State-of-the-Art Methods and Emerging Fluid Biomarkers in the Diagnostics of Dementia—A Short Review and Diagnostic Algorithm
Abstract
1. Introduction
2. ELISA-Based Detection of Markers in Biofluids
3. Single-Molecule Enzyme-Linked Immunosorbent Assay (SIMOA): The New Avenue to Peripheral Biomarkers in Neurodegenerative Dementias
4. Real-Time Quaking (RT-QuIC): A New and Promising State-of-the-Art Tool
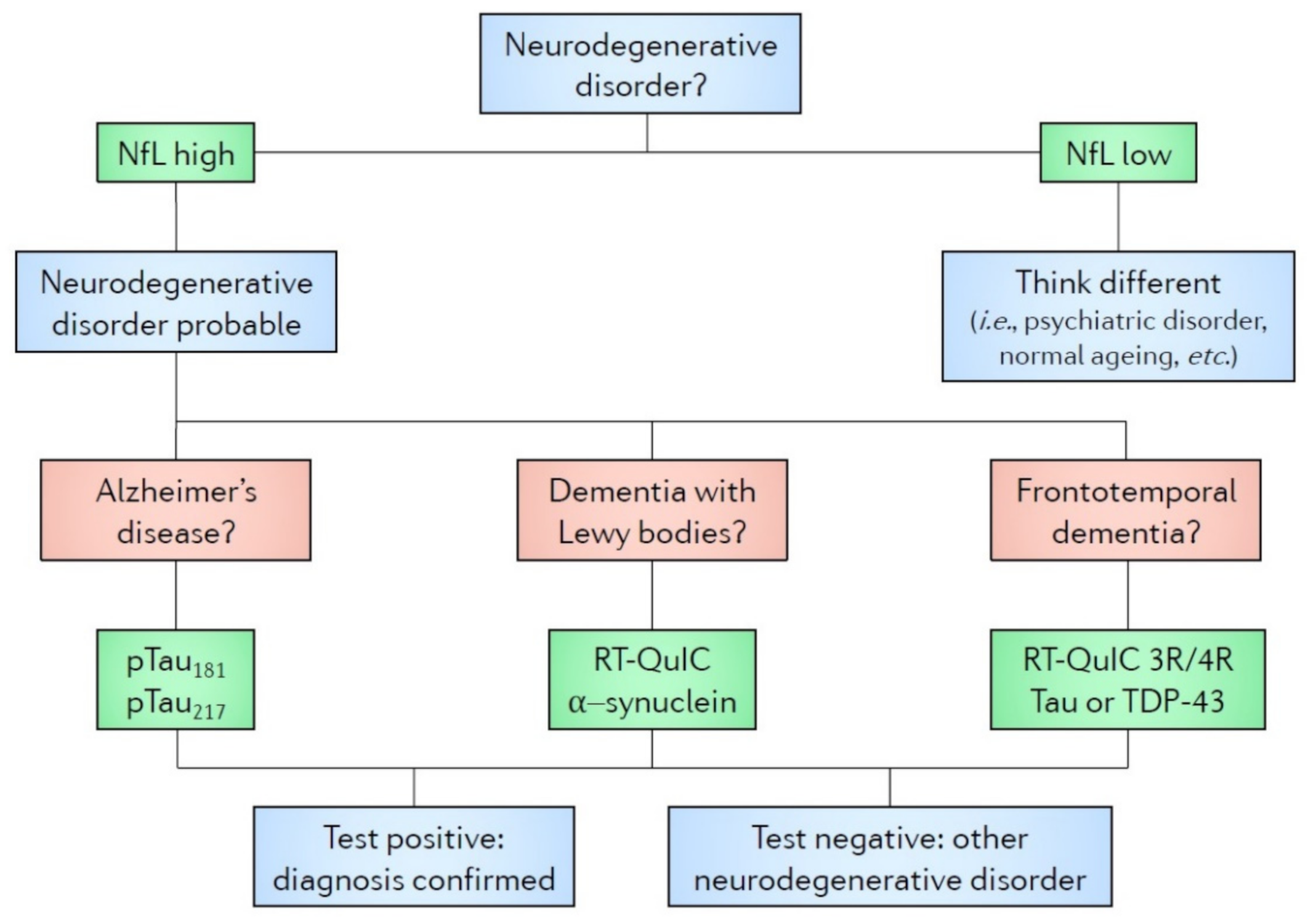
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Scheltens, P.; Blennow, K.; Breteler, M.M.B.; De Strooper, B.; Frisoni, G.B.; Salloway, S.; Van der Flier, W.M. Alzheimer’s disease. Lancet 2016, 388, 505–517. [Google Scholar] [CrossRef]
- Bang, J.; Spina, S.; Miller, B.L. Frontotemporal dementia. Lancet 2015, 386, 1672–1682. [Google Scholar] [CrossRef]
- Walker, Z.; Possin, K.L.; Boeve, B.F.; Aarsland, D. Lewy body dementias. Lancet 2015, 386, 1683–1697. [Google Scholar] [CrossRef]
- Warren, J.D.; Rohrer, J.D.; Rossor, M.N. Clinical review. Frontotemporal dementia. BMJ 2013, 347, f4827. [Google Scholar] [CrossRef]
- Kovacs, G.G.; Alafuzoff, I.; Al-Sarraj, S.; Arzberger, T.; Bogdanovic, N.; Capellari, S.; Ferrer, I.; Gelpi, E.; Kövari, V.; Kretzschmar, H.; et al. Mixed brain pathologies in dementia: The BrainNet Europe consortium experience. Dement. Geriatr. Cogn. Disord. 2008, 26, 343–350. [Google Scholar] [CrossRef]
- Alexovič, M.; Sabo, J.; Longuespée, R. Microproteomic sample preparation. Proteomics 2021. [Google Scholar] [CrossRef]
- Engvall, E.; Perlmann, P. Enzyme-Linked Immunosorbent Assay, Elisa. J. Immunol. 1972, 109, 129–135. [Google Scholar]
- Aydin, S. A short history, principles, and types of ELISA, and our laboratory experience with peptide/protein analyses using ELISA. Peptides 2015, 72, 4–15. [Google Scholar] [CrossRef]
- Vandermeeren, M.; Mercken, M.; Vanmechelen, E.; Six, J.; Voorde, A.; Martin, J.-J.; Cras, P. Detection of Proteins in Normal and Alzheimer’s Disease Cerebrospinal Fluid with a Sensitive Sandwich Enzyme-Linked Immunosorbent Assay. J. Neurochem. 1993, 61, 1828–1834. [Google Scholar] [CrossRef]
- Motter, R.; Vigo-Pelfrey, C.; Kholodenko, D.; Barbour, R.; Johnson-Wood, K.; Galasko, D.; Chang, L.; Miller, B.; Clark, C.; Green, R.; et al. Reduction of β-amyloid peptide42 in the cerebrospinal fluid of patients with Alzheimer’s disease. Ann. Neurol. 1995, 38, 643–648. [Google Scholar] [CrossRef]
- Bloudek, L.M.; Spackman, D.E.; Blankenburg, M.; Sullivan, S.D. Review and meta-analysis of biomarkers and diagnostic imaging in Alzheimer’s disease. J. Alzheimer’s Dis. 2011, 26, 627–645. [Google Scholar] [CrossRef]
- Song, B.; Ao, Q.; Wang, Z.; Liu, W.Q.; Niu, Y.; Shen, Q.; Zuo, H.C.; Zhang, X.F.; Gong, Y.D. Phosphorylation of tau protein over time in rats subjected to transient brain ischemia. Neural Regen. Res. 2013, 8, 3173–3182. [Google Scholar] [CrossRef]
- Kämälaïnen, A.; Herukka, S.K.; Hartikainen, P.; Helisalmi, S.; Moilanen, V.; Knuuttila, A.; Jansson, L.; Tienari, P.J.; Remes, A.M. Cerebrospinal fluid biomarkers for Alzheimer’s disease in patients with frontotemporal lobar degeneration and amyotrophic lateral sclerosis with the C9ORF72 repeat expansion. Dement. Geriatr. Cogn. Disord. 2015. [Google Scholar] [CrossRef]
- Chohan, G.; Pennington, C.; Mackenzie, J.M.; Andrews, M.; Everington, D.; Will, R.G.; Knight, R.S.G.; Green, A.J.E. The role of cerebrospinal fluid 14-3-3 and other proteins in the diagnosis of sporadic Creutzfeldt-Jakob disease in the UK: A 10-year review. J. Neurol. Neurosurg. Psychiatry 2010, 81, 1243–1248. [Google Scholar] [CrossRef]
- Bahl, J.M.C.; Heegaard, N.H.H.; Falkenhorst, G.; Laursen, H.; Høgenhaven, H.; Mølbak, K.; Jespersgaard, C.; Hougs, L.; Waldemar, G.; Johannsen, P.; et al. The diagnostic efficiency of biomarkers in sporadic Creutzfeldt-Jakob disease compared to Alzheimer’s disease. Neurobiol. Aging 2009, 30, 1834–1841. [Google Scholar] [CrossRef]
- Shea, Y.F.; Barker, W.; Greig-Gusto, M.T.; Loewenstein, D.A.; Duara, R.; Dekosky, S.T. Impact of Amyloid PET Imaging in the Memory Clinic: A Systematic Review and Meta-Analysis. J. Alzheimer’s Dis. 2018, 64, 323–335. [Google Scholar] [CrossRef]
