A Review of Biophysiological and Biochemical Indicators of Stress for Connected and Preventive Healthcare
Abstract
1. Introduction
2. Search Methodology
- In the first stage, studies related to mental and physical stress monitoring using biophysiological and biochemical parameters were included by reviewing the title and abstract of the papers, which resulted in 72 articles reporting the studies on human stress monitoring using biophysiological and biochemical methods.
- In the second stage, out of 72 articles, 38 original studies were selected for further review. A detailed review of the 38 selected manuscripts was performed to obtain information on the type of sensors, methods of stress induction, number of subjects volunteered for experimentation, and findings of authors about the use of the specified indicators of stress. Two additional papers identified during the review stage were included in this paper for a literature review.
3. Review of the Papers
3.1. Biophysiological Indicators
3.1.1. Stress Monitoring with a Single Sensor
3.1.2. Stress Monitoring with Two Sensors
3.1.3. Stress Monitoring with Multiple Sensors
3.2. Biochemical Indicators
3.2.1. Stress Monitoring Using a Single Sensor
3.2.2. Stress Monitoring Using Two Sensors
3.2.3. Stress Monitoring Using Multiple Sensors
4. Discussion and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Abbreviation | Meaning |
|---|---|
| PSS | Perceived Stress Scale |
| VASS | Visual Analogue Scale for Stress |
| TSST | Trier Social Stress Test |
| ML | Machine learning |
| SVM | Support vector machine |
| BN | Bayesian networks |
| ANN | Artificial neural network |
| DT | Decision tree |
| CAD | Computer-aided diagnostic |
| SRI | Stress Response Inventory |
| EDA | Electrodermal activity |
| ECG | Electrocardiography |
| EEG | Electroencephalograph |
| Resp | Respiration |
| BP | Blood pressure |
| PPG | Photoplethysmography |
| ST | Skin temperature |
| EMG | Electromyography |
| ACC | Accelerometer |
| AVP | Arginine vasopressin |
| ELISA | Enzyme-linked immunosorbent assay |
| GSR | Galvanic skin response |
| HRV | Heart rate variability |
| LDA | Linear discriminant analysis |
| MIST | Montreal Imaging Stress Task |
| kNN | K-nearest neighbors |
| RF | Random forest |
| TEB | Thoracic electrical bioimpedance |
| MLP | Multilayer perceptron classifier |
| TP | True positive |
| TN | True negative |
| PCA | Principal component analysis |
| GPS | Global positioning system |
| PDM | Principle dynamic modes |
| PSD | Power spectrum density |
| STAI | State–Trait Anxiety Inventory |
| IAPS | International Affective Picture System |
| FFT | Fast Fourier transform |
| PNN | Probabilistic neural network |
| RBF | Radial basis function |
| HPA | Hypothalamic–pituitary–adrenal |
| PSQ | Perceived Stress Questionnaire |
| DHEA | Dehydroepiandrosterone |
| LC-MS/MS | Liquid chromatography–mass spectrometry |
| RIA | Radioimmunoassay |
| NIRS | Near-infrared spectroscopy |
| oxy-Hb | Oxy-hemoglobin |
| ELS | Early life stress |
| fMRI | Functional magnetic resonance imaging |
| CTQ | Childhood Trauma Questionnaire |
| SRI-Q | Severe Respiratory Insufficiency Questionnaire |
| MSI | Mental Stress Index |
Appendix B
| Algorithm | Brief Detail |
|---|---|
| SVM | The support vector machine (SVM) classification technique is the most precise method of solving a classification problem. These are built around a perception of margin, i.e., data are separated into two classes, on each side of the hyperplane. The SVM classifier is a binary classifier; hence, for a multiclass classification problem, multiple machines are trained [56]. SVM aims at the maximization of the margin between instances (features) of the two classes and at the minimization of the generalization error, usually incurred in other classifiers. |
| BN | To represent probability relationships of input instances (features) in the form of graphs, a Bayesian network is used. The structure of the Bayesian network (BN) is a directed acyclic graph (DAG), and there is a one-to-one correspondence between its nodes. Arcs in the DAG show the influence of different features on each other. Conditional independences can be detected if there is no arc representing casual influences in between features or there are no descendant nodes from this node (feature). |
| ANN | An artificial neural network (ANN) is used for classification whenever instances in the training dataset cannot be linearly separated. The ANN is created by the connection of many neurons (units). Neurons of the network are divided into three layers: an input layer, which receives incoming information from training dataset, an output layer, which gives us the processed result (mostly probabilities), and a hidden layer, which is in between the input and output layers. |
