, C.; Crowson, C.S.; Kermani, T.A.; Mhuircheartaigh, O.N.; Alexanderson, H. Functional Index-3: A Valid and Reliable Functional Outcome Assessment Measure in Patients With Dermatomyositis and Polymyositis. J. Rheumatol. 2021, 48, 94–100. [Google Scholar] [CrossRef]
- Baughman, R.P.; Iannuzzi, M.C.; Lower, E.E.; Moller, D.R.; Balkissoon, R.C.; Winget, D.B.; Judson, M.A. Use of Fluticasone in Acute Symptomatic Pulmonary Sarcoidosis. Sarcoidosis Vasc. Diffus. Lung Dis. Off. J. WASOG 2002, 19, 198–204. [Google Scholar]
- Khan, N.A.; Donatelli, C.V.; Tonelli, A.R.; Wiesen, J.; Ribeiro Neto, M.L.; Sahoo, D.; Culver, D.A. Toxicity Risk from Glucocorticoids in Sarcoidosis Patients. Respir. Med. 2017, 132, 9–14. [Google Scholar] [CrossRef]
- Proesmans, V.L.J.; Drent, M.; Elfferich, M.D.P.; Wijnen, P.A.H.M.; Jessurun, N.T.; Bast, A. Self-Reported Gastrointestinal Side Effects of Antifibrotic Drugs in Dutch Idiopathic Pulmonary Fibrosis Patients. Lung 2019, 197, 551–558. [Google Scholar] [CrossRef]
- Goldman, C.; Judson, M.A. Corticosteroid Refractory Sarcoidosis. Respir. Med. 2020, 171, 106081. [Google Scholar] [CrossRef]
- Broos, C.E.; Wapenaar, M.; Looman, C.W.N.; In ’t Veen, J.C.C.M.; van den Toorn, L.M.; Overbeek, M.J.; Grootenboers, M.J.J.H.; Heller, R.; Mostard, R.L.; Poell, L.H.C.; et al. Daily Home Spirometry to Detect Early Steroid Treatment Effects in Newly Treated Pulmonary Sarcoidosis. Eur. Respir. J. 2018, 51, 1702089. [Google Scholar] [CrossRef]
- Pande, A.; Culver, D.A. Knowing When to Use Steroids, Immunosuppressants or Biologics for the Treatment of Sarcoidosis. Expert Rev. Respir. Med. 2020, 14, 285–298. [Google Scholar] [CrossRef] [PubMed]
- Caplan, A.; Fett, N.; Rosenbach, M.; Werth, V.P.; Micheletti, R.G. Prevention and Management of Glucocorticoid-Induced Side Effects: A Comprehensive Review: A Review of Glucocorticoid Pharmacology and Bone Health. J. Am. Acad. Dermatol. 2017, 76, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Baughman, R.P.; Janovcik, J.; Ray, M.; Sweiss, N.; Lower, E.E. Calcium and Vitamin D Metabolism in Sarcoidosis. Sarcoidosis Vasc. Diffus. Lung Dis. Off. J. WASOG 2013, 30, 113–120. [Google Scholar]
- Zhou, Y.; Lower, E.E. Balancing Altered Calcium Metabolism with Bone Health in Sarcoidosis. Semin. Respir. Crit. Care Med. 2020, 41, 618–625. [Google Scholar] [CrossRef]
- Saketkoo, L.A.; Karpinski, A.; Young, J.; Adell, R.; Walker, M.; Hennebury, T.; Wickremasinghe, M.; Russell, A.-M. Feasibility, Utility and Symptom Impact of Modified Mindfulness Training in Sarcoidosis. ERJ Open Res. 2018, 4, 00085–02017. [Google Scholar] [CrossRef]
- Buckley, L.; Guyatt, G.; Fink, H.A.; Cannon, M.; Grossman, J.; Hansen, K.E.; Humphrey, M.B.; Lane, N.E.; Magrey, M.; Miller, M.; et al. 2017 American College of Rheumatology Guideline for the Prevention and Treatment of Glucocorticoid-Induced Osteoporosis. Arthritis Rheumatol. 2017, 69, 1521–1537. [Google Scholar] [CrossRef]
- Duckworth, A.; Gibbons, M.A.; Allen, R.J.; Almond, H.; Beaumont, R.N.; Wood, A.R.; Lunnon, K.; Lindsay, M.A.; Wain, L.V.; Tyrrell, J.; et al. Telomere Length and Risk of Idiopathic Pulmonary Fibrosis and Chronic Obstructive Pulmonary Disease: A Mendelian Randomisation Study. Lancet Respir. Med. 2021, 9, 285–294. [Google Scholar] [CrossRef]
- Arsenis, N.C.; You, T.; Ogawa, E.F.; Tinsley, G.M.; Zuo, L. Physical Activity and Telomere Length: Impact of Aging and Potential Mechanisms of Action. Oncotarget 2017, 8, 45008–45019. [Google Scholar] [CrossRef] [PubMed]
- Sole-Smith, V. In Obesity Research, Fatphobia Is Always the X Factor. Sci. Am. 2021. Available online: https://www.scientificamerican.com/article/in-obesity-research-fatphobia-is-always-the-x-factor/ (accessed on 3 June 2021).