- Fantoni, E.R.; Chalkidou, A.; O’Brien, J.T.; Farrar, G.; Hammers, A. A Systematic Review and Aggregated Analysis on the Impact of Amyloid PET Brain Imaging on the Diagnosis, Diagnostic Confidence, and Management of Patients being Evaluated for Alzheimer’s Disease. J. Alzheimer’s Dis. 2018, 63, 783–796. [Google Scholar] [CrossRef]
- Molinuevo, J.L.; Blennow, K.; Dubois, B.; Engelborghs, S.; Lewczuk, P.; Perret-Liaudet, A.; Teunissen, C.E.; Parnetti, L. The clinical use of cerebrospinal fluid biomarker testing for Alzheimer’s disease diagnosis: A consensus paper from the Alzheimer’s Biomarkers Standardization Initiative. Alzheimer’s Dement. 2014, 10, 808–817. [Google Scholar] [CrossRef]
- Vanderstichele, H.; Bibl, M.; Engelborghs, S.; Le Bastard, N.; Lewczuk, P.; Molinuevo, J.L.; Parnetti, L.; Perret-Liaudet, A.; Shaw, L.M.; Teunissen, C.; et al. Standardization of preanalytical aspects of cerebrospinal fluid biomarker testing for Alzheimer’s disease diagnosis: A consensus paper from the Alzheimer’s Biomarkers Standardization Initiative. Alzheimer’s Dement. 2012, 8, 65–73. [Google Scholar] [CrossRef]
- Mattsson, N.; Andreasson, U.; Persson, S.; Arai, H.; Batish, S.D.; Bernardini, S.; Bocchio-Chiavetto, L.; Blankenstein, M.A.; Carrillo, M.C.; Chalbot, S.; et al. The Alzheimer’s Association external quality control program for cerebrospinal fluid biomarkers. Alzheimer’s Dement. 2011, 7, 386–395.e6. [Google Scholar] [CrossRef]
- Rissin, D.M.; Kan, C.W.; Campbell, T.G.; Howes, S.C.; Fournier, D.R.; Song, L.; Piech, T.; Patel, P.P.; Chang, L.; Rivnak, A.J.; et al. Single-molecule enzyme-linked immunosorbent assay detects serum proteins at subfemtomolar concentrations. Nat. Biotechnol. 2010, 28, 595–599. [Google Scholar] [CrossRef] [PubMed]
- Rissin, D.M.; Walt, D.R. Digital readout of target binding with attomole detection limits via enzyme amplification in femtoliter arrays. J. Am. Chem. Soc. 2006, 128, 6286–6287. [Google Scholar] [CrossRef] [PubMed]
- Janelidze, S.; Stomrud, E.; Palmqvist, S.; Zetterberg, H.; Van Westen, D.; Jeromin, A.; Song, L.; Hanlon, D.; Tan Hehir, C.A.; Baker, D.; et al. Plasma β-amyloid in Alzheimer’s disease and vascular disease. Sci. Rep. 2016, 6. [Google Scholar] [CrossRef] [PubMed]
- Verberk, I.M.W.; Slot, R.E.; Verfaillie, S.C.J.; Heijst, H.; Prins, N.D.; van Berckel, B.N.M.; Scheltens, P.; Teunissen, C.E.; van der Flier, W.M. Plasma Amyloid as Prescreener for the Earliest Alzheimer Pathological Changes. Ann. Neurol. 2018, 84, 648–658. [Google Scholar] [CrossRef]
- Li, Q.; Berndt, M.; Bush, A.; Rumble, B.; Mackenzie, I.; Friedhuber, A.; Beyreuther, K.; Masters, C. Membrane-associated forms of the beta A4 amyloid protein precursor of Alzheimer’s disease in human platelet and brain: Surface expression on the activated human platelet. Blood 1994, 84, 133–142. [Google Scholar] [CrossRef]
- Startin, C.M.; Ashton, N.J.; Hamburg, S.; Hithersay, R.; Wiseman, F.K.; Mok, K.Y.; Hardy, J.; Lleó, A.; Lovestone, S.; Parnetti, L.; et al. Plasma biomarkers for amyloid, tau, and cytokines in Down syndrome and sporadic Alzheimer’s disease. Alzheimer’s Res. Ther. 2019, 11. [Google Scholar] [CrossRef]
- Koychev, I.; Jansen, K.; Dette, A.; Shi, L.; Holling, H. Blood-Based ATN Biomarkers of Alzheimer’s Disease: A Meta-Analysis. J. Alzheimer’s Dis. 2020, 79, 177–195. [Google Scholar] [CrossRef]
- De Wolf, F.; Ghanbari, M.; Licher, S.; McRae-McKee, K.; Gras, L.; Weverling, G.J.; Wermeling, P.; Sedaghat, S.; Ikram, M.K.; Waziry, R.; et al. Plasma tau, neurofilament light chain and amyloid-β levels and risk of dementia; a population-based cohort study. Brain 2020, 143, 1220–1232. [Google Scholar] [CrossRef]
- Stockmann, J.; Verberk, I.M.W.; Timmesfeld, N.; Denz, R.; Budde, B.; Lange-Leifhelm, J.; Scheltens, P.; van der Flier, W.M.; Nabers, A.; Teunissen, C.E.; et al. Amyloid-β misfolding as a plasma biomarker indicates risk for future clinical Alzheimer’s disease in individuals with subjective cognitive decline. Alzheimer’s Res. Ther. 2020, 12, 169. [Google Scholar] [CrossRef]
- Keshavan, A.; Pannee, J.; Karikari, T.K.; Lantero Rodriguez, J.; Ashton, N.J.; Nicholas, J.M.; Cash, D.M.; Coath, W.; Lane, C.A.; Parker, T.D.; et al. Skip Nav Destination Article Navigation Population-based blood screening for preclinical Alzheimer’s disease in a British birth cohort at age 70. Brain 2020. [Google Scholar] [CrossRef]
