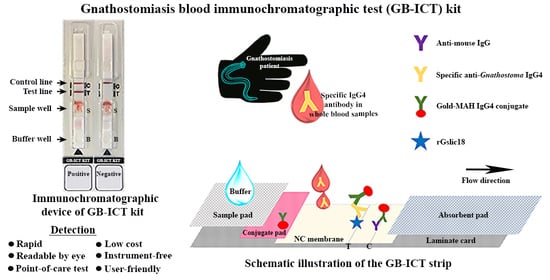
Development of Immunochromatographic Test Kit for Rapid Detection of Specific IgG4 Antibody in Whole-Blood Samples for Diagnosis of Human Gnathostomiasis
Abstract
1. Introduction
2. Materials and Methods
2.1. Clinical Samples
2.2. Preparation of Simulated WBSs
2.3. Preparation of Recombinant Antigen
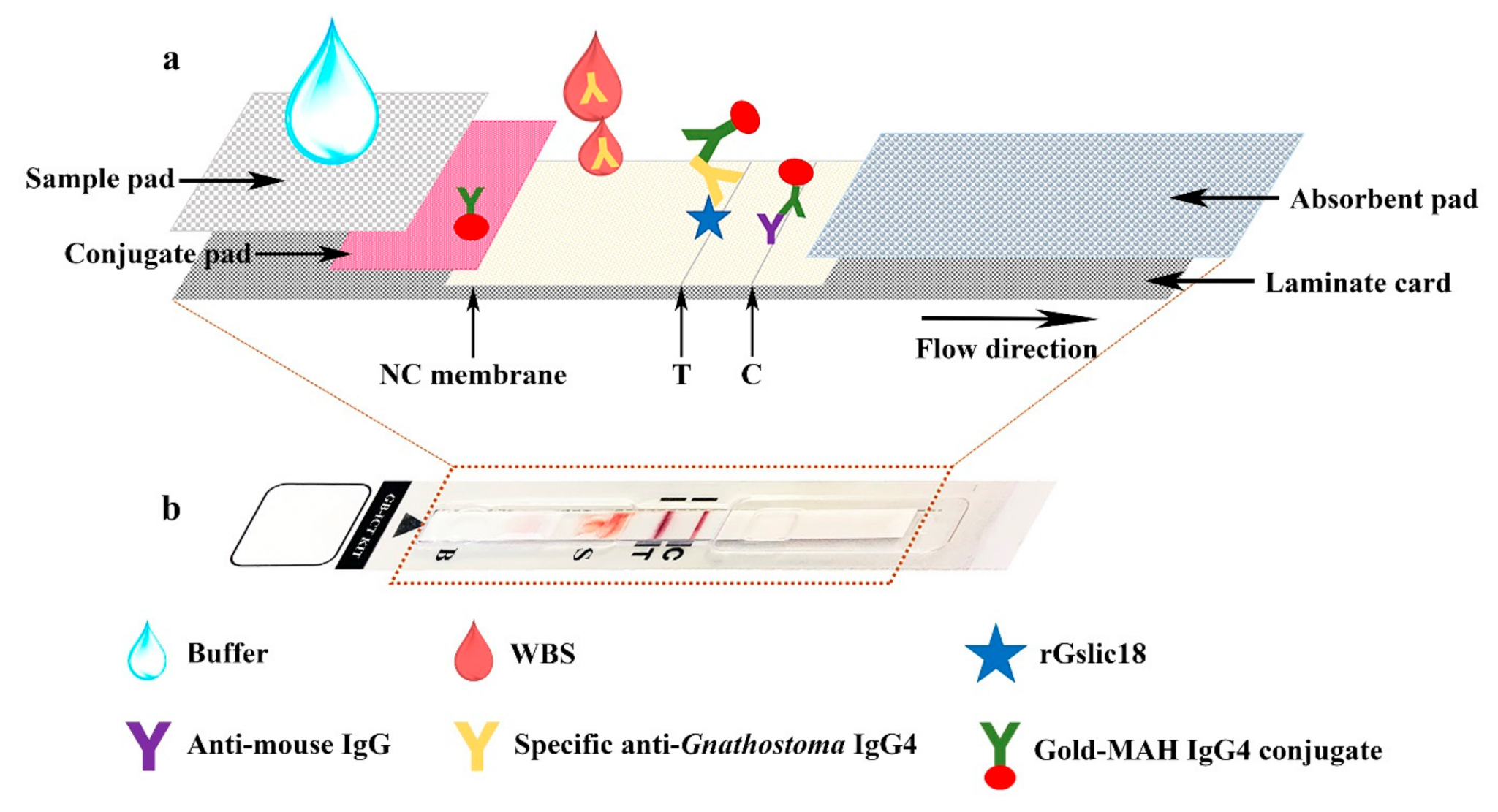
2.4. Preparation of An Immunochromatographic Device
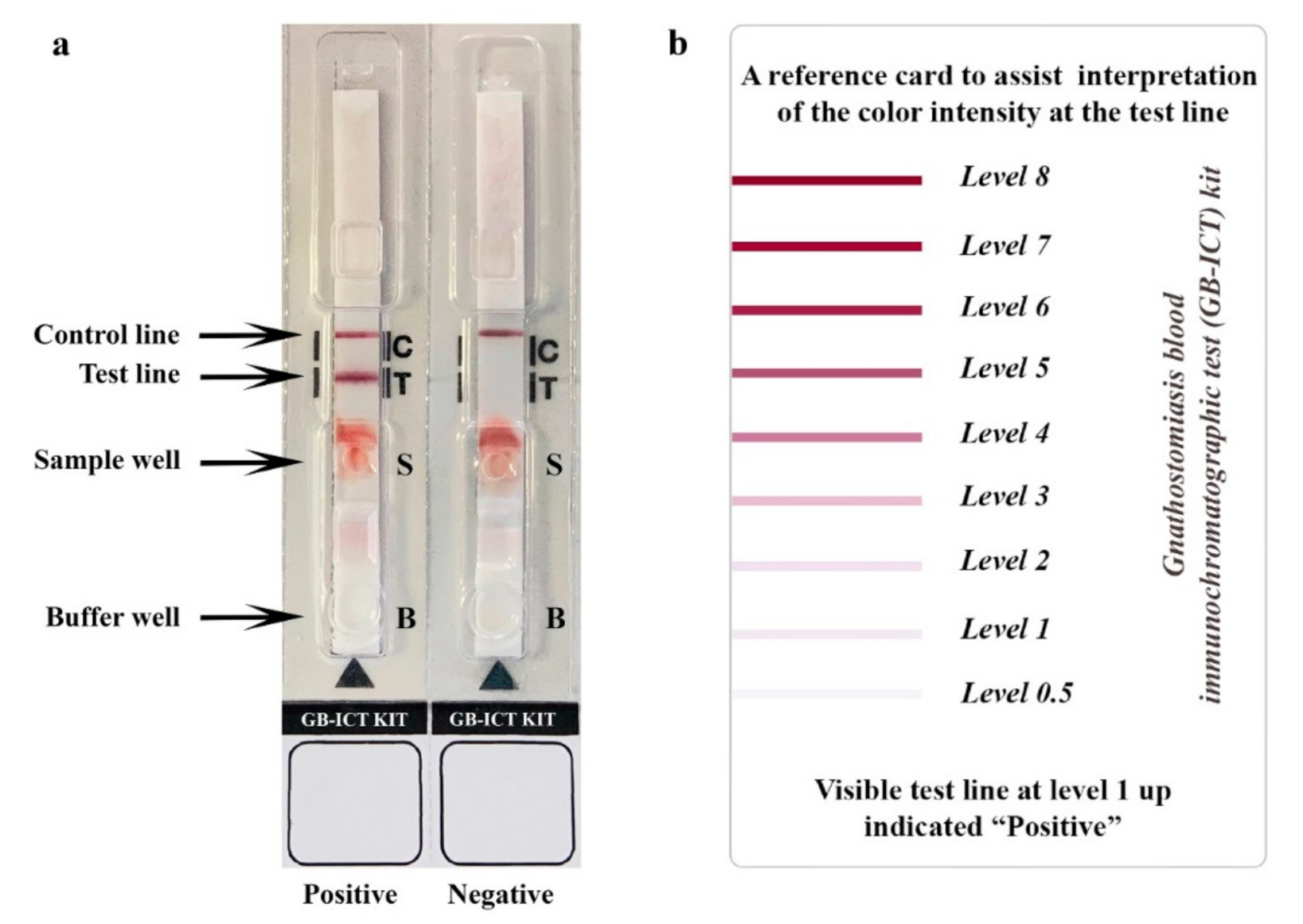
2.5. Immunochromatographic Testing Method
3. Results
3.1. GB-ICT Kit Optimization and Development
3.2. Evaluation of the GB-ICT Kit for Diagnosis of Human Gnathostomiasis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Moore, D.A.J.; McCroddan, J.; Dekumyoy, P.; Chiodini, P.L. Gnathostomiasis: An emerging imported disease. Emerg. Infect. Dis. 2003, 9, 647–650. [Google Scholar] [CrossRef] [PubMed]
- De Górgolas, M.; Santos-O’Connor, F.; Unzú, A.L.; Fernández-Guerrero, M.L.; Gárate, T.; Troyas Guarch, R.M.; Grobusch, M.P. Cutaneous and medullar gnathostomiasis in travelers to Mexico and Thailand. J. Travel Med. 2003, 10, 358–361. [Google Scholar] [CrossRef] [PubMed]
- Herman, J.S.; Chiodini, P.L. Gnathostomiasis, another emerging imported disease. Clin. Microbiol. Rev. 2009, 22, 484–492. [Google Scholar] [CrossRef] [PubMed]
- Nawa, Y.; Maleewong, W.; Intapan, P.M.; Diaz-Camacho, S.P. Gnathostoma. In Food Microbiology Series: Biology of Foodborne Parasites, 1st ed.; Xiao, L., Ryan, U., Feng, Y., Eds.; CRC Press: New York, NY, USA, 2015; pp. 405–426. [Google Scholar]
- Diaz, J.H. Gnathostomiasis: An emerging infection of raw fish consumers in Gnathostoma nematode-endemic and nonendemic countries. J. Travel Med. 2015, 22, 318–324. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.H.; Sun, M.M.; Elsheikha, H.M.; Fu, Y.T.; Sugiyama, H.; Ando, K.; Sohn, W.M.; Zhu, X.Q.; Yao, C. Human gnathostomiasis: A neglected food-borne zoonosis. Parasites Vectors 2020, 13, 616. [Google Scholar] [CrossRef] [PubMed]
- Boongird, P.; Phuapradit, P.; Siridej, N.; Chirachariyavej, T.; Chuahirun, S.; Vejjajiva, A. Neurological manifestations of gnathostomiasis. J. Neurol. Sci. 1977, 31, 279–291. [Google Scholar] [CrossRef]
