A Case Report of Tongue Lymphoepithelial Carcinoma with a Histological Diagnostic Dilemma
Abstract
1. Introduction
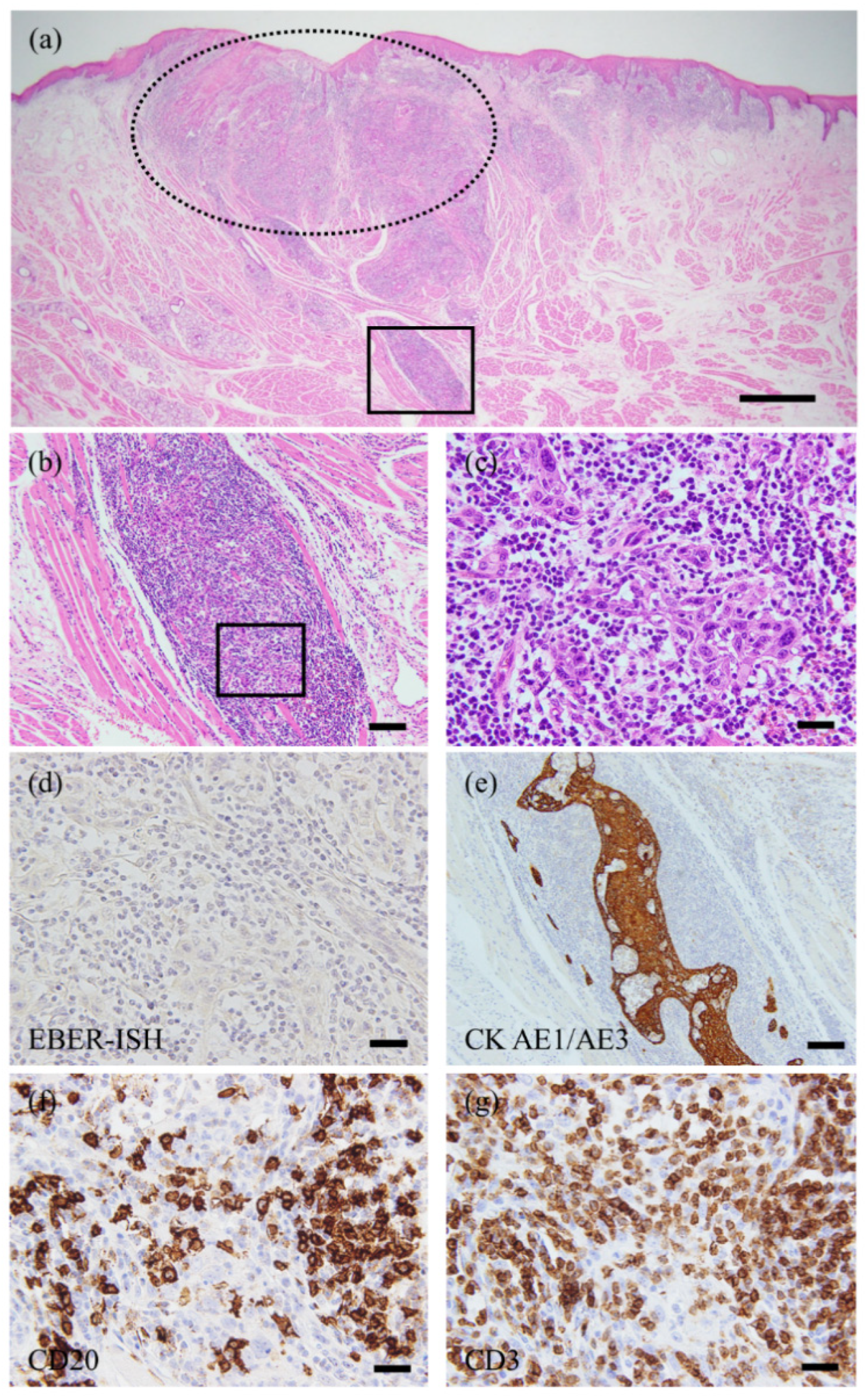
2. Case Presentation
3. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- El-Naggar, A.K.; Centre International de Recherche sur le cancer. WHO Classification of Head and Neck Tumours; IARC Publications: Lyon, France, 2017. [Google Scholar]
- Holmes, B.J.; Wenig, B.M. Virus-associated carcinomas of the head & neck: Update from the 2017 WHO classification. Ann. Diagn. Pathol. 2019, 38, 29–42. [Google Scholar] [CrossRef] [PubMed]
- Iezzoni, J.C.; Gaffey, M.J.; Weiss, L.M. The role of epstein-barr virus in lymphoepithelioma-like carcinomas. Am. J. Clin. Pathol. 1995, 103, 308–315. [Google Scholar] [CrossRef]
- Lanier, A.P.; Bornkamm, G.W.; Henle, W.; Henle, G.; Bender, T.R.; Talbot, M.L.; Dohan, P.H. Association of Epstein-Barr virus with nasopharyngeal carcinoma in alaskan native patients: Serum antibodies and tissue EBNA and DNA. Int. J. Cancer 1981, 28, 301–305. [Google Scholar] [CrossRef]
- Zong, Y.; Liu, K.; Zhong, B.; Chen, G.; Wu, W. Epstein-Barr virus infection of sinonasal lymphoepithelial carcinoma in Guangzhou. Chin. Med. J. 2001, 114, 132–136. [Google Scholar] [PubMed]
- Sckolnick, J.; Murphy, J.; Hunt, J.L. Microsatellite instability in nasopharyngeal and lymphoepithelial carcinomas of the head and neck. Am. J. Surg. Pathol. 2006, 30, 1250–1253. [Google Scholar] [CrossRef]
- Brierley, J.D.; Gospodarowicz, M.K.; Wittekind, C. TNM Classification of Malignant Tumours; John Wiley & Sons: New York, NY, USA, 2016; ISBN 978-1-119-26354-8. [Google Scholar]
- Bansberg, S.F.; Olsen, K.D.; Gaffey, T.A. Lymphoepithelioma of the Oropharynx. Otolaryngol. Head Neck Surg. 1989, 100, 303–307. [Google Scholar] [CrossRef] [PubMed]
- Dubey, P.; Ha, C.S.; Ang, K.K.; El-Naggar, A.K.; Knapp, C.; Byers, R.M.; Morrison, W.H. Nonnasopharyngeal lymphoepithelioma of the head and neck. Cancer 1998, 82, 1556–1562. [Google Scholar] [CrossRef]
- Merz, H.; Marnitz, S.; Erbersdobler, A.; Goektas, O. Schmincke’s Tumor, Carcinoma of the Base of the Tongue c T1-2, cN2c M0–A Case Report. Case Rep. Oncol. 2010, 3, 77–82. [Google Scholar] [CrossRef]