- Dage, J.L.; Wennberg, A.M.V.; Airey, D.C.; Hagen, C.E.; Knopman, D.S.; Machulda, M.M.; Roberts, R.O.; Jack, C.R.; Petersen, R.C.; Mielke, M.M. Levels of tau protein in plasma are associated with neurodegeneration and cognitive function in a population-based elderly cohort. Alzheimer’s Dement. 2016, 12, 1226–1234. [Google Scholar] [CrossRef]
- Zetterberg, H.; Wilson, D.; Andreasson, U.; Minthon, L.; Blennow, K.; Randall, J.; Hansson, O. Plasma tau levels in Alzheimer’s disease. Alzheimer’s Res. Ther. 2013, 5, 9. [Google Scholar] [CrossRef]
- Shi, M.; Kovac, A.; Korff, A.; Cook, T.J.; Ginghina, C.; Bullock, K.M.; Yang, L.; Stewart, T.; Zheng, D.; Aro, P.; et al. CNS tau efflux via exosomes is likely increased in Parkinson’s disease but not in Alzheimer’s disease. Alzheimer’s Dement. 2016, 12, 1125–1131. [Google Scholar] [CrossRef]
- Müller, S.; Preische, O.; Göpfert, J.C.; Yañez, V.A.C.; Joos, T.O.; Boecker, H.; Düzel, E.; Falkai, P.; Priller, J.; Buerger, K.; et al. Tau plasma levels in subjective cognitive decline: Results from the DELCODE study. Sci. Rep. 2017, 7, 9529. [Google Scholar] [CrossRef]
- Mattsson, N.; Zetterberg, H.; Janelidze, S.; Insel, P.S.; Andreasson, U.; Stomrud, E.; Palmqvist, S.; Baker, D.; Tan Hehir, C.A.; Jeromin, A.; et al. Plasma tau in Alzheimer disease. Neurology 2016, 87, 1827–1835. [Google Scholar] [CrossRef]
- Sugarman, M.A.; Zetterberg, H.; Blennow, K.; Tripodis, Y.; McKee, A.C.; Stein, T.D.; Martin, B.; Palmisano, J.N.; Steinberg, E.G.; Simkin, I.; et al. A longitudinal examination of plasma neurofilament light and total tau for the clinical detection and monitoring of Alzheimer’s disease. Neurobiol. Aging 2020, 94, 60–70. [Google Scholar] [CrossRef]
- Illán-Gala, I.; Lleo, A.; Karydas, A.; Staffaroni, A.M.; Zetterberg, H.; Sivasankaran, R.; Grinberg, L.T.; Spina, S.; Kramer, J.; Ramos, E.M.; et al. Plasma tau and neurofilament light in frontotemporal lobar degeneration and Alzheimer’s disease. Neurology 2021, 96, e671–e683. [Google Scholar] [CrossRef]
- Mielke, M.M.; Hagen, C.E.; Xu, J.; Chai, X.; Vemuri, P.; Lowe, V.J.; Airey, D.C.; Knopman, D.S.; Roberts, R.O.; Machulda, M.M.; et al. Plasma phospho-tau181 increases with Alzheimer’s disease clinical severity and is associated with tau- and amyloid-positron emission tomography. Alzheimer’s Dement. 2018, 14, 989–997. [Google Scholar] [CrossRef]
- Fossati, S.; Ramos Cejudo, J.; Debure, L.; Pirraglia, E.; Sone, J.Y.; Li, Y.; Chen, J.; Butler, T.; Zetterberg, H.; Blennow, K.; et al. Plasma tau complements CSF tau and P-tau in the diagnosis of Alzheimer’s disease. Alzheimer’s Dement. Diagn. Assess. Dis. Monit. 2019, 11, 483–492. [Google Scholar] [CrossRef]
- Benussi, A.; Karikari, T.K.; Ashton, N.; Gazzina, S.; Premi, E.; Benussi, L.; Ghidoni, R.; Rodriguez, J.L.; Emeršič, A.; Simrén, J.; et al. Diagnostic and prognostic value of serum NfL and p-Tau 181 in frontotemporal lobar degeneration. J. Neurol. Neurosurg. Psychiatry 2020, 91, 960–967. [Google Scholar] [CrossRef]
- Tatebe, H.; Kasai, T.; Ohmichi, T.; Kishi, Y.; Kakeya, T.; Waragai, M.; Kondo, M.; Allsop, D.; Tokuda, T. Quantification of plasma phosphorylated tau to use as a biomarker for brain Alzheimer pathology: Pilot case-control studies including patients with Alzheimer’s disease and down syndrome. Mol. Neurodegener. 2017, 12, 63. [Google Scholar] [CrossRef]
- Karikari, T.K.; Pascoal, T.A.; Ashton, N.J.; Janelidze, S.; Benedet, A.L.; Rodriguez, J.L.; Chamoun, M.; Savard, M.; Kang, M.S.; Therriault, J.; et al. Blood phosphorylated tau 181 as a biomarker for Alzheimer’s disease: A diagnostic performance and prediction modelling study using data from four prospective cohorts. Lancet Neurol. 2020, 19, 422–433. [Google Scholar] [CrossRef]
- Janelidze, S.; Mattsson, N.; Palmqvist, S.; Smith, R.; Beach, T.G.; Serrano, G.E.; Chai, X.; Proctor, N.K.; Eichenlaub, U.; Zetterberg, H.; et al. Plasma P-tau181 in Alzheimer’s disease: Relationship to other biomarkers, differential diagnosis, neuropathology and longitudinal progression to Alzheimer’s dementia. Nat. Med. 2020, 26, 379–386. [Google Scholar] [CrossRef]
- Thijssen, E.H.; La Joie, R.; Wolf, A.; Strom, A.; Wang, P.; Iaccarino, L.; Bourakova, V.; Cobigo, Y.; Heuer, H.; Spina, S.; et al. Diagnostic value of plasma phosphorylated tau181 in Alzheimer’s disease and frontotemporal lobar degeneration. Nat. Med. 2020, 26, 387–397. [Google Scholar] [CrossRef]