| DT | The decision tree classifies data by sorting input instances on the basis of feature values. Each node of the decision tree shows a classified feature from an input instance, while each branch shows an assumed nodal value. Classification of instances starts from the root and is sorted depending upon feature values. The best divisor of input training data becomes the root node of the decision tree. |
| kNN | The k-nearest neighbor (kNN) is one of the simplest instance-based learning algorithms. The kNN classifies all the proximity instances, in a database, into a single group and then, when a new instance (feature) comes, the classifier observes the properties of the instance and places it into the closest matched group (nearest neighbor). |
| Logistic regression | Logistic regression is one of the simplest machine learning algorithms mostly used for binary classification problems. This algorithm can be implemented easily and is used as a baseline algorithm for other two-class classifiers. There are three types of logistic regression algorithms. If the targeted features have only two outcomes, e.g., spam or not spam emails and diabetic or not diabatic, then this problem is solved by using binary logistic regression. If the targeted features have three or more than three nominal categories, then multinomial logistic regression is used (e.g., prediction of the type of clothing). If the targeted features have three or more than three ordinal categories, then ordinal logistic regression is used (e.g., rating any product between 1 and 5). |
| LDA | Linear discriminant analysis (LDA) is the most used dimensionality reduction algorithm. LDA is used during a preprocessing step in pattern classification and other machine learning applications. LDA is calculated in five steps [38]: (1) compute d-dimensional mean vectors for each class in a given dataset; (2) compute the between-class scatter matrix and within-class scatter matrix; (3) compute eigenvectors and their corresponding eigenvalues for both scatter matrices; (4) select linear discriminants for the new feature subspace by sorting the eigenvectors in descending order using eigenvalues; (5) transform the samples into the new subspace by simply doing matrix multiplication. |
| PCA | Principle component analysis (PCA) is generally used to reduce the dimensions of a high-dimension dataset to a small subspace before using it to train any learning or classification algorithms. PCA transforms data into a new coordinate system having a low-dimension subspace. In this coordinate system, the first axis represents the first principle component that represents the greatest aggregate of variance in a dataset. |
| RF | The random forest classification technique is a supervised learning technique. This classifier performs best if we need to predict only two target classes as the output. Random forest is typically implemented using multiple decision trees. The hyperparameters of each decision tree are tuned before training and testing of the random forest classifier. When combined, each decision tree votes for the popular output class, which is mostly used whenever we have an ensemble model. |
References
- Richard, L.; Hurst, T.; Lee, J. Lifetime exposure to abuse, current stressors, and health in federally qualified health center patients. J. Hum. Behav. Soc. Environ. 2019, 29, 593–607. [Google Scholar] [CrossRef]
- Everly, G.S.; Lating, J.M. The anatomy and physiology of the human stress response. In A Clinical Guide to the Treatment of the Human Stress Response; Springer: Berlin/Heidelberg, Germany, 2019; pp. 19–56. [Google Scholar]
- Hemmingsson, E. Early childhood obesity risk factors: Socioeconomic adversity, family dysfunction, offspring distress, and junk food self-medication. Curr. Obes. Rep. 2018, 7, 204–209. [Google Scholar] [CrossRef] [PubMed]
- Kivimäki, M.; Steptoe, A. Effects of stress on the development and progression of cardiovascular disease. Nat. Rev. Cardiol. 2018, 15, 215. [Google Scholar] [CrossRef]
- Mariotti, A. The effects of chronic stress on health: New insights into the molecular mechanisms of brain—Body communication. Futur. Sci. OA 2015, 1, FSO23. [Google Scholar] [CrossRef] [PubMed]
- Tamashiro, K.L.; Sakai, R.R.; Shively, C.A.; Karatsoreos, I.N.; Reagan, L.P. Chronic stress, metabolism, and metabolic syndrome. Stress 2011, 14, 468–474. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Others World Health Statistics Data Visualizations Dashboard. In Obtenido de World Health Statistics Data Visualizations Dashboard; WHO: Geneva, Switzerland, 2018; pp. 3–4. Available online: http//apps.who.int/gho/data/node.sdg (accessed on 20 February 2021).
- Aguiló, J.; Ferrer-Salvans, P.; Garc′ia-Rozo, A.; Armario, A.; Corb′i, Á.; Cambra, F.J.; Bailón, R.; González-Marcos, A.; Caja, G.; Aguiló, S.; et al. Project ES3: Attempting to quantify and measure the level of stress. Rev. Neurol. 2015, 61, 405–415. [Google Scholar]
