Targeted Swabbing of Implant-Associated Biofilm Formation—A Staining-Guided Sampling Approach for Optimizing Routine Microbiological Diagnostics
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Growth Conditions
2.2. Biofilm Growth on Titanium Alloy Specimens
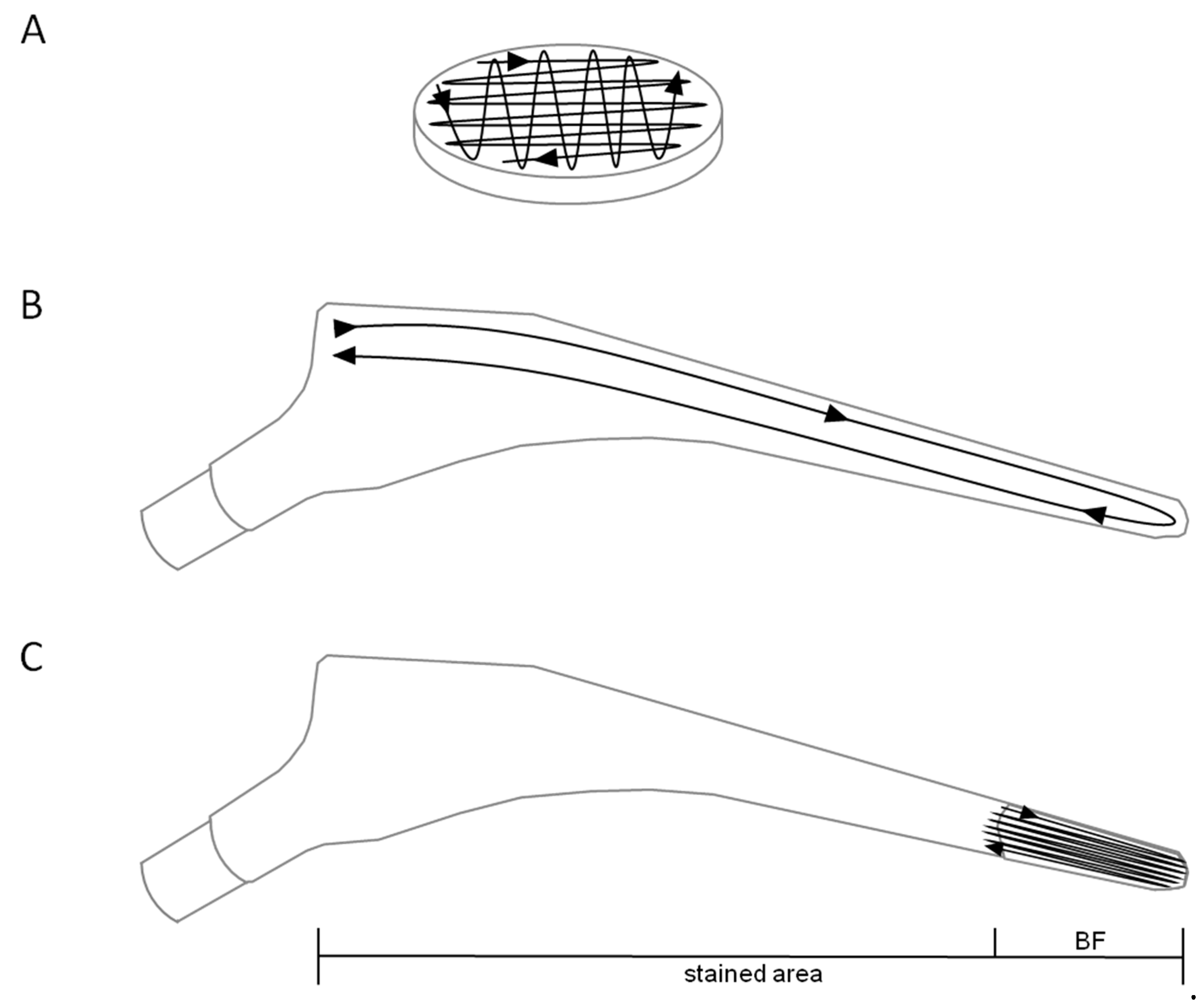
2.3. Biofilm Staining on Titanium Alloy Specimens
2.4. CFU Determination after Biofilm Staining on Titanium Alloy Specimens
2.5. Biofilm Growth and Staining on Hip Implant Material
2.6. Statistics and Reproducibility
2.7. Regeneration of Titanium Alloy Specimens and Implant Materials
3. Results
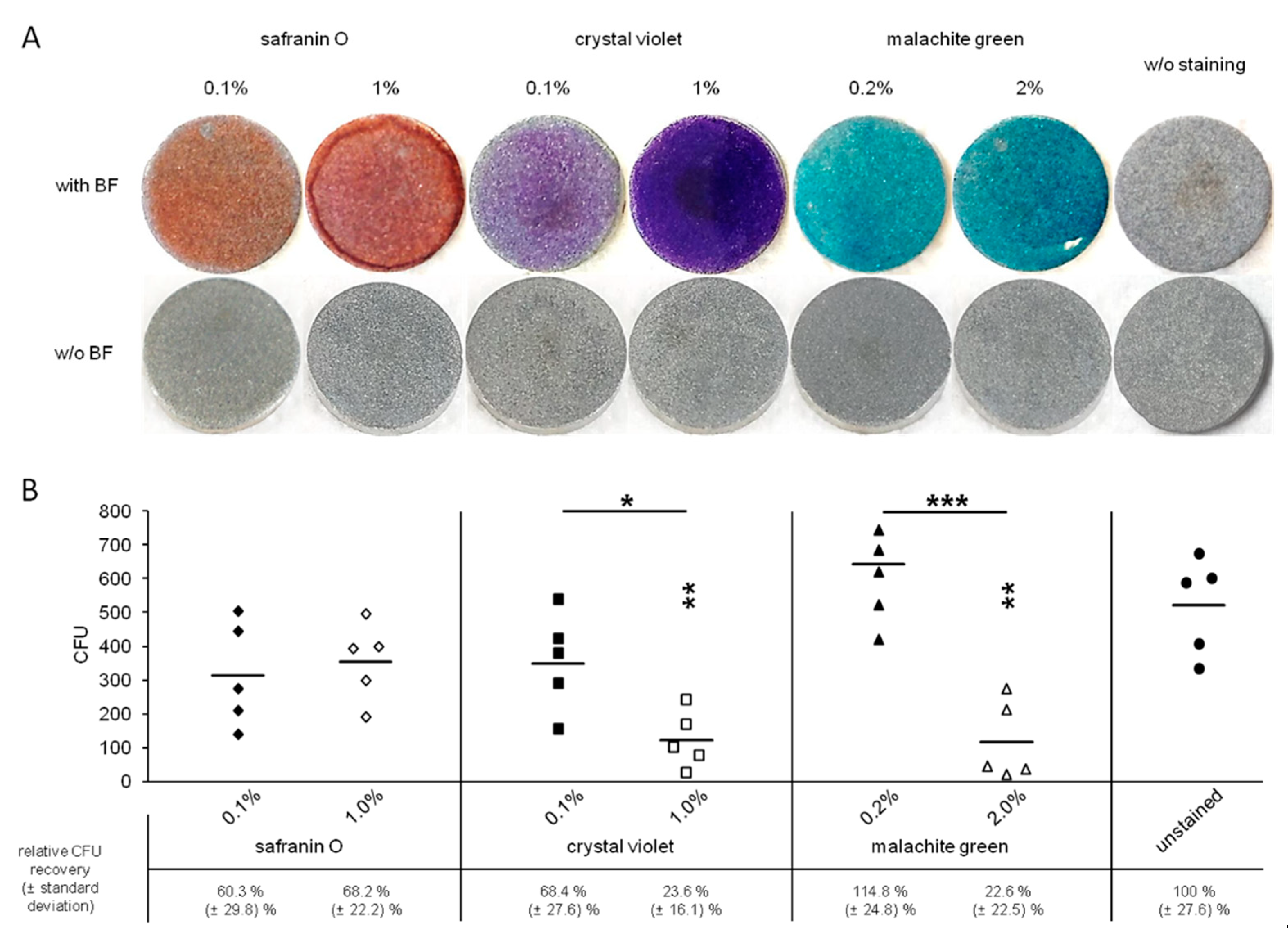
3.1. Visualization of Biofilms on Titanium Alloy Disc
3.2. Toxicity of the Staining Solutions
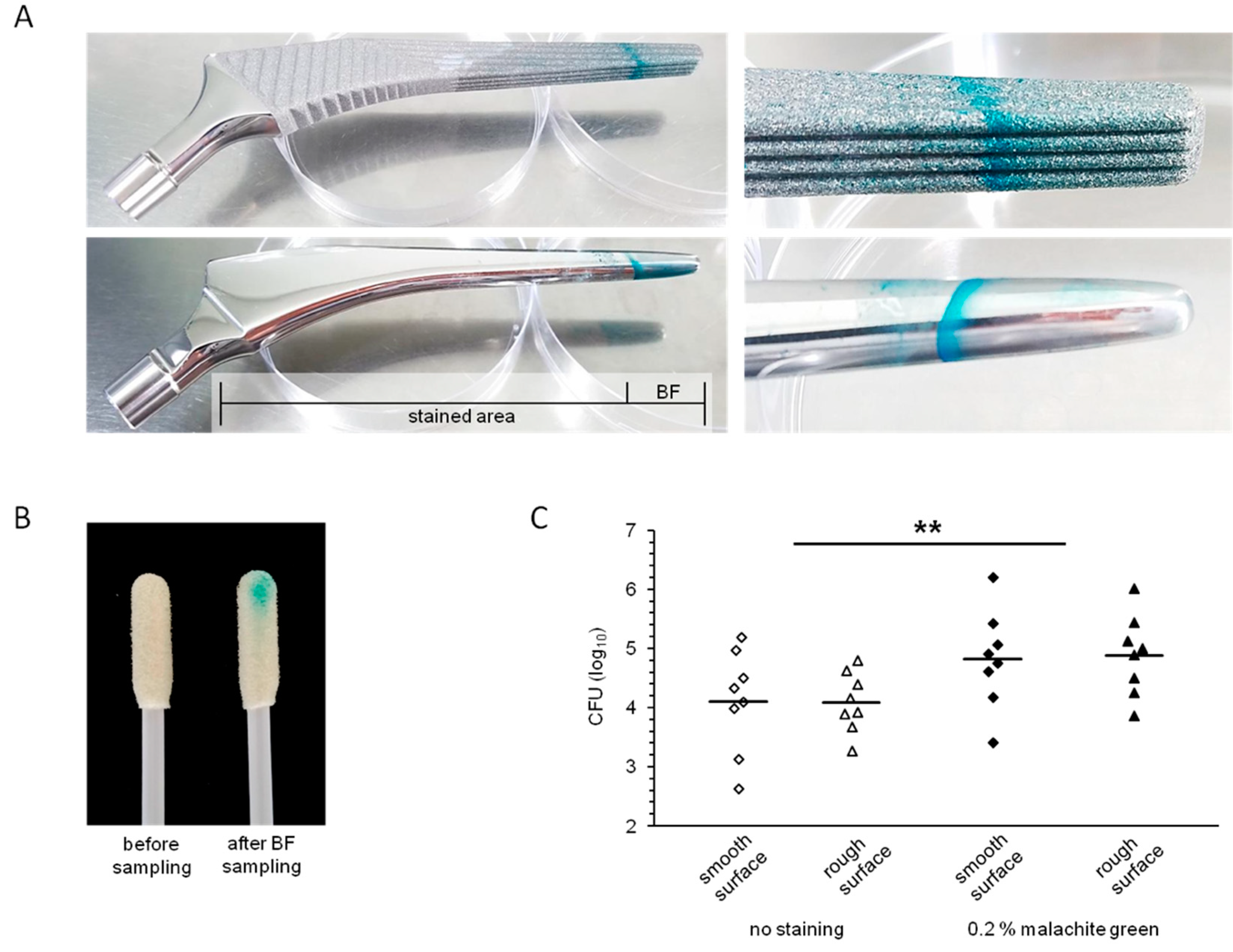
3.3. Visualization of Biofilms on Implant Materials
3.4. Recovery of Biofilm Bacteria from Implant Surfaces
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Costerton, J.W.; Stewart, P.S.; Greenberg, E.P. Bacterial Biofilms: A Common Cause of Persistent Infections. Science 1999, 284, 1318–1322. [Google Scholar] [CrossRef] [PubMed]
- Gristina, A.G. Biomaterial-centered infection: Microbial adhesion versus tissue integration. Science 1987, 237, 1588–1595. [Google Scholar] [CrossRef]
- Shimabukuro, M. Antibacterial Property and Biocompatibility of Silver, Copper, and Zinc in Titanium Dioxide Layers Incorporated by One-Step Micro-Arc Oxidation: A Review. Antibiotics 2020, 9, 716. [Google Scholar] [CrossRef] [PubMed]
- Fabritius, M.; Al-Munajjed, A.A.; Freytag, C.; Jülke, H.; Zehe, M.; LeMarchand, T.; Arts, J.J.; Schumann, D.; Alt, V.; Sternberg, K. Antimicrobial Silver Multilayer Coating for Prevention of Bacterial Colonization of Orthopedic Implants. Materials 2020, 13, 1415. [Google Scholar] [CrossRef]