- Hung, M.N.; Huang, H.W.; Dekumyoy, P.; Pakdee, W.; Lee, Y.S.; Ji, D.D. First case of neurognathostomiasis in Taiwan—A Thai laborer presenting with eosinophilic meningitis and intracranial hemorrhage. J. Formos. Med. Assoc. 2015, 114, 1280–1284. [Google Scholar] [CrossRef]
- Hamilton, W.L.; Agranoff, D. Imported gnathostomiasis manifesting as cutaneous larva migrans and Löffler’s syndrome. BMJ Case Rep. 2018, 2018. [Google Scholar] [CrossRef]
- Benavides, M.A.; Baldo, M.B.; Tauber, S.; Figueiras, S.F.; Incani, R.N.; Nawa, Y. Case report: Ocular gnathostomiasis in Venezuela most likely acquired in Texas. Am. J. Trop. Med. Hyg. 2018, 99, 1028–1032. [Google Scholar] [CrossRef]
- Maleewong, W.; Morakote, N.; Thamasonthi, W.; Charuchinda, K.; Tesana, S.; Khamboonruang, C. Serodiagnosis of human gnathostomiasis. Southeast Asian J. Trop. Med. Public Health 1988, 19, 201–205. [Google Scholar]
- Nopparatana, C.; Setasuban, P.; Chaicumpa, W.; Tapchaisri, P. Purification of Gnathostoma spinigerum specific antigen and immunodiagnosis of human gnathostomiasis. Int. J. Parasitol. 1991, 21, 677–687. [Google Scholar] [CrossRef]
- Tuntipopipat, S.; Chawengkirttikul, R.; Sirisinha, S. A simplified method for the fractionation of Gnathostoma–specific antigens for serodiagnosis of human gnathostomosis. J. Helminthol. 1993, 67, 297–304. [Google Scholar] [CrossRef]
- Saksirisampant, W.; Chawengkiattikul, R.; Kraivichain, K.; Nuchprayoon, S. Specific IgE antibody responses to somatic and excretory–secretory antigens of third stage G. spinigerum larvae in human gnathostomiasis. J. Med. Assoc. Thail. 2001, 84 (Suppl. S1), S173–S181. [Google Scholar]
- Tapchaisri, P.; Nopparatana, C.; Chaicumpa, W.; Setasuban, P. Specific antigen of Gnathostoma spinigerum for immunodiagnosis of human gnathostomiasis. Int. J. Parasitol. 1991, 21, 315–319. [Google Scholar] [CrossRef]
- Anantaphruti, M.T.; Nuamtanong, S.; Dekumyoy, P. Diagnostic values of IgG4 in human gnathostomiasis. Trop. Med. Int. Health 2005, 10, 1013–1021. [Google Scholar] [CrossRef]
- Laummaunwai, P.; Sawanyawisuth, K.; Intapan, P.M.; Chotmongkol, V.; Wongkham, C.; Maleewong, W. Evaluation of human IgG class and subclass antibodies to a 24 kDa antigenic component of Gnathostoma spinigerum for the serodiagnosis of gnathostomiasis. Parasitol. Res. 2007, 101, 703–708. [Google Scholar] [CrossRef]