- Verma, P.; Sood, S.K. A comprehensive framework for student stress monitoring in fog-cloud IoT environment: M-health perspective. Med. Biol. Eng. Comput. 2019, 57, 231–244. [Google Scholar] [CrossRef]
- Golgouneh, A.; Tarvirdizadeh, B. Fabrication of a portable device for stress monitoring using wearable sensors and soft computing algorithms. Neural Comput. Appl. 2019, 32, 7515–7537. [Google Scholar] [CrossRef]
- McEwen, B.S. Stressed or stressed out: What is the difference? J. Psychiatry Neurosci. 2005, 30, 315. [Google Scholar]
- Hellhammer, D.H.; Stone, A.A.; Hellhammer, J.; Broderick, J. Measuring stress. Encycl. Behav. Neurosci. 2010, 2, 186–191. [Google Scholar]
- Goyal, A.; Singh, S.; Vir, D.; Pershad, D. Automation of stress recognition using subjective or objective measures. Psychol. Stud. 2016, 61, 348–364. [Google Scholar] [CrossRef]
- Petrakova, L.; Boy, K.; Mittmann, L.; Möller, L.; Engler, H.; Schedlowski, M. Salivary alpha-amylase and noradrenaline responses to corticotropin-releasing hormone administration in humans. Biol. Psychol. 2017, 127, 34–39. [Google Scholar] [CrossRef]
- Allen, A.P.; Kennedy, P.J.; Cryan, J.F.; Dinan, T.G.; Clarke, G. Biological and psychological markers of stress in humans: Focus on the Trier Social Stress Test. Neurosci. Biobehav. Rev. 2014, 38, 94–124. [Google Scholar] [CrossRef]
- Alberdi, A.; Aztiria, A.; Basarab, A. Towards an automatic early stress recognition system for office environments based on multimodal measurements: A review. J. Biomed. Inform. 2016, 59, 49–75. [Google Scholar] [CrossRef]
- Cvetković, B.; Gjoreski, M.; Šorn, J.; Maslov, P.; Kosiedowski, M.; Bogdański, M.; Stroiński, A.; Luštrek, M. Real-time physical activity and mental stress management with a wristband and a smartphone. In Proceedings of the UbiComp ‘17: Proceedings of the 2017 ACM International Joint Conference on Pervasive and Ubiquitous Computing and Proceedings of the 2017 ACM International Symposium on Wearable Computers, Maui, HI, USA, 11–15 September 2017; pp. 225–228. [Google Scholar]
- Seoane, F.; Mohino-Herranz, I.; Ferreira, J.; Alvarez, L.; Buendia, R.; Ayllón, D.; Llerena, C.; Gil-Pita, R. Wearable biomedical measurement systems for assessment of mental stress of combatants in real time. Sensors 2014, 14, 7120–7141. [Google Scholar] [CrossRef]
- Han, L.; Zhang, Q.; Chen, X.; Zhan, Q.; Yang, T.; Zhao, Z. Detecting work-related stress with a wearable device. Comput. Ind. 2017, 90, 42–49. [Google Scholar] [CrossRef]
- Katsis, C.D.; Katertsidis, N.S.; Fotiadis, D.I. An integrated system based on physiological signals for the assessment of affective states in patients with anxiety disorders. Biomed. Signal Process. Control 2011, 6, 261–268. [Google Scholar] [CrossRef]
- Gjoreski, M.; Luštrek, M.; Gams, M.; Gjoreski, H. Monitoring stress with a wrist device using context. J. Biomed. Inform. 2017, 73, 159–170. [Google Scholar] [CrossRef] [PubMed]
- van den Broek, E.L.; van der Sluis, F.; Dijkstra, T. Cross-validation of bimodal health-related stress assessment. Pers. Ubiquitous Comput. 2013, 17, 215–227. [Google Scholar] [CrossRef]
- Seo, S.-H.; Lee, J.-T.; Crisan, M. Stress and EEG. In Convergence and Hybrid Information Technologies; IntechOpen: London, UK, 2010; Volume 27. [Google Scholar]
- Zontone, P.; Affanni, A.; Bernardini, R.; Piras, A.; Rinaldo, R. Stress detection through electrodermal activity (EDA) and electrocardiogram (ECG) analysis in car drivers. In Proceedings of the 2019 27th European Signal Processing Conference (EUSIPCO), A Coruña, Spain, 2–6 September 2019; pp. 1–5. [Google Scholar]
- Affanni, A. Wireless sensors system for stress detection by means of ECG and EDA acquisition. Sensors 2020, 20, 2026. [Google Scholar] [CrossRef] [PubMed]
- Giannakakis, G.; Grigoriadis, D.; Giannakaki, K.; Simantiraki, O.; Roniotis, A.; Tsiknakis, M. Review on psychological stress detection using biosignals. IEEE Trans. Affect. Comput. 2019, 1. [Google Scholar] [CrossRef]
- Pourmohammadi, S.; Maleki, A. Stress detection using ECG and EMG signals: A comprehensive study. Comput. Methods Programs Biomed. 2020, 193, 105482. [Google Scholar] [CrossRef]
- Oka, T. Psychogenic fever: How psychological stress affects body temperature in the clinical population. Temperature 2015, 2, 368–378. [Google Scholar] [CrossRef] [PubMed]
- Herborn, K.A.; Graves, J.L.; Jerem, P.; Evans, N.P.; Nager, R.; McCafferty, D.J.; McKeegan, D.E.F. Skin temperature reveals the intensity of acute stress. Physiol. Behav. 2015, 152, 225–230. [Google Scholar] [CrossRef] [PubMed]
- de Arriba-Pérez, F.; Santos-Gago, J.M.; Caeiro-Rodr′iguez, M.; Ramos-Merino, M. Study of stress detection and proposal of stress-related features using commercial-off-the-shelf wrist wearables. J. Ambient Intell. Humaniz. Comput. 2019, 10, 4925–4945. [Google Scholar] [CrossRef]