- Lantero Rodriguez, J.; Karikari, T.K.; Suárez-Calvet, M.; Troakes, C.; King, A.; Emersic, A.; Aarsland, D.; Hye, A.; Zetterberg, H.; Blennow, K.; et al. Plasma p-tau181 accurately predicts Alzheimer’s disease pathology at least 8 years prior to post-mortem and improves the clinical characterisation of cognitive decline. Acta Neuropathol. 2020, 140, 267–278. [Google Scholar] [CrossRef]
- Simrén, J.; Leuzy, A.; Karikari, T.K.; Hye, A.; Benedet, A.L.; Lantero-Rodriguez, J.; Mattsson-Carlgren, N.; Schöll, M.; Mecocci, P.; Vellas, B.; et al. The diagnostic and prognostic capabilities of plasma biomarkers in Alzheimer’s disease. Alzheimer’s Dement. 2021, 1–12. [Google Scholar] [CrossRef]
- Karikari, T.K.; Emeršič, A.; Vrillon, A.; Lantero-Rodriguez, J.; Ashton, N.J.; Kramberger, M.G.; Dumurgier, J.; Hourregue, C.; Čučnik, S.; Brinkmalm, G.; et al. Head-to-head comparison of clinical performance of CSF phospho-tau T181 and T217 biomarkers for Alzheimer’s disease diagnosis. Alzheimer’s Dement. 2020. [Google Scholar] [CrossRef]
- Ashton, N.J.; Pascoal, T.A.; Karikari, T.K.; Benedet, A.L.; Lantero-Rodriguez, J.; Brinkmalm, G.; Snellman, A.; Schöll, M.; Troakes, C.; Hye, A.; et al. Plasma p-tau231: A new biomarker for incipient Alzheimer’s disease pathology. Acta Neuropathol. 2021. [Google Scholar] [CrossRef]
- Chhatwal, J.P.; Schultz, A.P.; Dang, Y.; Ostaszewski, B.; Liu, L.; Yang, H.S.; Johnson, K.A.; Sperling, R.A.; Selkoe, D.J. Plasma N-terminal tau fragment levels predict future cognitive decline and neurodegeneration in healthy elderly individuals. Nat. Commun. 2020, 11, 6024. [Google Scholar] [CrossRef]
- Cicognola, C.; Brinkmalm, G.; Wahlgren, J.; Portelius, E.; Gobom, J.; Cullen, N.C.; Hansson, O.; Parnetti, L.; Constantinescu, R.; Wildsmith, K.; et al. Novel tau fragments in cerebrospinal fluid: Relation to tangle pathology and cognitive decline in Alzheimer’s disease. Acta Neuropathol. 2019, 137, 279–296. [Google Scholar] [CrossRef]
- Cicognola, C.; Hansson, O.; Scheltens, P.; Kvartsberg, H.; Zetterberg, H.; Teunissen, C.E.; Blennow, K. Cerebrospinal fluid N-224 tau helps discriminate Alzheimer’s disease from subjective cognitive decline and other dementias. Alzheimers. Res. Ther. 2021, 13, 38. [Google Scholar] [CrossRef] [PubMed]
- Yuan, A.; Rao, M.V.; Veeranna; Nixon, R.A. Neurofilaments at a glance. J. Cell Sci. 2012, 125, 3257–3263. [Google Scholar] [CrossRef] [PubMed]
- Kuhle, J.; Barro, C.; Andreasson, U.; Derfuss, T.; Lindberg, R.; Sandelius, Å.; Liman, V.; Norgren, N.; Blennow, K.; Zetterberg, H. Comparison of three analytical platforms for quantification of the neurofilament light chain in blood samples: ELISA, electrochemiluminescence immunoassay and Simoa. Clin. Chem. Lab. Med. 2016, 54, 1655–1661. [Google Scholar] [CrossRef] [PubMed]
- Hansson, O.; Janelidze, S.; Hall, S.; Magdalinou, N.; Lees, A.J.; Andreasson, U.; Norgren, N.; Linder, J.; Forsgren, L.; Constantinescu, R.; et al. Blood-based NfL: A biomarker for differential diagnosis of parkinsonian disorder. Neurology 2017, 88, 930–937. [Google Scholar] [CrossRef]
- Zhou, W.; Zhang, J.; Ye, F.; Xu, G.; Su, H.; Su, Y.; Zhang, X. Plasma neurofilament light chain levels in Alzheimer’s disease. Neurosci. Lett. 2017, 650, 60–64. [Google Scholar] [CrossRef]
- Lin, Y.S.; Lee, W.J.; Wang, S.J.; Fuh, J.L. Levels of plasma neurofilament light chain and cognitive function in patients with Alzheimer or Parkinson disease. Sci. Rep. 2018, 8. [Google Scholar] [CrossRef]
- Ashton, N.J.; Leuzy, A.; Lim, Y.M.; Troakes, C.; Hortobágyi, T.; Höglund, K.; Aarsland, D.; Lovestone, S.; Schöll, M.; Blennow, K.; et al. Increased plasma neurofilament light chain concentration correlates with severity of post-mortem neurofibrillary tangle pathology and neurodegeneration. Acta Neuropathol. Commun. 2019, 7, 5. [Google Scholar] [CrossRef]
- Mattsson, N.; Andreasson, U.; Zetterberg, H.; Blennow, K.; Alzheimer’s Disease Neuroimaging Initiative. Association of Plasma Neurofilament Light With Neurodegeneration in Patients With Alzheimer Disease. JAMA Neurol. 2017, 74, 557. [Google Scholar] [CrossRef]
- Lewczuk, P.; Ermann, N.; Andreasson, U.; Schultheis, C.; Podhorna, J.; Spitzer, P.; Maler, J.M.; Kornhuber, J.; Blennow, K.; Zetterberg, H. Plasma neurofilament light as a potential biomarker of neurodegeneration in Alzheimer’s disease. Alzheimer’s Res. Ther. 2018, 10. [Google Scholar] [CrossRef]