- Intapan, P.M.; Khotsri, P.; Kanpittaya, J.; Chotmongkol, V.; Sawanyawisuth, K.; Maleewong, W. Immunoblot diagnostic test for neurognathostomiasis. Am. J. Trop. Med. Hyg. 2010, 83, 927–929. [Google Scholar] [CrossRef]
- Janwan, P.; Intapan, P.M.; Yamasaki, H.; Laummaunwai, P.; Sawanyawisuth, K.; Wongkham, C.; Tayapiwatana, C.; Kitkhuandee, A.; Lulitanond, V.; Nawa, Y.; et al. Application of recombinant Gnathostoma spinigerum matrix metalloproteinase-like protein for serodiagnosis of human gnathostomiasis by immunoblotting. Am. J. Trop. Med. Hyg. 2013, 89, 63–67. [Google Scholar] [CrossRef]
- Janwan, P.; Intapan, P.M.; Yamasaki, H.; Rodpai, R.; Laummaunwai, P.; Thanchomnang, T.; Sanpool, O.; Kobayashi, K.; Takayama, K.; Kobayashi, Y.; et al. Development and usefulness of an immunochromatographic device to detect antibodies for rapid diagnosis of human gnathostomiasis. Parasites Vectors 2016, 9, 14. [Google Scholar] [CrossRef]
- Janwan, P.; Intapan, P.M.; Yamasaki, H.; Laummaunwai, P.; Sawanyawisuth, K.; Wongkham, C.; Tayapiwatana, C.; Kitkhuandee, A.; Lulitanond, V.; Nawa, Y.; et al. A recombinant matrix metalloproteinase protein from Gnathostoma spinigerum for serodiagnosis of neurognathostomiasis. Korean J. Parasitol. 2013, 51, 751–754. [Google Scholar] [CrossRef]
- Saenseeha, S.; Janwan, P.; Yamasaki, H.; Laummaunwai, P.; Tayapiwatana, C.; Kitkhuandee, A.; Maleewong, W.; Intapan, P.M. A dot-ELISA test using a Gnathostoma spinigerum recombinant matrix metalloproteinase protein for the serodiagnosis of human gnathostomiasis. Southeast Asian J. Trop. Med. Public Health 2014, 45, 990–996. [Google Scholar]
- O’Farrell, B. Lateral flow immunoassay systems: Evolution from the current state of the art to the next generation of highly sensitive, quantitative rapid assays. In The Immunoassay Handbook, 4th ed.; Wild, D., John, R., Sheehan, C., Binder, S., He, J., Eds.; Elsevier Science: Oxford, UK, 2013; pp. 89–107. [Google Scholar]
- Koczula, K.M.; Gallotta, A. Lateral flow assays. Essays Biochem. 2016, 60, 111–120. [Google Scholar]
- Elkins, D.B.; Haswell-Elkins, M.; Anderson, R.M. The epidemiology and control of intestinal helminths in the Pulicat Lake region of Southern India. I. Study design and pre–and post–treatment observations on Ascaris lumbricoides infection. Trans. R. Soc. Trop. Med. Hyg. 1986, 80, 774–792. [Google Scholar] [CrossRef]