- Reims, H.; Sevre, K.; Fossum, E.; Høieggen, A.; Eide, I.; Kjeldsen, S. Plasma catecholamines, blood pressure responses and perceived stress during mental arithmetic stress in young men. Blood Press. 2004, 13, 287–294. [Google Scholar] [CrossRef]
- Drummond, J.B.; Soares, B.S.; Pedrosa, W.; Vieira, E.L.M.; Teixeira, A.L.; Christ-Crain, M.; Ribeiro-Oliveira, A. Copeptin response to hypoglycemic stress is linked to prolactin activation in children. Pituitary 2020, 23, 681–690. [Google Scholar] [CrossRef] [PubMed]
- Valentin, B.; Grottke, O.; Skorning, M.; Bergrath, S.; Fischermann, H.; Rörtgen, D.; Mennig, M.-T.; Fitzner, C.; Müller, M.P.; Kirschbaum, C.; et al. Cortisol and alpha-amylase as stress response indicators during pre-hospital emergency medicine training with repetitive high-fidelity simulation and scenarios with standardized patients. Scand. J. Trauma. Resusc. Emerg. Med. 2015, 23, 1–8. [Google Scholar] [CrossRef]
- Thau, L.; Sharma, S. Physiology, Cortisol; StatPearls: Treasure Island, FL, USA, 2020. [Google Scholar]
- Kim, K.H.; Bang, S.W.; Kim, S.R. Emotion recognition system using short-term monitoring of physiological signals. Med. Biol. Eng. Comput. 2004, 42, 419–427. [Google Scholar] [CrossRef]
- Sun, F.-T.; Kuo, C.; Cheng, H.-T.; Buthpitiya, S.; Collins, P.; Griss, M. Activity-aware mental stress detection using physiological sensors. In Proceedings of the International Conference on Mobile Computing, Applications, and Services, Santa Clara, CA, USA, 25–28 October 2010; pp. 282–301. [Google Scholar]
- Chen, L.; Zhao, Y.; Ye, P.; Zhang, J.; Zou, J. Detecting driving stress in physiological signals based on multimodal feature analysis and kernel classifiers. Expert Syst. Appl. 2017, 85, 279–291. [Google Scholar] [CrossRef]
- Kim, J.; Park, J.; Park, J. Development of a statistical model to classify driving stress levels using galvanic skin responses. Hum. Factors Ergon. Manuf. Serv. Ind. 2020, 30, 321–328. [Google Scholar] [CrossRef]
- Healey, J.; Picard, R. SmartCar: Detecting driver stress. In Proceedings of the 15th International Conference on Pattern Recognition. ICPR-2000, Barcelona, Spain, 3–7 September 2000; Volume 4, pp. 218–221. [Google Scholar]
- Healey, J.A.; Picard, R.W. Detecting stress during real-world driving tasks using physiological sensors. IEEE Trans. Intell. Transp. Syst. 2005, 6, 156–166. [Google Scholar] [CrossRef]
- Mahmoud, R.; Shanableh, T.; Bodala, I.P.; Thakor, N.V.; Al-Nashash, H. Novel classification system for classifying cognitive workload levels under vague visual stimulation. IEEE Sens. J. 2017, 17, 7019–7028. [Google Scholar] [CrossRef]
- Shi, Y.; Nguyen, M.H.; Blitz, P.; French, B.; Fisk, S.; la Torre, F.; Smailagic, A.; Siewiorek, D.P.; al′Absi, M.; Ertin, E.; et al. Personalized stress detection from physiological measurements. In Proceedings of the International Symposium on Quality of Life Technology, Las Vegas, NV, USA, 28–29 June 2010; pp. 28–29. [Google Scholar]
- Wijsman, J.; Grundlehner, B.; Liu, H.; Hermens, H.; Penders, J. Towards mental stress detection using wearable physiological sensors. In Proceedings of the 2011 Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Boston, MA, USA, 30 August–3 September 2011; pp. 1798–1801. [Google Scholar]
- Sandulescu, V.; Andrews, S.; Ellis, D.; Bellotto, N.; Mozos, O.M. Stress detection using wearable physiological sensors. In Proceedings of the International Work-Conference on the Interplay between Natural and Artificial Computation, Elche, Spain, 1–5 June 2015; pp. 526–532. [Google Scholar]
- Choi, M.; Koo, G.; Seo, M.; Kim, S.W. Wearable device-based system to monitor a driver’s stress, fatigue, and drowsiness. IEEE Trans. Instrum. Meas. 2017, 67, 634–645. [Google Scholar] [CrossRef]
- Vinkers, C.H.; Penning, R.; Hellhammer, J.; Verster, J.C.; Klaessens, J.H.G.M.; Olivier, B.; Kalkman, C.J. The effect of stress on core and peripheral body temperature in humans. Stress 2013, 16, 520–530. [Google Scholar] [CrossRef]
- Uesato, M.; Nabeya, Y.; Akai, T.; Inoue, M.; Watanabe, Y.; Kawahira, H.; Mamiya, T.; Ohta, Y.; Motojima, R.; Kagaya, A.; et al. Salivary amylase activity is useful for assessing perioperative stress in response to pain in patients undergoing endoscopic submucosal dissection of gastric tumors under deep sedation. Gastric Cancer 2010, 13, 84–89. [Google Scholar] [CrossRef]
- Ullmann, E.; Barthel, A.; Petrowski, K.; Stalder, T.; Kirschbaum, C.; Bornstein, S.R. Pilot study of adrenal steroid hormones in hair as an indicator of chronic mental and physical stress. Sci. Rep. 2016, 6, 1–7. [Google Scholar] [CrossRef]
- Arza, A.; Garzón-Rey, J.M.; Lázaro, J.; Gil, E.; Lopez-Anton, R.; de la Camara, C.; Laguna, P.; Bailon, R.; Aguiló, J. Measuring acute stress response through physiological signals: Towards a quantitative assessment of stress. Med. Biol. Eng. Comput. 2019, 57, 271–287. [Google Scholar] [CrossRef]