- Tonelli, R.; Cocconcelli, E.; Lanini, B.; Romagnoli, I.; Florini, F.; Castaniere, I.; Andrisani, D.; Cerri, S.; Luppi, F.; Fantini, R.; et al. Effectiveness of Pulmonary Rehabilitation in Patients with Interstitial Lung Disease of Different Etiology: A Multicenter Prospective Study. BMC Pulm. Med. 2017, 17, 130. [Google Scholar] [CrossRef] [PubMed]
- Grünig, E.; MacKenzie, A.; Peacock, A.J.; Eichstaedt, C.A.; Benjamin, N.; Nechwatal, R.; Ulrich, S.; Saxer, S.; Bussotti, M.; Sommaruga, M.; et al. Standardized Exercise Training Is Feasible, Safe, and Effective in Pulmonary Arterial and Chronic Thromboembolic Pulmonary Hypertension: Results from a Large European Multicentre Randomized Controlled Trial. Eur. Heart J. 2020. [Google Scholar] [CrossRef] [PubMed]
- Nakazawa, A.; Cox, N.S.; Holland, A.E. Current Best Practice in Rehabilitation in Interstitial Lung Disease. Ther. Adv. Respir. Dis. 2017, 11, 115–128. [Google Scholar] [CrossRef] [PubMed]
- Kerti, M.; Balogh, Z.; Kelemen, K.; Varga, J.T. The Relationship between Exercise Capacity and Different Functional Markers in Pulmonary Rehabilitation for COPD. Int. J. Chron. Obstr. Pulm. Dis. 2018, 13, 717–724. [Google Scholar] [CrossRef] [PubMed]
- Guber, E.; Wand, O.; Epstein Shochet, G.; Romem, A.; Shitrit, D. The Short- and Long-Term Impact of Pulmonary Rehabilitation in Subjects with Sarcoidosis: A Prospective Study and Review of the Literature. Respiration 2021, 100, 1–9. [Google Scholar] [CrossRef]
- Yoon, H.S.; Cha, Y.J.; You, J.; Sung, H. Effects of Dynamic Core-Postural Chain Stabilization on Diaphragm Movement, Abdominal Muscle Thickness, and Postural Control in Patients with Subacute Stroke: A Randomized Control Trial. Neurorehabilitation 2020, 46, 381–389. [Google Scholar] [CrossRef]
- Lewis, A.; Cave, P.; Hopkinson, N. Singing for Lung Health: Service Evaluation of the British Lung Foundation Programme. Perspect. Public Health 2018, 138, 215–222. [Google Scholar] [CrossRef]
- Kang, J.; Scholp, A.; Jiang, J.J. A Review of the Physiological Effects and Mechanisms of Singing. J. Voice Off. J. Voice Found. 2018, 32, 390–395. [Google Scholar] [CrossRef]
- Idrose, A.M.; Juliana, N.; Azmani, S.; Yazit, N.A.A.; Muslim, M.S.A.; Ismail, M.; Amir, S.N. Singing Improves Oxygen Saturation in Simulated High-Altitude Environment. J. Voice 2020. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Shi, K.; Yan, J.; He, Z.; Wang, Y.; Yi, Q.; Huang, H. A Modified 6-Form Tai Chi for Patients with COPD. Complement. Ther. Med. 2018, 39, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Saketkoo, L.A.; Alexanderson, H.; Lammi, M.R.; LeSage, D.; Jensen, K.; Scholand, M.B.; Volkmann, E.R.; Russell, A.-M. An Ode to the Primal Tonic of Dance—Congratulating the Life of Breath Project. Lancet Respir. Med. 2020, 8, e90–e91. [Google Scholar] [CrossRef]
| Category | Manifestation | Source of Disability/Potential Causes to be Investigated |
|---|---|---|
| CONSTITUTIONAL | Fevers | Sub-clinical infection, systemic sarcoid |
| Weight Loss | Systemic disease, medication-related nausea, depressive symptoms | |
| Weight Gain | CNS/endocrine involvement, impairment-related deconditioning, GCs | |
| Fatigue | Anxiety, body pain, DMARDS/GCs, dyspnea, headache, hormonal (e.g., thyroid, testosterone), GC or anxiety-related insomnia, GC or sarcoid myopathy, HF, hypercalcemia, nutritional, OSA, PH, systemic disease | |
| Insomnia/Poor sleep | Anxiety, body pain, depressive symptoms, dyspnea, GCs, HF, hypercalcemia, OSA, periodic leg syndrome, restless leg syndrome | |