- Chernozem, R.V.; Surmeneva, M.A.; Krause, B.; Baumbach, T.; Ignatov, V.P.; Prymak, O.; Loza, K.; Epple, M.; Ennen-Roth, F.; Wittmar, A.; et al. Functionalization of titania nano-tubes with electrophoretically deposited silver and calcium phosphate nanoparticles: Structure, composition and anti-bacterial assay. Mater. Sci. Eng. 2019, 97, 420–430. [Google Scholar] [CrossRef] [PubMed]
- Dudareva, M.; Barrett, L.; Figtree, M.; Scarborough, M.; Watanabe, M.; Newnham, R.; Wallis, R.; Oakley, S.; Kendrick, B.; Stubbs, D.; et al. Sonication versus Tissue Sampling for Diagnosis of Prosthetic Joint and Other Orthopedic Device-Related Infections. J. Clin. Microbiol. 2018, 56. [Google Scholar] [CrossRef]
- Gomez-Urena, E.O.; Tande, A.J.; Osmon, D.R.; Berbari, E.F. Diagnosis of Prosthetic Joint Infection: Cultures, Biomarker and Criteria. Infect. Dis. Clin. 2017, 31, 219–235. [Google Scholar] [CrossRef]
- Stylianakis, A.; Schinas, G.; Thomaidis, P.C.; Papaparaskevas, J.; Ziogas, D.C.; Gamaletsou, M.N.; Daikos, G.L.; Pneumaticos, S.; Sipsas, N.V. Combination of conventional culture, vial culture, and broad-range PCR of sonication fluid for the diagnosis of prosthetic joint infection. Diagn. Microbiol. Infect. Dis. 2018, 92, 13–18. [Google Scholar] [CrossRef]
- Gomes, A.; Van Oosten, M.; Bijker, K.L.B.; Boiten, K.E.; Salomon, E.N.; Rosema, S.; Rossen, J.W.A.; Natour, E.; Douglas, Y.L.; Kampinga, G.A.; et al. Sonication of heart valves detects more bacteria in infective endocarditis. Sci. Rep. 2018, 8, 1–9. [Google Scholar] [CrossRef]