- Intapan, P.M.; Khotsri, P.; Kanpittaya, J.; Chotmongkol, V.; Maleewong, W.; Morakote, N. Evaluation of IgG4 and total IgG antibodies against cysticerci and peptide antigens for the diagnosis of human neurocysticercosis by ELISA. Asian Pac. J. Allergy Immunol. 2008, 26, 237–244. [Google Scholar]
- Morakote, N.; Khamboonruang, C.; Siriprasert, V.; Suphawitayanukul, S.; Marcanantachoti, S.; Thamasonthi, W. The value of enzyme–linked immunosorbent assay (ELISA) for diagnosis of human trichinosis. Trop. Med. Parasitol. 1991, 42, 172–174. [Google Scholar]
- Maleewong, W.; Sombatsawat, P.; Intapan, P.M.; Wongkham, C.; Chotmongkol, V. Immunoblot evaluation of the specificity of the 29–kDa antigen from young adult female worms Angiostrongylus cantonensis for immunodiagnosis of human angiostrongyliasis. Asian Pac. J. Allergy Immunol. 2001, 19, 267–273. [Google Scholar] [PubMed]
- Sadaow, L.; Yamasaki, H.; Morishima, Y.; Sanpool, O.; Rodpai, R.; Janwan, P.; Boonroumkaew, P.; Maleewong, W.; Intapan, P.M. Effectiveness of Fasciola gigantica excretory–secretory and recombinant cathepsin L antigens for rapid diagnosis of human fascioliasis using immunochromatographic devices. Parasitol. Res. 2020, 119, 3691–3698. [Google Scholar] [CrossRef]
- Galen, R.S. Predictive value and efficiency of laboratory testing. Pediatr. Clin. N. Am. 1980, 27, 861–869. [Google Scholar] [CrossRef]
- Cook, G.C. The clinical significance of gastrointestinal helminths—A review. Trans. R. Soc. Trop. Med. Hyg. 1986, 80, 675–685. [Google Scholar] [CrossRef]
- Finsterer, J.; Auer, H. Parasitoses of the human central nervous system. J. Helminthol. 2013, 87, 257–270. [Google Scholar] [CrossRef]
- Cheepsattayakorn, A.; Cheepsattayakorn, R. Parasitic pneumonia and lung involvement. BioMed Res. Int. 2014, 2014, 874021. [Google Scholar] [CrossRef] [PubMed]
- Pal, M.; Ayele, Y.; Hadush, A.; Kundu, P.; Jadhav, V.J. Public health significance of foodborne helminthiasis: A systematic review. J. Exp. Food Chem. 2018, 4, 1–6. [Google Scholar] [CrossRef]
- Rodrigues, R.M.; de Oliveira, M.C.; Sopelete, M.C.; Silva, D.A.O.; Campos, D.M.B.; Taketomi, E.A.; Costa-Cruz, J.M. IgG1, IgG4, and IgE antibody responses in human strongyloidiasis by ELISA using Strongyloides ratti saline extract as heterologous antigen. Parasitol. Res. 2007, 101, 1209–1214. [Google Scholar] [CrossRef] [PubMed]