| PSYCHOLOGICAL | Anxiety | Dyspnea, impaired coping, GC, hypercalcemia, poor sleep quality, uncertainty about future health/finances, vitamin D dysregulation |
| Cognitive impairment | Anxiety, body pain, CNS involvement eye discomfort, GC, fatigue, headaches, hypercalcemia | |
| Depressed feelings | Anxiety, body pain, cutaneous disfigurement; CNS involvement, fatigue, GCs, hypercalcemia, impaired coping, progressive/irreversible impairment, social isolation, uncertainty about future health/finances, vitamin D dysregulation | |
| NEUROLOGICAL | Seizures | CNS involvement |
| Headache | CNS, endocrine or ocular involvement, dyspnea, DMARDs/GCs, hypercalcemia, insomnia, OSA | |
| Weakness | CNS/spinal cord involvement, fatigue, GC or sarcoid myopathy, or large fiber neuropathy, hypercalcemia | |
| Falls/Gait imbalance | CNS/spinal cord, involvement myopathy, small and/or large fiber neuropathy involvement | |
| Numbness/Tingling | CNS/PNS, small fiber neuropathy, hypercalcemia | |
| Dysautonomia (palpitations, sweating abnormalities, orthostatic intolerance, bloating, constipation, diarrhea) | Hypercalcemia, small fiber neuropathy associated symptoms | |
| OCULAR | Acuity impairment | Glaucoma, medication, ocular muscle or nerve involvement, synechiae, uveitis |
| Dryness | Lacrimal gland involvement, medication related | |
| Pain, pressure | Glaucoma, uveitis; CNS, lacrimal gland or sinus involvement, | |
| Tearing | ||
| OTOLARYNGOLOGICAL | Sinus congestion | GC/DMARD related infection; sarcoid sinus involvement |
| CARDIAC | Dyspnea, exercise intolerance, palpitations | Conduction abnormalities, HF, hypercalcemia, PH |
| PULMONARY | Dyspnea, cough, exercise intolerance | Infection, ILD, PH |
| GASTROINTESTINAL | Dyspepsia | GC/DMARD related |
| Nausea, vomiting | CNS, GC/DMARD related, hypercalcemia, renal calculi | |
| ENDOCRINE | Fatigue | Testosterone, thyroid-related, sarcoid or treatment related hyperglycemia, SIADH |
| Bone fracture | Systemic disease, GCs, primary or secondary osteoporosis, hormone deficiency | |
| Weight gain/loss | hypothalamic involvement, inflammation, GC-related | |
| DERMATOLOGICAL | Disfigurement | Dyspigmentation, lupus pernio, facial lesions |
| Pruritus | Lesion-related, medication-related | |
| Pain | Erythema nodosum, ulceration, deep subcutaneous granulomas | |
| MUSCULOSKELETAL | ||
| Body pain | Boney lesions, joint, hypercalcemia, muscle, neurological, poor sleep | |
| Bone fracture | See above | |
| Exercise intolerance | Cardiac or pulmonary involvement (e.g., HF, ILD, PH), Hypercalcemia, Muscle weakness, Deconditioning, Underlying Infection | |
| Myopathy/Myalgia | Sarcoidosis or GC related | |
| Weakness | CNS or muscle involvement |
| Central Nervous System |
|---|
| Steroid induced psychosis |
| Changes in mood and behavior |
| Dysphoria |
| Insomnia |
| Changes in memory |
| Cerebral atrophy |
| Ocular |
| Glaucoma |
| Cataracts |
| Cardiovascular |
| Hypertension |
| Dyslipidemia |
| Thrombosis |
| Vascular frailty |
| Gastrointestinal |
| Peptic ulcer |
| Gastrointestinal bleeding |
| Pancreatitis |
| Hepatic steatosis |
| Renal |
| Increased sodium retention |
| Increased potassium excretion |
| Endocrine |
| Diabetes mellitus |
| Weight gain |
| Cushing’s syndrome |
| Adrenal suppression |
| Hypogonadism |
| Male gynecomastia |
| Musculoskeletal |
| Osteoporosis |
| Bone necrosis |
| Muscle atrophy |
| Myopathy |
| Skin |
| Striae rubrae distensae |
| Skin frailty and tearing |
| Delayed wound healing |
| Glucocorticoid induced acne |
| Secondary infections, including candidiasis |
| Perioral dermatitis |