- Liu, S.; Huang, Z.; Zhang, L.; Pan, J.; Lei, Q.; Meng, Y.; Li, Z. Plasma Neurofilament Light Chain May Be a Biomarker for the Inverse Association Between Cancers and Neurodegenerative Diseases. Front. Aging Neurosci. 2020, 12. [Google Scholar] [CrossRef]
- Ma, W.; Zhang, J.; Xu, J.; Feng, D.; Wang, X.; Zhang, F. Elevated Levels of Serum Neurofilament Light Chain Associated with Cognitive Impairment in Vascular Dementia. Dis. Markers 2020, 2020, 6612871. [Google Scholar] [CrossRef]
- Cajanus, A.; Katisko, K.; Kontkanen, A.; Jääskeläinen, O.; Hartikainen, P.; Haapasalo, A.; Herukka, S.K.; Vanninen, R.; Solje, E.; Hall, A.; et al. Serum neurofilament light chain in FTLD: Association with C9orf72, clinical phenotype, and prognosis. Ann. Clin. Transl. Neurol. 2020, 7, 903–910. [Google Scholar] [CrossRef]
- Mielke, M.M.; Syrjanen, J.A.; Blennow, K.; Zetterberg, H.; Vemuri, P.; Skoog, I.; Machulda, M.M.; Kremers, W.K.; Knopman, D.S.; Jack, C.; et al. Plasma and CSF neurofilament light: Relation to longitudinal neuroimaging and cognitive measures. Neurology 2019, 93, E252–E260. [Google Scholar] [CrossRef]
- Katisko, K.; Cajanus, A.; Jääskeläinen, O.; Kontkanen, A.; Hartikainen, P.; Korhonen, V.E.; Helisalmi, S.; Haapasalo, A.; Koivumaa-Honkanen, H.; Herukka, S.K.; et al. Serum neurofilament light chain is a discriminative biomarker between frontotemporal lobar degeneration and primary psychiatric disorders. J. Neurol. 2020, 267, 162–167. [Google Scholar] [CrossRef]
- Shahim, P.; Gren, M.; Liman, V.; Andreasson, U.; Norgren, N.; Tegner, Y.; Mattsson, N.; Andreasen, N.; Öst, M.; Zetterberg, H.; et al. Serum neurofilament light protein predicts clinical outcome in traumatic brain injury. Sci. Rep. 2016, 6. [Google Scholar] [CrossRef]
- Thebault, S.; Abdoli, M.; Fereshtehnejad, S.M.; Tessier, D.; Tabard-Cossa, V.; Freedman, M.S. Serum neurofilament light chain predicts long term clinical outcomes in multiple sclerosis. Sci. Rep. 2020, 10. [Google Scholar] [CrossRef]
- Benedet, A.L.; Leuzy, A.; Pascoal, T.A.; Ashton, N.J.; Mathotaarachchi, S.; Savard, M.; Therriault, J.; Kang, M.S.; Chamoun, M.; Schöll, M.; et al. Stage-specific links between plasma neurofilament light and imaging biomarkers of Alzheimer’s disease. Brain 2021, 143, 3793–3804. [Google Scholar] [CrossRef]
- Nyberg, L.; Lundquist, A.; Nordin Adolfsson, A.; Andersson, M.; Zetterberg, H.; Blennow, K.; Adolfsson, R. Elevated plasma neurofilament light in aging reflects brain white-matter alterations but does not predict cognitive decline or Alzheimer’s disease. Alzheimer’s Dement. Diagn. Assess. Dis. Monit. 2020, 12, e12050. [Google Scholar] [CrossRef]
- Verberk, I.M.W.; Thijssen, E.; Koelewijn, J.; Mauroo, K.; Vanbrabant, J.; De Wilde, A.; Zwan, M.D.; Verfaillie, S.C.J.; Ossenkoppele, R.; Barkhof, F.; et al. Combination of plasma amyloid beta(1-42/1-40)and glial fibrillary acidic protein strongly associates with cerebral amyloid pathology. Alzheimer’s Res. Ther. 2020, 12, 118. [Google Scholar] [CrossRef]
- Benussi, A.; Ashton, N.J.; Karikari, T.K.; Gazzina, S.; Premi, E.; Benussi, L.; Ghidoni, R.; Rodriguez, J.L.; Emeršič, A.; Binetti, G.; et al. Serum Glial Fibrillary Acidic Protein (GFAP) Is a Marker of Disease Severity in Frontotemporal Lobar Degeneration. J. Alzheimer’s Dis. 2020, 29, 1–13. [Google Scholar] [CrossRef]
- Heller, C.; Foiani, M.S.; Moore, K.; Convery, R.; Bocchetta, M.; Neason, M.; Cash, D.M.; Thomas, D.; Greaves, C.V.; Woollacott, I.O.C.; et al. Plasma glial fibrillary acidic protein is raised in progranulin-associated frontotemporal dementia. J. Neurol. Neurosurg. Psychiatry 2020, 91, 263–270. [Google Scholar] [CrossRef]
- Chatterjee, P.; Pedrini, S.; Stoops, E.; Goozee, K.; Villemagne, V.L.; Asih, P.R.; Verberk, I.M.W.; Dave, P.; Taddei, K.; Sohrabi, H.R.; et al. Plasma glial fibrillary acidic protein is elevated in cognitively normal older adults at risk of Alzheimer’s disease. Transl. Psychiatry 2021, 11. [Google Scholar] [CrossRef]
- Högel, H.; Rissanen, E.; Barro, C.; Matilainen, M.; Nylund, M.; Kuhle, J.; Airas, L. Serum glial fibrillary acidic protein correlates with multiple sclerosis disease severity. Mult. Scler. J. 2020, 26, 210–219. [Google Scholar] [CrossRef]