- Cashdan, E. Hormones and competitive aggression in women. Aggress. Behav. Off. J. Int. Soc. Res. Aggress. 2003, 29, 107–115. [Google Scholar] [CrossRef]
- Rohleder, N.; Nater, U.M.; Wolf, J.M.; Ehlert, U.; Kirschbaum, C. Psychosocial stress-induced activation of salivary alpha-amylase: An indicator of sympathetic activity. Ann. N. Y. Acad. Sci. 2004, 1032, 258–263. [Google Scholar] [CrossRef]
- Tasaka, A.; Tahara, Y.; Sugiyama, T.; Sakurai, K. Influence of chewing rate on salivary stress hormone levels. Nihon Hotetsu Shika Gakkai Zasshi 2008, 52, 482–487. [Google Scholar] [CrossRef] [PubMed]
- Hjortskov, N.; Garde, A.H.; Ørbæk, P.; Hansen, Å.M. Evaluation of salivary cortisol as a biomarker of self-reported mental stress in field studies. Stress Health J. Int. Soc. Investig. Stress 2004, 20, 91–98. [Google Scholar] [CrossRef]
- Bitkina, O.V.; Kim, J.; Park, J.; Park, J.; Kim, H.K. Identifying traffic context using driving stress: A longitudinal preliminary case study. Sensors 2019, 19, 2152. [Google Scholar] [CrossRef]
- Li, T.; Chen, Y.; Chen, W. Daily stress monitoring using heart rate variability of bathtub ecg signals. In Proceedings of the 2018 40th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Honolulu, HI, USA, 18–21 July 2018; pp. 2699–2702. [Google Scholar]
- Liu, Y.; Du, S. Psychological stress level detection based on electrodermal activity. Behav. Brain Res. 2018, 341, 50–53. [Google Scholar] [CrossRef] [PubMed]
- Mohino-Herranz, I.; Gil-Pita, R.; Ferreira, J.; Rosa-Zurera, M.; Seoane, F. Assessment of mental, emotional and physical stress through analysis of physiological signals using smartphones. Sensors 2015, 15, 25607–25627. [Google Scholar] [CrossRef] [PubMed]
- Lim, T.-S.; Loh, W.-Y.; Shih, Y.-S. A comparison of prediction accuracy, complexity, and training time of thirty-three old and new classification algorithms. Mach. Learn. 2000, 40, 203–228. [Google Scholar] [CrossRef]
- Choi, J.; Gutierrez-Osuna, R. Using heart rate monitors to detect mental stress. In Proceedings of the BSN’09: Proceedings of the 2009 Sixth International Workshop on Wearable and Implantable Body Sensor Networks, Washington, DC, USA, 3–5 June 2009; pp. 219–223. [Google Scholar]
- Linden, W. What do arithmetic stress tests measure? Protocol variations and cardiovascular responses. Psychophysiology 1991, 28, 91–102. [Google Scholar] [CrossRef]
- Tulen, J.H.M.; Moleman, P.; Van Steenis, H.G.; Boomsma, F. Characterization of stress reactions to the Stroop Color Word Test. Pharmacol. Biochem. Behav. 1989, 32, 9–15. [Google Scholar] [CrossRef]
- Hosseini, S.A.; Khalilzadeh, M.A. Emotional stress recognition system using EEG and psychophysiological signals: Using new labelling process of EEG signals in emotional stress state. In Proceedings of the 2010 International Conference on Biomedical Engineering and Computer Science, Wuhan, China, 23–25 April 2010; pp. 1–6. [Google Scholar]
- Scotti, S.; Mauri, M.; Cerutti, S.; Mainardi, L.; Villamira, M. Quantitative evaluation of distant student psychophysical responses during the e-learning processes. In Proceedings of the 2005 IEEE Engineering in Medicine and Biology 27th Annual Conference, Shanghai, China, 31 August–3 September 2005; pp. 1196–1199. [Google Scholar]
- Karthikeyan, P.; Murugappan, M.; Yaacob, S. Detection of human stress using short-term ECG and HRV signals. J. Mech. Med. Biol. 2013, 13, 1350038. [Google Scholar] [CrossRef]
- Muaremi, A.; Bexheti, A.; Gravenhorst, F.; Arnrich, B.; Tröster, G. Monitoring the impact of stress on the sleep patterns of pilgrims using wearable sensors. In Proceedings of the IEEE-EMBS International Conference on Biomedical and Health Informatics (BHI), Valencia, Spain, 1–4 June 2014; pp. 185–188. [Google Scholar]
- Mozos, O.M.; Sandulescu, V.; Andrews, S.; Ellis, D.; Bellotto, N.; Dobrescu, R.; Ferrandez, J.M. Stress detection using wearable physiological and sociometric sensors. Int. J. Neural Syst. 2017, 27, 1650041. [Google Scholar] [CrossRef]
- Lee, D.S.; Chong, T.W.; Lee, B.G. Stress events detection of driver by wearable glove system. IEEE Sens. J. 2016, 17, 194–204. [Google Scholar] [CrossRef]
- Euro Truck Simulator 2. Available online: https://eurotrucksimulator2.com/ (accessed on 19 March 2021).
- Can, Y.S.; Chalabianloo, N.; Ekiz, D.; Ersoy, C. Continuous stress detection using wearable sensors in real life: Algorithmic programming contest case study. Sensors 2019, 19, 1849. [Google Scholar] [CrossRef] [PubMed]
- Gardner, M.; Lightman, S.; Kuh, D.; Comijs, H.; Deeg, D.; Gallacher, J.; Geoffroy, M.-C.; Kivimaki, M.; Kumari, M.; Power, C.; et al. Dysregulation of the hypothalamic pituitary adrenal (HPA) axis and cognitive capability at older ages: Individual participant meta-analysis of five cohorts. Sci. Rep. 2019, 9, 1–13. [Google Scholar] [CrossRef]