- Adjobimey, T.; Hoerauf, A. Induction of immunoglobulin G4 in human filariasis: An indicator of immunoregulation. Ann. Trop. Med. Parasitol. 2010, 104, 455–464. [Google Scholar] [CrossRef]
- Prodjinotho, U.F.; von Horn, C.; Debrah, A.Y.; Batsa Debrah, L.; Albers, A.; Layland, L.E.; Hoerauf, A.; Adjobimey, T. Pathological manifestations in lymphatic filariasis correlate with lack of inhibitory properties of IgG4 antibodies on IgE–activated granulocytes. PLoS Negl. Trop. Dis. 2017, 11, e0005777. [Google Scholar] [CrossRef]
- Arifin, N.; Hanafiah, K.M.; Ahmad, H.; Noordin, R. Serodiagnosis and early detection of Strongyloides stercoralis infection. J. Microbiol. Immunol. 2019, 52, 371–378. [Google Scholar] [CrossRef]
- Watanabe, T.; Ohkubo, Y.; Matsuoka, H.; Kimura, H.; Sakai, Y.; Ohkaru, Y.; Tanaka, T.; Kitaura, Y. Development of a simple whole blood panel test for detection of human heart–type fatty acid–binding protein. Clin. Biochem. 2001, 34, 257–263. [Google Scholar] [CrossRef]
- Golden, A.; Steel, C.; Yokobe, L.; Jackson, E.; Barney, R.; Kubofcik, J.; Peck, R.; Unnasch, T.R.; Nutman, T.B.; de los Santos, T.; et al. Extended result reading window in lateral flow tests detecting exposure to Onchocerca volvulus: A new technology to improve epidemiological surveillance tools. PLoS ONE 2013, 8, e69231. [Google Scholar] [CrossRef]
- Lu, Z.; Rey, E.; Vemulapati, S.; Srinivasan, B.; Mehta, S.; Erickson, D. High-yield paper-based quantitative blood separation system. Lab Chip 2018, 18, 3865–3871. [Google Scholar] [CrossRef]
- Foxdal, P.; Bergqvist, Y.; Eckerbom, S.; Sandhagen, B. Improving lactate analysis with the YSI 2300 GL: Hemolyzing blood samples makes results comparable with those for deproteinized whole blood. Clin. Chem. 1992, 38, 2110–2114. [Google Scholar] [CrossRef]
- Ono, T.; Sugiyama, K.; Kuroda, T.; Kawamura, M.; Arao, S.; Nariuchi, H. A quantitative immunochromatography assay of whole blood samples for antigen–specific IgE—A new method for point of care testing for allergens. Allergol. Int. 2005, 54, 393–399. [Google Scholar] [CrossRef][Green Version]
| Type of Simulated WBSs | Number Positive/Total Number |
|---|---|
| Healthy volunteers | 0/40 |
| Confirmed gnathostomiasis | 7/7 |
| Clinically suspected gnathostomiasis | 46/46 |
| Angiostrongyliasis cantonensis | 1/13 |
| Trichinellosis spiralis | 3/14 |