| Telangiectasia |
| Skin atrophy |
| Seborrheic dermatitis |
| Immune |
| Reactivation of latent viruses |
| Increased risk of infection |
| Immunosuppression |
| Serious Infection |
| Candidiasis: oral/cutaneous/vaginal |
| Bone Health [148,149,150] |
| Counsel patients about the risk of osteoporosis and screen for risk factors. |
| Baseline DEXA scan (for patients anticipated to need glucocorticoids for >3 months). Initiation of bisphosphonate for prevention according to American College of Rheumatology guidelines. |
| Calcium supplementation is controversial in sarcoidosis and vitamin D supplementation, only if 1,25 dihydroxy is low. |
| Counseling on lifestyle modifications—smoking cessation, weight-bearing activities. |
| Baseline height as surrogate for vertebral height/compression fracture. |
| Gastrointestinal [148] |
| Counsel on gastric protection, take with food, H2 Blocker, or PPI depending on risk level. Assess for risk factors for PUD–history of PUD, heavy smokers, heavy alcohol use, age >65 years old, other medications that increase risk of PUD. |
| For patients on glucocorticoids and nonsteroidal anti-inflammatory drugs, start PPI. |
| For patients with multiple risk factors for PUD, consider addition of PPI. |
| Endocrinology [148] |
| In patients with diabetes, glucose monitoring with sliding scale insulin instructions. Consider screening for diabetes–hemoglobin A1C, basic metabolic panel, or fingerstick glucose. |
| Monitoring fingerstick glucose or basic metabolic panel in patients. |
| Consider prescribing home glucometer for patients on long-term high dose glucocorticoids. |
| Monitoring of electrolytes. |
| Cardiovascular [148] |
| Baseline lipid panel. |
| Blood pressure monitoring and treatment of hypertension if indicated. |
| Immunizations [148] |
| Inquire about vaccination history. |
| Live vaccines should be given 2–4 weeks prior to initiation of glucocorticoids if possible. |
| Administer vaccines according to standard schedule as indicated; withholding live vaccines. |
| Psychiatric [148] |
| Inquire about history of neuropsychiatric disease, suicidal ideation, and self-harm. |
| Referral to psychiatrist if indicated. |
| Counsel family members on risk of mood and behavior changes and advise physician if any changes are noted. |
| Dose glucocorticoids in the morning to reduce insomnia. Monitor for insomnia, manage insomnia as needed. |
| Ocular [148] |
| Assess for personal and/or family history of glaucoma or cataracts. |
| Obtain baseline ophthalmologic exam for patients who may need long-term glucocorticoid treatment. |
| Infectious [148] |
| Consider PCP prophylaxis for patients taking the equivalent of ≥20 mg prednisone for ≥4 weeks, especially if a second risk factor is present—hematologic malignancy, interstitial lung disease, or use of other immunosuppressant medication. |
| Inquire about infection history and risk factors for bacterial, fungal, and viral infections, and screen if indicated. |
| Hypersensitivity to any component of formulation |
| Concurrent administration of live or live-attenuated vaccines |
| Depression, uncontrolled anxiety, or history of psychosis |
| History of peptic ulcer disease or gastrointestinal bleed |
| Osteoporosis |
| Current or recent joint infection |
| Glaucoma/elevated intraocular pressure |
| Cataract |
| Diabetes mellitus/uncontrolled hyperglycemia |
| Uncontrolled hypertension |
| Elevated BMI/metabolic syndrome |
| Uncontrolled bacterial or viral infection |
| Systemic fungal infection |
| Strategies to Improve and Preserve HRQoL |