- Izakovicova, P.; Borens, O.; Trampuz, A. Periprosthetic joint infection: Current concepts and outlook. EFORT Open Rev. 2019, 4, 482–494. [Google Scholar] [CrossRef]
- Janz, V.; Wassilew, G.I.; Kribus, M.; Trampuz, A.; Perka, C. Improved identification of polymicrobial infection in total knee arthroplasty through sonicate fluid cultures. Arch. Orthop. Trauma Surg. 2015, 135, 1453–1457. [Google Scholar] [CrossRef]
- Podbielski, A.; Abele-Horn, M.; Bücker, A.; Devide, A.; Donat, M.; Ellenrieder, M.; Erbersdobler, A.; Frommelt, L.; Gärtner, B.; Haenle, M.; et al. Microbiological analytics. In Microbiological Diagnostics of Arthritis and Osteomyeli-tis (Part I and II), MiQ: Quality Standards in Microbiological-infectiological Diagnostics, 2nd ed.; 2014. [Google Scholar]
- Rothenberg, A.C.; Wilson, A.E.; Hayes, J.P.; O’Malley, M.J.; Klatt, B.A. Sonication of Arthroplasty Implants Improves Accuracy of Periprosthetic Joint Infection Cultures. Clin. Orthop. Relat. Res. 2017, 475, 1827–1836. [Google Scholar] [CrossRef]
- Trampuz, A.; Piper, K.E.; Jacobson, M.J.; Hanssen, A.D.; Unni, K.K.; Osmon, D.R.; Mandrekar, J.N.; Cockerill, F.R.; Steckelberg, J.M.; Greenleaf, J.F.; et al. Sonication of Removed Hip and Knee Prostheses for Diagnosis of Infection. N. Engl. J. Med. 2007, 357, 654–663. [Google Scholar] [CrossRef] [PubMed]
- O’Toole, G.A. Microtiter Dish Biofilm Formation Assay. J. Vis. Exp. 2011, 47, e2437. [Google Scholar] [CrossRef]
- Pilz, M.; Staats, K.; Tobudic, S.; Assadian, O.; Presterl, E.; Windhager, R.; Holinka, J. Zirconium Nitride Coating Reduced Staphylococcus epidermidis Biofilm Formation on Orthopaedic Implant Surfaces: An In Vitro Study. Clin. Orthop. Relat. Res. 2019, 477, 461–466. [Google Scholar] [CrossRef]