- Nass, R.D.; Akgün, K.; Elger, C.; Reichmann, H.; Wagner, M.; Surges, R.; Ziemssen, T. Serum biomarkers of cerebral cellular stress after self limited tonic clonic seizures: An exploratory study. Seizure 2021, 85, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Huebschmann, N.A.; Luoto, T.M.; Karr, J.E.; Berghem, K.; Blennow, K.; Zetterberg, H.; Ashton, N.J.; Simrén, J.; Posti, J.P.; Gill, J.M.; et al. Comparing Glial Fibrillary Acidic Protein (GFAP) in Serum and Plasma Following Mild Traumatic Brain Injury in Older Adults. Front. Neurol. 2020, 11, 1054. [Google Scholar] [CrossRef]
- Atarashi, R.; Satoh, K.; Sano, K.; Fuse, T.; Yamaguchi, N.; Ishibashi, D.; Matsubara, T.; Nakagaki, T.; Yamanaka, H.; Shirabe, S.; et al. Ultrasensitive human prion detection in cerebrospinal fluid by real-time quaking-induced conversion. Nat. Med. 2011, 17, 175–178. [Google Scholar] [CrossRef]
- Wilham, J.M.; Orrú, C.D.; Bessen, R.A.; Atarashi, R.; Sano, K.; Race, B.; Meade-White, K.D.; Taubner, L.M.; Timmes, A.; Caughey, B. Rapid end-point quantitation of prion seeding activity with sensitivity comparable to bioassays. PLoS Pathog. 2010, 6, e1001217. [Google Scholar] [CrossRef]
- Saijo, E.; Groveman, B.R.; Kraus, A.; Metrick, M.; Orrù, C.D.; Hughson, A.G.; Caughey, B. Ultrasensitive rt-quic seed amplification assays for disease-associated tau, α-synuclein, and prion aggregates. In Methods in Molecular Biology; Humana Press Inc.: Totowa, NY, USA, 2019; Volume 1873, pp. 19–37. [Google Scholar]
- Orrú, C.D.; Groveman, B.R.; Hughson, A.G.; Zanusso, G.; Coulthart, M.B.; Caughey, B. Rapid and sensitive RT-QuIC detection of human creutzfeldt-jakob disease using cerebrospinal fluid. MBio 2015, 6, e02451-14. [Google Scholar] [CrossRef]
- Groveman, B.R.; Orrú, C.D.; Hughson, A.G.; Bongianni, M.; Fiorini, M.; Imperiale, D.; Ladogana, A.; Pocchiari, M.; Zanusso, G.; Caughey, B. Extended and direct evaluation of RT-QuIC assays for Creutzfeldt-Jakob disease diagnosis. Ann. Clin. Transl. Neurol. 2017, 4, 139–144. [Google Scholar] [CrossRef]
- Franceschini, A.; Baiardi, S.; Hughson, A.G.; McKenzie, N.; Moda, F.; Rossi, M.; Capellari, S.; Green, A.; Giaccone, G.; Caughey, B.; et al. High diagnostic value of second generation CSF RT-QuIC across the wide spectrum of CJD prions. Sci. Rep. 2017, 7. [Google Scholar] [CrossRef]
- Foutz, A.; Appleby, B.S.; Hamlin, C.; Liu, X.; Yang, S.; Cohen, Y.; Chen, W.; Blevins, J.; Fausett, C.; Wang, H.; et al. Diagnostic and prognostic value of human prion detection in cerebrospinal fluid. Ann. Neurol. 2017, 81, 79–92. [Google Scholar] [CrossRef] [PubMed]
- Orrú, C.D.; Bongianni, M.; Tonoli, G.; Ferrari, S.; Hughson, A.G.; Groveman, B.R.; Fiorini, M.; Pocchiari, M.; Monaco, S.; Caughey, B.; et al. A Test for Creutzfeldt–Jakob Disease Using Nasal Brushings. N. Engl. J. Med. 2014, 371, 519–529. [Google Scholar] [CrossRef] [PubMed]
- Bongianni, M.; Orrù, C.; Groveman, B.R.; Sacchetto, L.; Fiorini, M.; Tonoli, G.; Triva, G.; Capaldi, S.; Testi, S.; Ferrari, S.; et al. Diagnosis of Human Prion Disease Using Real-Time Quaking-Induced Conversion Testing of Olfactory Mucosa and Cerebrospinal Fluid Samples. JAMA Neurol. 2017, 74, 155. [Google Scholar] [CrossRef]
- Orrú, C.D.; Yuan, J.; Appleby, B.S.; Li, B.; Li, Y.; Winner, D.; Wang, Z.; Zhan, Y.A.; Rodgers, M.; Rarick, J.; et al. Prion seeding activity and infectivity in skin samples from patients with sporadic Creutzfeldt-Jakob disease. Sci. Transl. Med. 2017, 9. [Google Scholar] [CrossRef]
- Orrú, C.D.; Groveman, B.R.; Foutz, A.; Bongianni, M.; Cardone, F.; McKenzie, N.; Culeux, A.; Poleggi, A.; Grznarova, K.; Perra, D.; et al. Ring trial of 2nd generation RT-QuIC diagnostic tests for sporadic CJD. Ann. Clin. Transl. Neurol. 2020, 7, 2262–2271. [Google Scholar] [CrossRef]
- Hermann, P.; Laux, M.; Glatzel, M.; Matschke, J.; Knipper, T.; Goebel, S.; Treig, J.; Schulz-Schaeffer, W.; Cramm, M.; Schmitz, M.; et al. Validation and utilization of amended diagnostic criteria in Creutzfeldt-Jakob disease surveillance. Neurology 2018, 91, e331–e338. [Google Scholar] [CrossRef]
- Fairfoul, G.; McGuire, L.I.; Pal, S.; Ironside, J.W.; Neumann, J.; Christie, S.; Joachim, C.; Esiri, M.; Evetts, S.G.; Rolinski, M.; et al. Alpha-synuclein RT-QuIC in the CSF of patients with alpha-synucleinopathies. Ann. Clin. Transl. Neurol. 2016, 3, 812–818. [Google Scholar] [CrossRef]