- Gardner, M.P.; Lightman, S.L.; Gallacher, J.; Hardy, R.; Kuh, D.; Ebrahim, S.; Bayer, A.; Ben-Shlomo, Y.; Team, H.S. Diurnal cortisol patterns are associated with physical performance in the Caerphilly Prospective Study. Int. J. Epidemiol. 2011, 40, 1693–1702. [Google Scholar] [CrossRef]
- Morris, M.C.; Compas, B.E.; Garber, J. Relations among posttraumatic stress disorder, comorbid major depression, and HPA function: A systematic review and meta-analysis. Clin. Psychol. Rev. 2012, 32, 301–315. [Google Scholar] [CrossRef] [PubMed]
- Russell, E.; Koren, G.; Rieder, M.; Van Uum, S. Hair cortisol as a biological marker of chronic stress: Current status, future directions and unanswered questions. Psychoneuroendocrinology 2012, 37, 589–601. [Google Scholar] [CrossRef] [PubMed]
- Sauvé, B.; Koren, G.; Walsh, G.; Tokmakejian, S.; Van Uum, S.H.M. Measurement of cortisol in human hair as a biomarker of systemic exposure. Clin. Investig. Med. 2007, 30, E183–E191. [Google Scholar] [CrossRef] [PubMed]
- Gow, R.; Thomson, S.; Rieder, M.; Van Uum, S.; Koren, G. An assessment of cortisol analysis in hair and its clinical applications. Forensic Sci. Int. 2010, 196, 32–37. [Google Scholar] [CrossRef] [PubMed]
- Sunthad, K.; Niitsu, Y.; Inoue, M.; Yokemura, T. Brain’s Stress Observation System Using 2-Channels NIRS Based on Classroom Activity. In Proceedings of the 2019 IEEE International Conference on Consumer Electronics (ICCE), Las Vegas, NV, USA, 11–13 January 2019; pp. 1–4. [Google Scholar]
- Muthukrishna, M.; Henrich, J. Innovation in the collective brain. Philos. Trans. R. Soc. B Biol. Sci. 2016, 371, 20150192. [Google Scholar] [CrossRef] [PubMed]
- Russell, E.; Koren, G.; Rieder, M.; Van Uum, S.H.M. The detection of cortisol in human sweat: Implications for measurement of cortisol in hair. Ther. Drug Monit. 2014, 36, 30–34. [Google Scholar] [CrossRef] [PubMed]
- Bönke, L.; Aust, S.; Fan, Y.; Wirth, K.; Khawli, E.; Stevense, A.; Herrera, A.; Loayza, A.; Bajbouj, M.; Grimm, S. Examining the effect of Early Life Stress on autonomic and endocrine indicators of individual stress reactivity. Neurobiol. Stress 2019, 10, 100142. [Google Scholar] [CrossRef] [PubMed]
- Klinitzke, G.; Romppel, M.; Häuser, W.; Brähler, E.; Glaesmer, H. The German Version of the Childhood Trauma Questionnaire (CTQ): Psychometric characteristics in a representative sample of the general population. Psychother. Psychosom. Med. Psychol. 2011, 62, 47–51. [Google Scholar]
- Wu, W.; Pirbhulal, S.; Zhang, H.; Mukhopadhyay, S.C. Quantitative Assessment for Self-Tracking of Acute Stress based on Triangulation Principle in a Wearable Sensor System. IEEE J. Biomed. Health Inform. 2018, 23, 703–713. [Google Scholar] [CrossRef]
- Koh, K.B.; Park, J.K.; Kim, C.H.; Cho, S. Development of the stress response inventory and its application in clinical practice. Psychosom. Med. 2001, 63, 668–678. [Google Scholar] [CrossRef]
- Giles, G.E.; Mahoney, C.R.; Brunyé, T.T.; Taylor, H.A.; Kanarek, R.B. Stress effects on mood, HPA axis, and autonomic response: Comparison of three psychosocial stress paradigms. PLoS ONE 2014, 9, e113618. [Google Scholar] [CrossRef]
- Ahmed, N.; de la Torre, B.; Wahlgren, N.G. Salivary cortisol, a biological marker of stress, is positively associated with 24-hour systolic blood pressure in patients with acute ischaemic stroke. Cerebrovasc. Dis. 2004, 18, 206–213. [Google Scholar] [CrossRef]
- House, A.; Dennis, M.; Hawton, K.; Warlow, C. Methods of identifying mood disorders in stroke patients: Experience in the Oxfordshire Community Stroke Project. Age Ageing 1989, 18, 371–379. [Google Scholar] [CrossRef]
- O′Brien, W.H.; Haynes, S.N. Behavioral assessment in the psychiatric setting. In Handbook of Behavior Therapy in the Psychiatric Setting; Springer: Berlin/Heidelberg, Germany, 1993; pp. 39–71. [Google Scholar]
- Oken, B.S.; Chamine, I.; Wakeland, W. A systems approach to stress, stressors and resilience in humans. Behav. Brain Res. 2015, 282, 144–154. [Google Scholar] [CrossRef] [PubMed]
- Redon, P.; Shahzad, A.; Iqbal, T.; Wijns, W. Development of a New Detection Algorithm to Identify Acute Coronary Syndrome Using Electrochemical Biosensors for Real-World Long-Term Monitoring. Bioengineering 2021, 8, 28. [Google Scholar] [CrossRef] [PubMed]
- Minaee, S. Popular Machine Learning Metrics. Part 1: Classification & Regression Evaluation Metrics 2019. Available online: https//towardsdatascience.com/20-popular-machine-learning-metrics-part-1-classification-regressionevaluation-metrics-1ca3e282a2ce (accessed on 10 March 2021).
- Jordan, J. Evaluating a Machine Learning Model. 2017. Available online: https//www.jeremyjordan.me/evaluating-a-machine-learning-model/ (accessed on 14 March 2021).
- Descoins, A. Why Accuracy Alone Is a Bad Measure for Classification Tasks, and What We Can Do about It 2013. Available online: https//tryolabs.com/blog/2013/03/25/why-accuracyalone-bad-measure-classification-tasksand-what-we-can-do-about-it/ (accessed on 14 March 2021).