| Strongyloidiasis | 1/15 |
| Capillariasis philippinensis | 0/10 |
| Ascariasis | 0/10 |
| Trichuriasis | 0/10 |
| Hookworm infection | 0/10 |
| Paragonimiasis heterotremus | 2/13 |
| Fascioliasis gigantica | 0/10 |
| Opisthorchiasis viverrini | 1/15 |
| Sparganosis | 0/5 |
| Taeniasis saginata | 2/15 |
| Cysticercosis | 2/15 |
| Total number of tests | 248 |
| Numbers of true positive, true negative, false positive, and false negative results | 53, 183, 12, 0 |
| Accuracy (%) [95% CI] | 95.2 [91.7–97.5] |
| Sensitivity (%) [95% CI] | 100 [93.3–100] |
| Specificity (%) [95% CI] | 93.8 [89.5–96.8] |
| Positive predictive value (%) [95% CI] | 81.5 [70.0–90.1] |
| Negative predictive value (%) [95% CI] | 100 [98.0–100] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Janwan, P.; Intapan, P.M.; Sadaow, L.; Rodpai, R.; Yamasaki, H.; Boonroumkaew, P.; Sanpool, O.; Thanchomnang, T.; Sadee, P.; Maleewong, W. Development of Immunochromatographic Test Kit for Rapid Detection of Specific IgG4 Antibody in Whole-Blood Samples for Diagnosis of Human Gnathostomiasis. Diagnostics 2021, 11, 862. https://doi.org/10.3390/diagnostics11050862
Janwan P, Intapan PM, Sadaow L, Rodpai R, Yamasaki H, Boonroumkaew P, Sanpool O, Thanchomnang T, Sadee P, Maleewong W. Development of Immunochromatographic Test Kit for Rapid Detection of Specific IgG4 Antibody in Whole-Blood Samples for Diagnosis of Human Gnathostomiasis. Diagnostics. 2021; 11(5):862. https://doi.org/10.3390/diagnostics11050862
Chicago/Turabian StyleJanwan, Penchom, Pewpan M. Intapan, Lakkhana Sadaow, Rutchanee Rodpai, Hiroshi Yamasaki, Patcharaporn Boonroumkaew, Oranuch Sanpool, Tongjit Thanchomnang, Phuangphaka Sadee, and Wanchai Maleewong. 2021. "Development of Immunochromatographic Test Kit for Rapid Detection of Specific IgG4 Antibody in Whole-Blood Samples for Diagnosis of Human Gnathostomiasis" Diagnostics 11, no. 5: 862. https://doi.org/10.3390/diagnostics11050862
APA StyleJanwan, P., Intapan, P. M., Sadaow, L., Rodpai, R., Yamasaki, H., Boonroumkaew, P., Sanpool, O., Thanchomnang, T., Sadee, P., & Maleewong, W. (2021). Development of Immunochromatographic Test Kit for Rapid Detection of Specific IgG4 Antibody in Whole-Blood Samples for Diagnosis of Human Gnathostomiasis. Diagnostics, 11(5), 862. https://doi.org/10.3390/diagnostics11050862