|---|
| Screening with review of systems; investigate and address other potential sarcoidosis manifestations. |
| Continually keep in mind that new or worsening symptoms may not be sarcoidosis. |
| Screening for treatment side effects to improve adherence and tolerability. |
| Screening for fatigue, assessment, and intervention for predominant causes. |
| Screening for impaired sleep quality and OSA. |
| Screening for and treating depression and anxiety. |
| Ensuring patient health literacy as a priority of treatment. |
| Essential clinician communication using shared-decision making regarding their interpretation of test results, disease severity, anticipated medication response, and prognosis. |
| Ascertain, through reflecting back to the patient’s understanding and opinion. |
| Recognition that disease activity may not be reflected in the results of the ‘objective’ tests and that lack of evidence on ‘objective’ testing does not preclude disease activity. |
| Anticipatory guidance regarding medication side effects. |
| Encouragement to perceive exercise as medicine, regardless of ability. |
| Referral for physical training and pulmonary rehabilitation for those with reduced exercise tolerance and unexplained fatigue. Patient engagement depends on their report of debilitation at any given time, as certain types of flares are globally incapacitating. |
| Publicize patient support group meetings as well as reliable patient education sources. |
| Preventive strategies for complications of disease or treatment: immunizations; medication monitoring, bone, and gastric protection; prophylactic antibiotic, as appropriate. |
| Opportunities for patients to learn skills for well-being, such as coping, mindfulness techniques, and organizational skills. |
| Strata of Engagement | Advisement | Comments |
|---|---|---|
| No cardiopulmonary involvement | Unrestricted but targeted to and guided by patient needs, and tolerance | Gradual increment of intensity, repetitions, and duration |
| Mild cardiopulmonary symptoms | Moderate aerobic intensity with moderate-load resistance exercises | Gradual increment of intensity, repetitions, and duration |
| Severe cardiopulmonary symptoms | Individualized modification of intensity and duration, with supplemental oxygen as needed | Can be intensified up to 75–80% of a patient’s projected maximal load |
| Desaturation with exercise | As above for severe | |
| Increase of systolic pulmonary artery pressure with exercise | Load reduction on systemic and pulmonary circulation is an important consideration | Rapid changes in pulmonary hemodynamics interval training may increase risk of syncope |
| Concept | Advisement | Comments |
|---|---|---|
| Exercise Initiation | All patients screened for clinically significant ILD and PH | |
| Assess current activity levels with FITT | FITT = Frequency, Intensity, Type, Time, an exercise program/prescription created by patient or clinician | |
| Consider assessing patient goals with PSFS | PSFS = Patient Specific Functional Scale, a patient created scale of their unique priorities | |
| Given the fairly high prevalence of sarcoid myopathy and neuropathy, aerobic and muscle testing prior start of exercise | Submaximal ergometer cycle test or treadmill test and muscle tests like TST, 30-sec CST and FI-2 | |
| Sustaining exercise | Anticipatory guidance of fluctuating fatigue/pain challenging exercise | Encourage mindfulness practice and pleasure principals during exercise to redirect frustration and disappointments |