- Wang, L.M.; Silva, M.A.; D’Costa, Z.; Bockelmann, R.; Soonawalla, Z.; Liu, S.; O’Neill, E.; Mukherjee, S.; McKenna, W.G.; Muschel, R.; et al. The prognostic role of desmoplastic stroma in pancreatic ductal adenocarcinoma. Oncotarget 2016, 7, 4183–4194. [Google Scholar] [CrossRef]
- Ueno, H.; Kanemitsu, Y.; Sekine, S.; Ishiguro, M.; Ito, E.; Hashiguchi, Y.; Kondo, F.; Shimazaki, H.; Mochizuki, S.; Kajiwara, Y.; et al. Desmoplastic Pattern at the Tumor Front Defines Poor-prognosis Subtypes of Colorectal Cancer. Am. J. Surg. Pathol. 2017, 41, 1506–1512. [Google Scholar] [CrossRef]
- Ao, T.; Kajiwara, Y.; Yonemura, K.; Shinto, E.; Mochizuki, S.; Okamoto, K.; Aosasa, S.; Ueno, H. Prognostic significance of histological categorization of desmoplastic reaction in colorectal liver metastases. Virchows Archiv 2019, 475, 341–348. [Google Scholar] [CrossRef]
- Almangush, A.; Bello, I.O.; Heikkinen, I.; Hagström, J.; Haglund, C.; Kowalski, L.P.; Nieminen, P.; Coletta, R.D.; Mäkitie, A.A.; Salo, T.; et al. Stromal categorization in early oral tongue cancer. Virchows Archiv 2021, 478, 925–932. [Google Scholar] [CrossRef]
- Izumo, T. Oral premalignant lesions: From the pathological viewpoint. Int. J. Clin. Oncol. 2011, 16, 15–26. [Google Scholar] [CrossRef]
- Arsenic, R.; Kurrer, M.O. Differentiated dysplasia is a frequent precursor or associated lesion in invasive squamous cell carcinoma of the oral cavity and pharynx. Virchows Archiv 2013, 462, 609–617. [Google Scholar] [CrossRef] [PubMed]
- Ranganathan, K.; Kavitha, L. Oral epithelial dysplasia: Classifications and clinical relevance in risk assessment of oral potentially malignant disorders. J. Oral Maxillofac. Pathol. 2019, 23, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Mahomed, F.; Grayson, W. A rare case of lymphoepithelial carcinoma of the lip. Oral Surgery, Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2008, 105, e49–e52. [Google Scholar] [CrossRef] [PubMed]
- Gultekin, M.; Omer, G.; Sefik, H.; Mustaf, C.; Murat, G. Lymphoepithelial Carcinoma of the Lower Lip: Report of a Case. Int. J. Hematol. Oncol. 2014, 24, 70–72. [Google Scholar] [CrossRef]
- Almeida, L.Y.; Silveira, H.A.; Silva, E.V.; Barbeiro, C.D.O.; De Paula, J.A.; Bufalino, A.; Ribeiro-Silva, A.; León, J.E. EBV-negative lymphoepithelial-like carcinoma of the lower lip. Autops. Case Rep. 2019, 10, e2020138. [Google Scholar] [CrossRef]