- Pumeesat, P.; Muangkaew, W.; Ampawong, S.; Luplertlop, N. Candida albicans biofilm development under increased tem-perature. New Microbiol. 2017, 40, 279–283. [Google Scholar]
- Redanz, S.; Standar, K.; Podbielski, A.; Kreikemeyer, B. Heterologous Expression of sahH Reveals That Biofilm Formation Is Autoinducer-2-independent in Streptococcus sanguinis but Is Associated with an Intact Activated Methionine Cycle*. J. Biol. Chem. 2012, 287, 36111–36122. [Google Scholar] [CrossRef]
- Standar, K.; Kreikemeyer, B.; Redanz, S.; Münter, W.L.; Laue, M.; Podbielski, A. Setup of an In Vitro Test System for Basic Studies on Biofilm Behavior of Mixed-Species Cultures with Dental and Periodontal Pathogens. PLoS ONE 2010, 5, e13135. [Google Scholar] [CrossRef] [PubMed]
- De Alencar, C.R.; De Oliveira, G.C.; Tripodi, C.D.; Gonçalves, P.S.; Ionta, F.Q.; Honorio, H.M.; Oliveira, T.M.; Rios, D.; Junior, C.D.T. Dental Plaque Disclosing as an Auxiliary Method for Professional Dental Prophylaxis in Early Childhood. Int. J. Clin. Pediatr. Dent. 2019, 12, 189–193. [Google Scholar] [CrossRef] [PubMed]
- Duarte, C.A.; Lascala, N.T.; Muench, A. Influence of bacterial plaque disclosing agents on patient oral hygiene motivation under direct supervision and direction. Revista de Odontologia da Universidade de São Paulo 1990, 4, 278–283. [Google Scholar] [PubMed]
- Srivastava, S.; Sinha, R.; Roy, D. Toxicological effects of malachite green. Aquat. Toxicol. 2004, 66, 319–329. [Google Scholar] [CrossRef]
- Zaatreh, S.; Wegner, K.; Strauß, M.; Pasold, J.; Mittelmeier, W.; Podbielski, A.; Kreikemeyer, B.; Bader, R. Co-Culture of S. epidermidis and Human Osteoblasts on Implant Surfaces: An Advanced In Vitro Model for Implant-Associated Infections. PLoS ONE 2016, 11, e0151534. [Google Scholar] [CrossRef]