| Education on stretching safety | Emphasis on consistency of practice and expectations of incremental improvement | |
| Developing alternate options for inclement weather or GI exacerbations | Indoor options Online class options | |
| Consider monitoring achievement with PSFS | ||
| Start gently and escalate with improvement | ||
| Recommendation for home general physical activity (e.g., walks) 30 min/5 days weekly | ||
| Aerobic and muscle testing after exercise period to evaluate intervention | Submaximal ergometer cycle test or treadmill test and muscle tests like TST, 30-sec CST and FI-2 |
| Expansive patient perspective investigations. |
| Isolate determinants of racial disparities in sarcoidosis, develop proactive interventions for targeted reversal of identified disparities. |
| Characterize presentations and descriptors to help identify type and cause of fatigue in sarcoidosis. |
| Develop protocol for management of fatigue according to type and cause. |
| Investigations to characterize ‘flare’ types, relational causes and recovery. |
| Integrated mind–body strategy impacts on inflammation, pulmonary functioning, fatigue, and HRQoL. |
| Exercise and physical activity impact on inflammation and other biomarkers, HRQoL. |
| Testing muscle endurance with FI-2/FI-3 in correlation with fatigue and other assessment parameters. |
| Identify optimal parameters of physical training in sarcoidosis. |
| Impact of sarcoidosis-related psychological distress on patient perceptions of their loved ones’ anxiety and emotional distress. |
| Impact of home-based prescriptions for physical activity and exercise on depressive symptoms, fatigue, inflammation, and activity levels in patients with sarcoidosis. |
| Examine HRQoL in the context of morbidity and survival in sarcoidosis. |
| Examine HRQoL interventions on symptom distress and survival. |
| Examine exercise on HRQoL and survival as stratified for disease severity. |
| Development of sarcoidosis-specific patient-reported experience measure (PREM). |
| Development of sarcoidosis-specific patient engagement measure (PEM). |
Develop and test CME practice modules on:
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saketkoo, L.A.; Russell, A.-M.; Jensen, K.; Mandizha, J.; Tavee, J.; Newton, J.; Rivera, F.; Howie, M.; Reese, R.; Goodman, M.; et al. Health-Related Quality of Life (HRQoL) in Sarcoidosis: Diagnosis, Management, and Health Outcomes. Diagnostics 2021, 11, 1089. https://doi.org/10.3390/diagnostics11061089
Saketkoo LA, Russell A-M, Jensen K, Mandizha J, Tavee J, Newton J, Rivera F, Howie M, Reese R, Goodman M, et al. Health-Related Quality of Life (HRQoL) in Sarcoidosis: Diagnosis, Management, and Health Outcomes. Diagnostics. 2021; 11(6):1089. https://doi.org/10.3390/diagnostics11061089
Chicago/Turabian StyleSaketkoo, Lesley Ann, Anne-Marie Russell, Kelly Jensen, Jessica Mandizha, Jinny Tavee, Jacqui Newton, Frank Rivera, Mike Howie, Rodney Reese, Melanie Goodman, and et al. 2021. "Health-Related Quality of Life (HRQoL) in Sarcoidosis: Diagnosis, Management, and Health Outcomes" Diagnostics 11, no. 6: 1089. https://doi.org/10.3390/diagnostics11061089
APA StyleSaketkoo, L. A., Russell, A.-M., Jensen, K., Mandizha, J., Tavee, J., Newton, J., Rivera, F., Howie, M., Reese, R., Goodman, M., Hart, P., Strookappe, B., De Vries, J., Rosenbach, M., Scholand, M. B., Lammi, M. R., Elfferich, M., Lower, E., Baughman, R. P., ... Drent, M. (2021). Health-Related Quality of Life (HRQoL) in Sarcoidosis: Diagnosis, Management, and Health Outcomes. Diagnostics, 11(6), 1089. https://doi.org/10.3390/diagnostics11061089