- Shimizu, S.; Miyazaki, A.; Nakamori, K.; Nakai, H.; Ogi, K.; Hasegawa, T.; Hiratsuka, H. Immunophenotypic analysis of tumor infiltrating lymphocytes in Epstein-Barr virus-negative lymphoepithelial carcinoma of the oral cavity: Report of a case. J. Oral Maxillofac. Surg. Med. Pathol. 2017, 29, 581–586. [Google Scholar] [CrossRef]
- Izumo, T.; Kirita, T.; Ariji, E.; Ozeki, S.; Okada, N.; Okabe, S.; Okazaki, Y.; Omura, K.; Kusama, M.; Sato, T.; et al. General rules for clinical and pathological studies on oral cancer: A synopsis. Jpn. J. Clin. Oncol. 2012, 42, 1099–1109. [Google Scholar] [CrossRef] [PubMed]
- Chow, T.; Lui, Y.; Sze, W.; Yuen, N.; Kwok, S.; Chow, T. Lymphoepithelioma-like carcinoma of oral cavity: Report of three cases and literature review. Int. J. Oral Maxillofac. Surg. 2002, 31, 212–218. [Google Scholar] [CrossRef] [PubMed]
- Rytkönen, A.E.; Hirvikoski, P.P.; Salo, T.A. Lymphoepithelial carcinoma: Two case reports and a systematic review of oral and sinonasal cases. Head Neck Pathol. 2011, 5, 327–334. [Google Scholar] [CrossRef] [PubMed]
- Wenig, B.M. Lymphoepithelial-like carcinomas of the head and neck. Semin. Diagn. Pathol. 2015, 32, 74–86. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Takeda, D.; Shigeoka, M.; Sugano, T.; Yatagai, N.; Hasegawa, T.; Akashi, M. A Case Report of Tongue Lymphoepithelial Carcinoma with a Histological Diagnostic Dilemma. Diagnostics 2021, 11, 1039. https://doi.org/10.3390/diagnostics11061039
Takeda D, Shigeoka M, Sugano T, Yatagai N, Hasegawa T, Akashi M. A Case Report of Tongue Lymphoepithelial Carcinoma with a Histological Diagnostic Dilemma. Diagnostics. 2021; 11(6):1039. https://doi.org/10.3390/diagnostics11061039
Chicago/Turabian StyleTakeda, Daisuke, Manabu Shigeoka, Tenyu Sugano, Nanae Yatagai, Takumi Hasegawa, and Masaya Akashi. 2021. "A Case Report of Tongue Lymphoepithelial Carcinoma with a Histological Diagnostic Dilemma" Diagnostics 11, no. 6: 1039. https://doi.org/10.3390/diagnostics11061039
APA StyleTakeda, D., Shigeoka, M., Sugano, T., Yatagai, N., Hasegawa, T., & Akashi, M. (2021). A Case Report of Tongue Lymphoepithelial Carcinoma with a Histological Diagnostic Dilemma. Diagnostics, 11(6), 1039. https://doi.org/10.3390/diagnostics11061039