- Groveman, B.R.; Orrù, C.D.; Hughson, A.G.; Raymond, L.D.; Zanusso, G.; Ghetti, B.; Campbell, K.J.; Safar, J.; Galasko, D.; Caughey, B. Rapid and ultra-sensitive quantitation of disease-associated α-synuclein seeds in brain and cerebrospinal fluid by αSyn RT-QuIC. Acta Neuropathol. Commun. 2018, 6. [Google Scholar] [CrossRef]
- Bongianni, M.; Ladogana, A.; Capaldi, S.; Klotz, S.; Baiardi, S.; Cagnin, A.; Perra, D.; Fiorini, M.; Poleggi, A.; Legname, G.; et al. α-Synuclein RT-QuIC assay in cerebrospinal fluid of patients with dementia with Lewy bodies. Ann. Clin. Transl. Neurol. 2019, 6, 2120–2126. [Google Scholar] [CrossRef]
- Van Rumund, A.; Green, A.J.E.; Fairfoul, G.; Esselink, R.A.J.; Bloem, B.R.; Verbeek, M.M. α-Synuclein real-time quaking-induced conversion in the cerebrospinal fluid of uncertain cases of parkinsonism. Ann. Neurol. 2019, 85, 777–781. [Google Scholar] [CrossRef]
- Manne, S.; Kondru, N.; Jin, H.; Serrano, G.E.; Anantharam, V.; Kanthasamy, A.; Adler, C.H.; Beach, T.G.; Kanthasamy, A.G. Blinded RT-QuIC Analysis of α-Synuclein Biomarker in Skin Tissue from Parkinson’s Disease Patients. Mov. Disord. 2020, 35. [Google Scholar] [CrossRef] [PubMed]
- Sano, K.; Atarashi, R.; Satoh, K.; Ishibashi, D.; Nakagaki, T.; Iwasaki, Y.; Yoshida, M.; Murayama, S.; Mishima, K.; Nishida, N. Prion-Like Seeding of Misfolded α-Synuclein in the Brains of Dementia with Lewy Body Patients in RT-QUIC. Mol. Neurobiol. 2018, 55, 3916–3930. [Google Scholar] [CrossRef] [PubMed]
- Han, J.Y.; Jang, H.S.; Green, A.J.E.; Choi, Y.P. RT-QuIC-based detection of alpha-synuclein seeding activity in brains of dementia with Lewy Body patients and of a transgenic mouse model of synucleinopathy. Prion 2020, 14, 88–94. [Google Scholar] [CrossRef] [PubMed]
- Rossi, M.; Candelise, N.; Baiardi, S.; Capellari, S.; Giannini, G.; Orrù, C.D.; Antelmi, E.; Mammana, A.; Hughson, A.G.; Calandra-Buonaura, G.; et al. Ultrasensitive RT-QuIC assay with high sensitivity and specificity for Lewy body-associated synucleinopathies. Acta Neuropathol. 2020, 140, 49–62. [Google Scholar] [CrossRef]
- Saijo, E.; Ghetti, B.; Zanusso, G.; Oblak, A.; Furman, J.L.; Diamond, M.I.; Kraus, A.; Caughey, B. Ultrasensitive and selective detection of 3-repeat tau seeding activity in Pick disease brain and cerebrospinal fluid. Acta Neuropathol. 2017, 133, 751–765. [Google Scholar] [CrossRef]
- Fitzpatrick, A.W.P.; Falcon, B.; He, S.; Murzin, A.G.; Murshudov, G.; Garringer, H.J.; Crowther, R.A.; Ghetti, B.; Goedert, M.; Scheres, S.H.W. Cryo-EM structures of tau filaments from Alzheimer’s disease. Nature 2017, 547, 185–190. [Google Scholar] [CrossRef]
- Goedert, M.; Eisenberg, D.S.; Crowther, R.A. Propagation of Tau Aggregates and Neurodegeneration. Annu. Rev. Neurosci. 2017, 40, 189–210. [Google Scholar] [CrossRef]
- Kraus, A.; Saijo, E.; Metrick, M.A.; Newell, K.; Sigurdson, C.J.; Zanusso, G.; Ghetti, B.; Caughey, B. Seeding selectivity and ultrasensitive detection of tau aggregate conformers of Alzheimer disease. Acta Neuropathol. 2019, 137, 585–598. [Google Scholar] [CrossRef]
- Saijo, E.; Metrick, M.A.; Koga, S.; Parchi, P.; Litvan, I.; Spina, S.; Boxer, A.; Rojas, J.C.; Galasko, D.; Kraus, A.; et al. 4-Repeat tau seeds and templating subtypes as brain and CSF biomarkers of frontotemporal lobar degeneration. Acta Neuropathol. 2020, 139, 63–77. [Google Scholar] [CrossRef]
- Metrick, M.A.; Ferreira, N.D.C.; Saijo, E.; Kraus, A.; Newell, K.; Zanusso, G.; Vendruscolo, M.; Ghetti, B.; Caughey, B. A single ultrasensitive assay for detection and discrimination of tau aggregates of Alzheimer and Pick diseases. Acta Neuropathol. Commun. 2020, 8. [Google Scholar] [CrossRef]
- Tennant, J.M.; Henderson, D.M.; Wisniewski, T.M.; Hoover, E.A. RT-QuIC detection of tauopathies using full-length tau substrates. Prion 2020, 14, 249–256. [Google Scholar] [CrossRef]
- Birsa, N.; Bentham, M.P.; Fratta, P. Cytoplasmic functions of TDP-43 and FUS and their role in ALS. Semin Cell Dev Biol. 2020, 99, 193–201. [Google Scholar] [CrossRef]
- Scialò, C.; Tran, T.H.; Salzano, G.; Novi, G.; Caponnetto, C.; Chiò, A.; Calvo, A.; Canosa, A.; Moda, F.; Caroppo, P.; et al. TDP-43 real-time quaking induced conversion reaction optimization and detection of seeding activity in CSF of amyotrophic lateral sclerosis and frontotemporal dementia patients. Brain Commun. 2020, 2. [Google Scholar] [CrossRef]