- Clinical and Laboratory Standards Institute (CLSI). Quality Control of Microbiological Transport Systems; approved stand-ard-second edition; CLSI document M40-A2; CLSI: Wayne, PA, USA, 2014. [Google Scholar]
- Warnke, P.; Redanz, S.; Zaatreh, S.; Podbielski, A. Augmented recovery of microorganisms from swabs by homogenization: A novel standardizable high-throughput approach. Diagn. Microbiol. Infect. Dis. 2016, 84, 16–18. [Google Scholar] [CrossRef]
- Warnke, P.; Warning, L.; Podbielski, A. Some Are More Equal - A Comparative Study on Swab Uptake and Release of Bacterial Suspensions. PLoS ONE 2014, 9, e102215. [Google Scholar] [CrossRef]
- Warnke, P.; Frickmann, H.; Ottl, P.; Podbielski, A. Nasal Screening for MRSA: Different Swabs – Different Results! PLoS ONE 2014, 9, e111627. [Google Scholar] [CrossRef] [PubMed]
- Ellenrieder, M.; Lenz, R.; Haenle, M.; Bader, R.; Mittelmeier, W. Two-stage revision of implant-associated infections after total hip and knee arthroplasty. GMS Krankenhhyg Interdiszip 2011, 6. [Google Scholar] [CrossRef]
- Li, L.; Mendis, N.; Trigui, H.; Oliver, J.D.; Faucher, S.P. The importance of the viable but non-culturable state in human bacterial pathogens. Front. Microbiol. 2014, 5, 258. [Google Scholar] [CrossRef] [PubMed]
- Pasquaroli, S.; Zandri, G.; Vignaroli, C.; Vuotto, C.; Donelli, G.; Biavasco, F. Antibiotic pressure can induce the viable but non-culturable state in Staphylococcus aureus growing in biofilms. J. Antimicrob. Chemother. 2013, 68, 1812–1817. [Google Scholar] [CrossRef] [PubMed]