- Korhonen, T.; Katisko, K.; Cajanus, A.; Hartikainen, P.; Koivisto, A.M.; Haapasalo, A.; Remes, A.M.; Solje, E. Comparison of Prodromal Symptoms of Patients with Behavioral Variant Frontotemporal Dementia and Alzheimer Disease. Dement. Geriatr. Cogn. Disord. 2020, 49, 98–106. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Matarrubia, M.; Matías-Guiu, J.A.; Cabrera-Martín, M.N.; Moreno-Ramos, T.; Valles-Salgado, M.; Carreras, J.L.; Matías-Guiu, J. Episodic Memory Dysfunction in Behavioral Variant Frontotemporal Dementia: A Clinical And FDG-PET Study. J. Alzheimers. Dis. 2017, 57, 1251–1264. [Google Scholar] [CrossRef] [PubMed]
- Foguem, C.; Manckoundia, P. Lewy Body Disease: Clinical and Pathological “Overlap Syndrome” Between Synucleinopathies (Parkinson Disease) and Tauopathies (Alzheimer Disease). Curr. Neurol. Neurosci. Rep. 2018, 18, 24. [Google Scholar] [CrossRef] [PubMed]
- Orad, R.I.; Shiner, T. Differentiating dementia with Lewy bodies from Alzheimer’s disease and Parkinson’s disease dementia: An update on imaging modalities. J. Neurol. 2021. [Google Scholar] [CrossRef] [PubMed]
- Cajanus, A.; Hall, A.; Koikkalainen, J.; Solje, E.; Tolonen, A.; Urhemaa, T.; Liu, Y.; Haanpää, R.M.; Hartikainen, P.; Helisalmi, S.; et al. Automatic MRI Quantifying Methods in Behavioral-Variant Frontotemporal Dementia Diagnosis. Dement. Geriatr. Cogn. Dis. Extra 2018. [Google Scholar] [CrossRef]
- Shaw, L.M.; Vanderstichele, H.; Knapik-Czajka, M.; Clark, C.M.; Aisen, P.S.; Petersen, R.C.; Blennow, K.; Soares, H.; Simon, A.; Lewczuk, P.; et al. Cerebrospinal fluid biomarker signature in Alzheimer’s disease neuroimaging initiative subjects. Ann. Neurol. 2009, 65, 403–413. [Google Scholar] [CrossRef]
- Visser, P.J.; Verhey, F.; Knol, D.L.; Scheltens, P.; Wahlund, L.-O.; Freund-Levi, Y.; Tsolaki, M.; Minthon, L.; Wallin, Å.K.; Hampel, H.; et al. Prevalence and prognostic value of CSF markers of Alzheimer’s disease pathology in patients with subjective cognitive impairment or mild cognitive impairment in the DESCRIPA study: A prospective cohort study. Lancet Neurol. 2009, 8, 619–627. [Google Scholar] [CrossRef]
- Feneberg, E.; Gray, E.; Ansorge, O.; Talbot, K.; Turner, M.R. towards a TDP-43-Based Biomarker for ALS and FTLD. Mol. Neurobiol. 2018, 55, 7789–7801. [Google Scholar] [CrossRef] [PubMed]
- Hill, J.W.; Futterman, R.; Duttagupta, S.; Mastey, V.; Lloyd, J.R.; Fillit, H. Alzheimer’s disease and related dementias increase costs of comorbidities in managed medicare. Neurology 2002, 58, 62–70. [Google Scholar] [CrossRef] [PubMed]
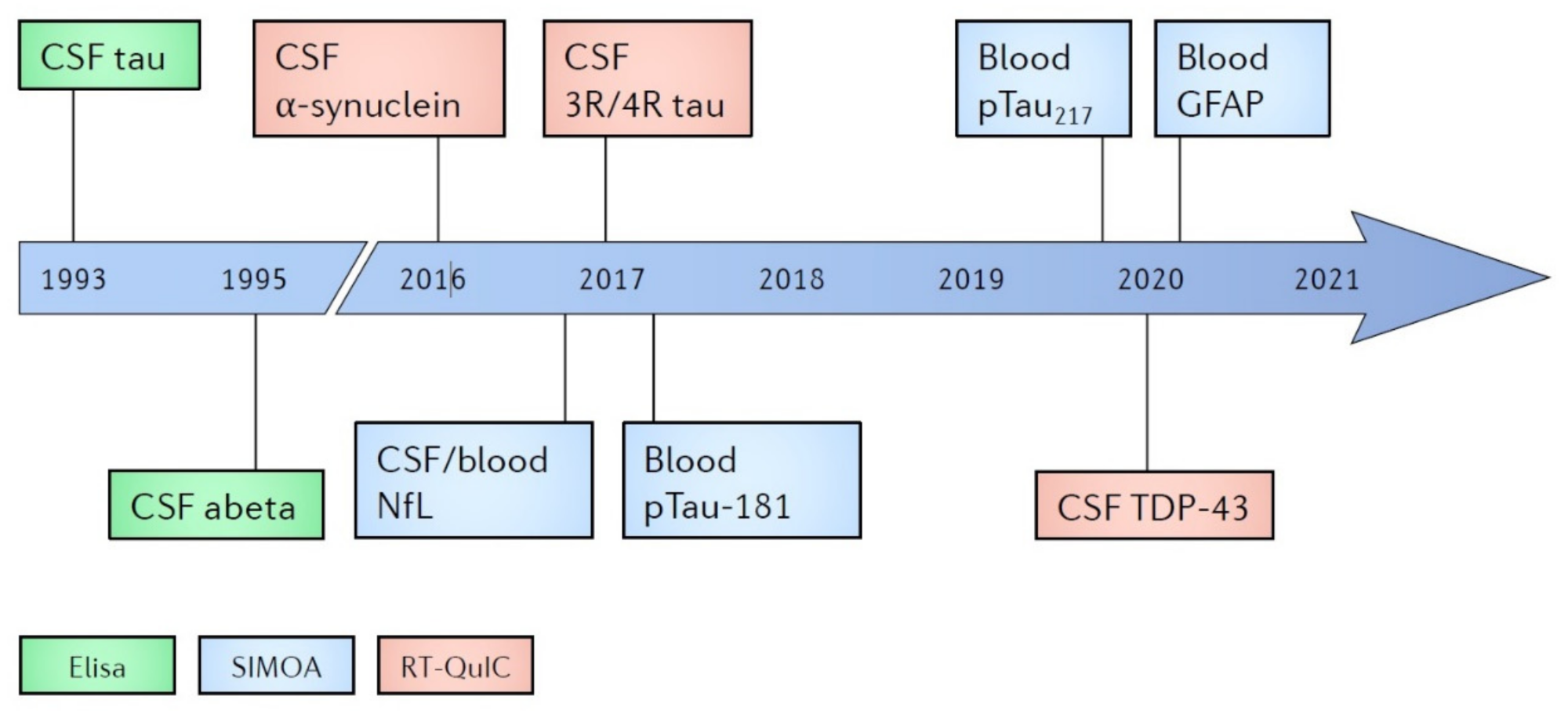
| Marker | Diagnostic | Neurodegeneration | Prognostic |
|---|---|---|---|
| ELISA | |||
| CSF Aβ42 CSF tau | AD | ||
| Yes | |||
| SIMOA | |||
| CSF and blood NfL Blood GFAP Blood pTau181 Blood pTau217 | Yes | AD, FTD | |
| Yes | |||
| AD | AD | ||
| AD | |||
| RT-QuIC | |||
| CSF α-synuclein | DLB | ||
| CSF 3R/4R tau | AD, FTD | ||
| CSF TDP-43 | FTD | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Solje, E.; Benussi, A.; Buratti, E.; Remes, A.M.; Haapasalo, A.; Borroni, B. State-of-the-Art Methods and Emerging Fluid Biomarkers in the Diagnostics of Dementia—A Short Review and Diagnostic Algorithm. Diagnostics 2021, 11, 788. https://doi.org/10.3390/diagnostics11050788
Solje E, Benussi A, Buratti E, Remes AM, Haapasalo A, Borroni B. State-of-the-Art Methods and Emerging Fluid Biomarkers in the Diagnostics of Dementia—A Short Review and Diagnostic Algorithm. Diagnostics. 2021; 11(5):788. https://doi.org/10.3390/diagnostics11050788
Chicago/Turabian StyleSolje, Eino, Alberto Benussi, Emanuele Buratti, Anne M. Remes, Annakaisa Haapasalo, and Barbara Borroni. 2021. "State-of-the-Art Methods and Emerging Fluid Biomarkers in the Diagnostics of Dementia—A Short Review and Diagnostic Algorithm" Diagnostics 11, no. 5: 788. https://doi.org/10.3390/diagnostics11050788
APA StyleSolje, E., Benussi, A., Buratti, E., Remes, A. M., Haapasalo, A., & Borroni, B. (2021). State-of-the-Art Methods and Emerging Fluid Biomarkers in the Diagnostics of Dementia—A Short Review and Diagnostic Algorithm. Diagnostics, 11(5), 788. https://doi.org/10.3390/diagnostics11050788