- Wood, T.K. Strategies for combating persister cell and biofilm infections. Microb. Biotechnol. 2017, 10, 1054–1056. [Google Scholar] [CrossRef] [PubMed]
- Schäfer, P.; Fink, B.; Sandow, D.; Margull, A.; Berger, I.; Frommelt, L. Prolonged Bacterial Culture to Identify Late Periprosthetic Joint Infection: A Promising Strategy. Clin. Infect. Dis. 2008, 47, 1403–1409. [Google Scholar] [CrossRef] [PubMed]
- Flurin, L.; Greenwood-Quaintance, K.E.; Patel, R. Microbiology of polymicrobial prosthetic joint infection. Diagn. Microbiol. Infect. Dis. 2019, 94, 255–259. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Redanz, S.; Enz, A.; Podbielski, A.; Warnke, P. Targeted Swabbing of Implant-Associated Biofilm Formation—A Staining-Guided Sampling Approach for Optimizing Routine Microbiological Diagnostics. Diagnostics 2021, 11, 1038. https://doi.org/10.3390/diagnostics11061038
Redanz S, Enz A, Podbielski A, Warnke P. Targeted Swabbing of Implant-Associated Biofilm Formation—A Staining-Guided Sampling Approach for Optimizing Routine Microbiological Diagnostics. Diagnostics. 2021; 11(6):1038. https://doi.org/10.3390/diagnostics11061038
Chicago/Turabian StyleRedanz, Sylvio, Andreas Enz, Andreas Podbielski, and Philipp Warnke. 2021. "Targeted Swabbing of Implant-Associated Biofilm Formation—A Staining-Guided Sampling Approach for Optimizing Routine Microbiological Diagnostics" Diagnostics 11, no. 6: 1038. https://doi.org/10.3390/diagnostics11061038
APA StyleRedanz, S., Enz, A., Podbielski, A., & Warnke, P. (2021). Targeted Swabbing of Implant-Associated Biofilm Formation—A Staining-Guided Sampling Approach for Optimizing Routine Microbiological Diagnostics. Diagnostics, 11(6), 1038. https://doi.org/10.3390/diagnostics11061038