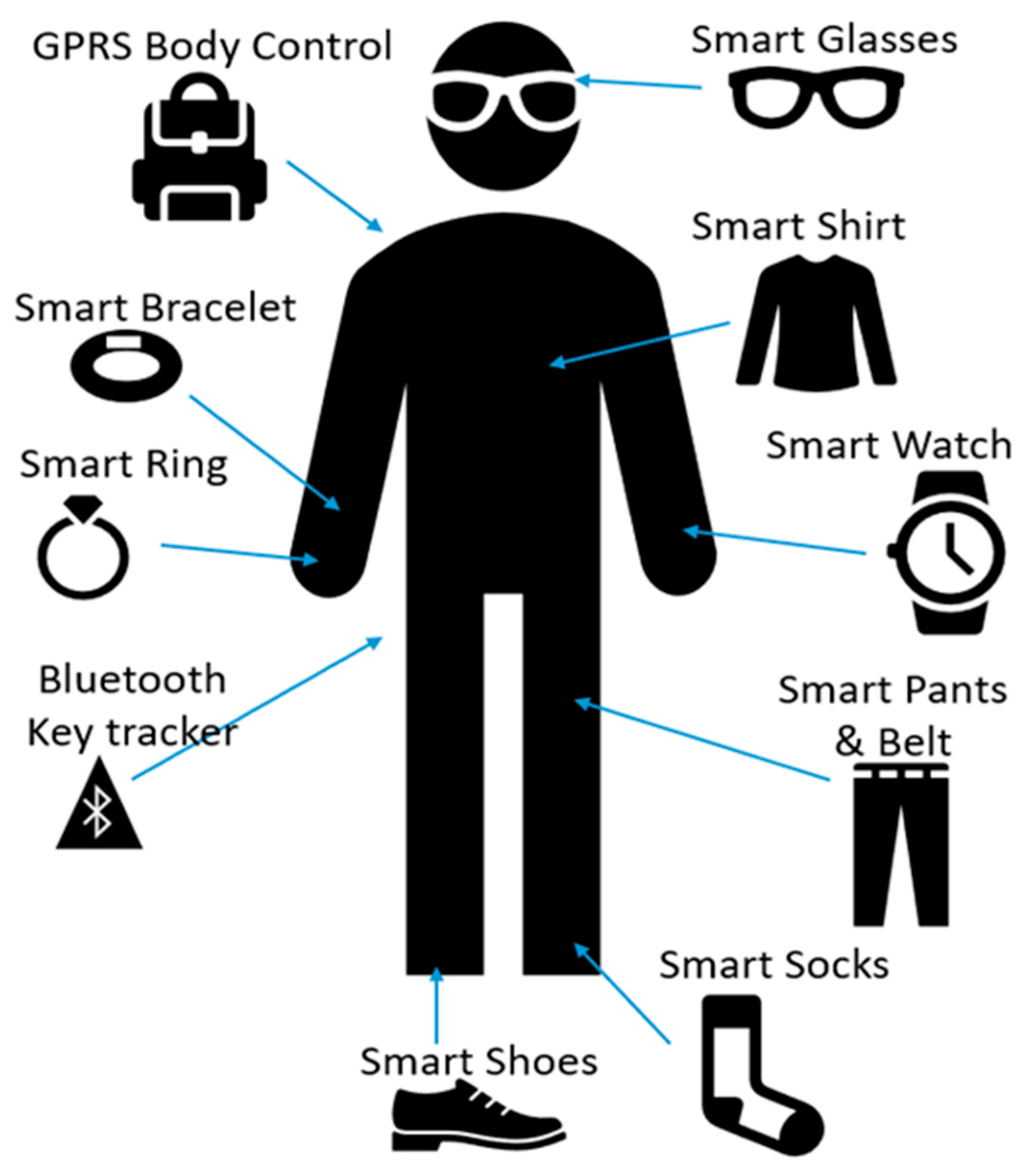
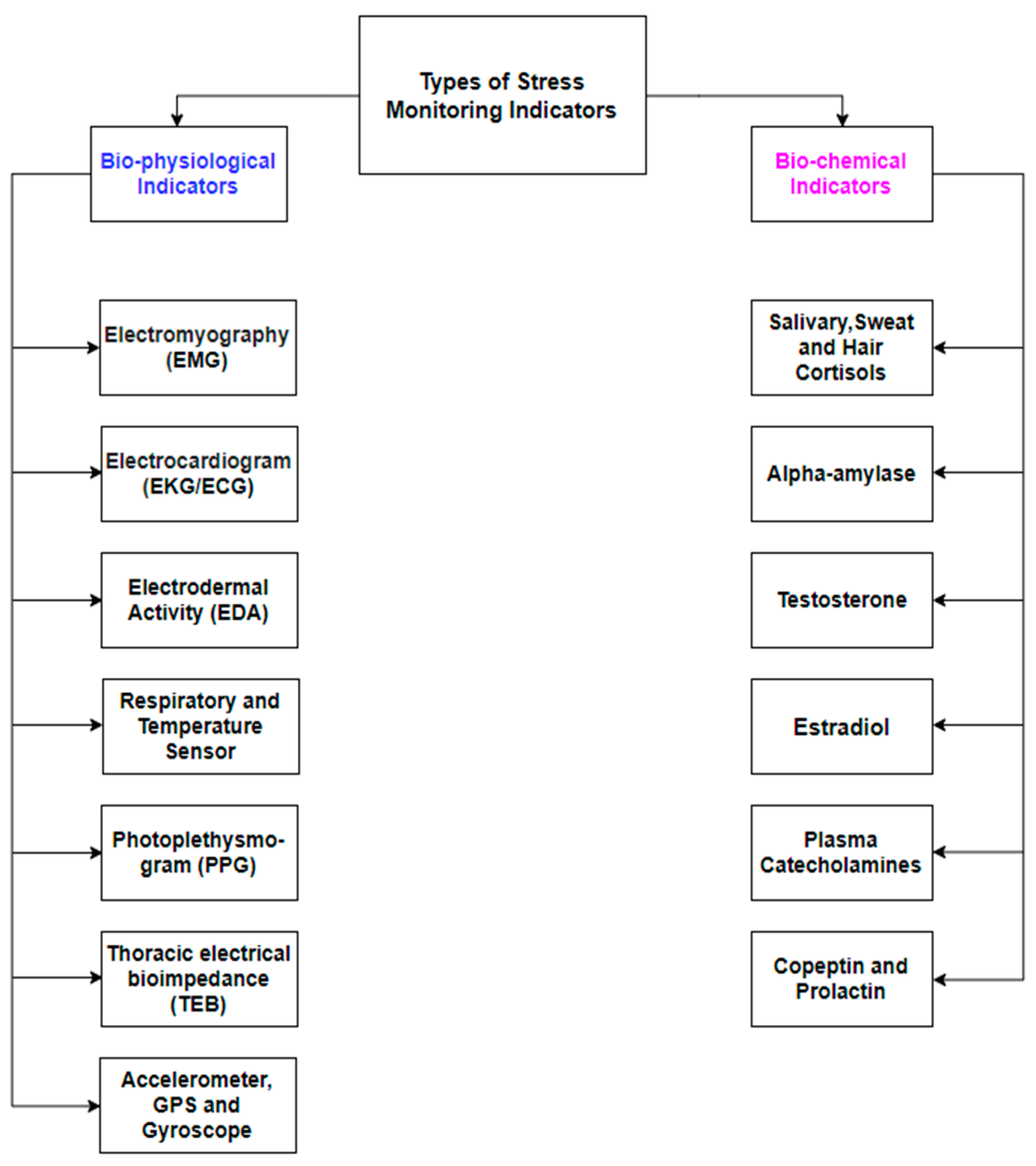
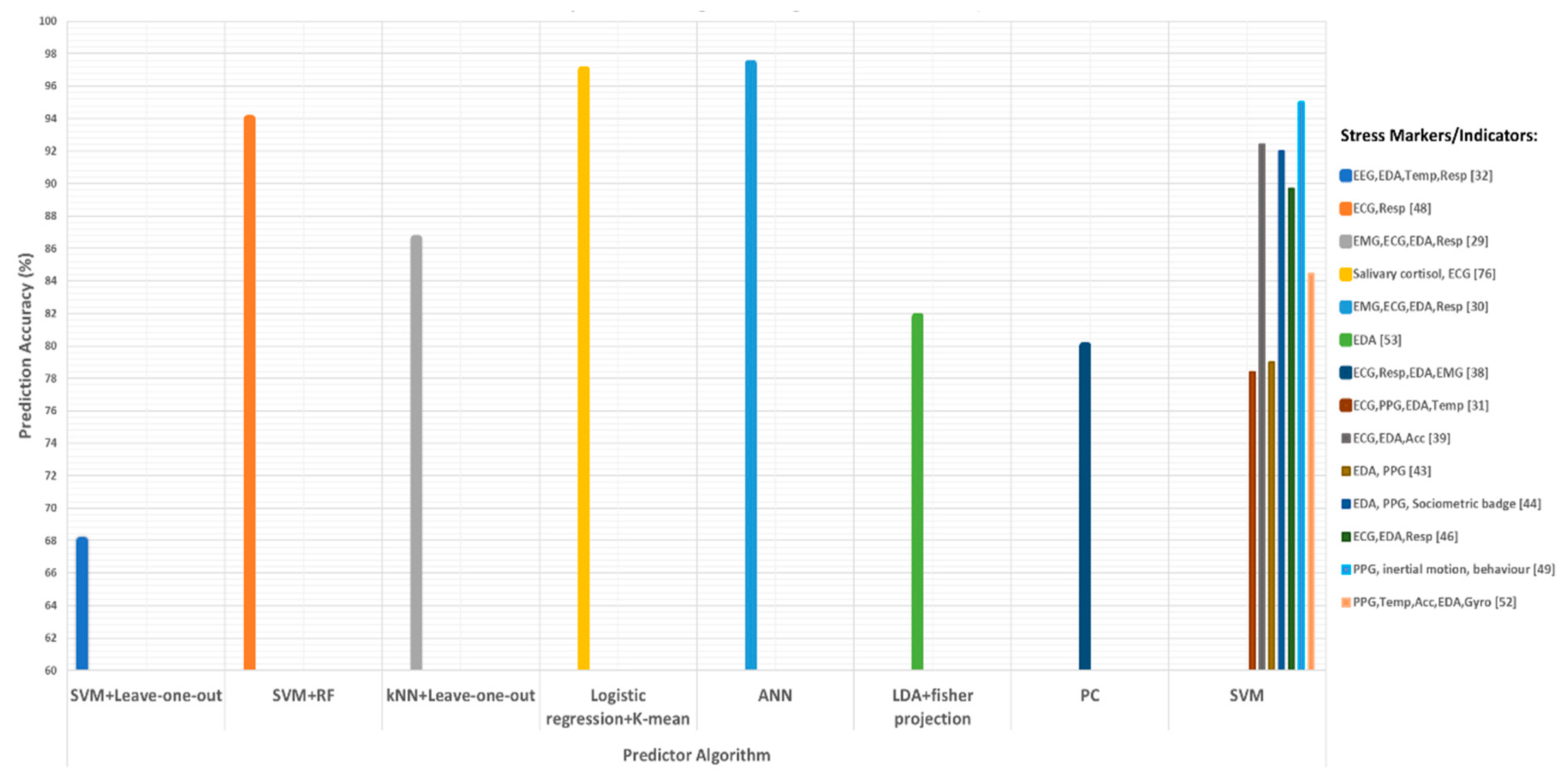
| Test Name | Stress Assessment Method |
|---|---|
| Mental Arithmetic Test | Participants are asked to solve arithmetic questions (subtraction, multiplication) within a time frame to induce stress. |
| Trier Social Stress Test (TSST) | Requires participants to deliver a speech on any given topic with a short time to prepare. After the speech, the participants are also asked to perform some verbal calculation. Both tasks are performed in the presence of an evaluating audience. |
| Stroop Test | Participants are shown the names of different colors written in various font colors and are asked to tell the font color rather than reading the word. |
| Perceived Stress Scale (PSS) | Participants fill out the questionnaire by rating the questions about their feelings and thoughts. The total score varies from 0 (no stress) to 40 (highest stress). |
| Visual Analogue Scale for Stress (VASS) | In this test, participants are asked different questions during a given task or experiment to rate their stress on the scale as no stress, moderate stress, or high stress rather than a numerical value. Most of the time, a 5-point (smiley) scale is used for stress assessment. |
| Stress Response Inventory (SRI) | The Stress Response Inventory consists of 39 questions scored in the range of 0 to 156. These questions are categorized into 7 factors, i.e., tension, fatigue, depression, aggression, anger, somatization, and frustration. A high score means high perceived stress. |
| COPE Inventory | There are 28 items of self-reporting questions designed to measure the efficiency of the ways in which participants cope with a stressful event. A score is given to each question on a scale of 1 (low stress) to 4 (high stress). The total scoring determines the participants stress coping style, i.e., approach coping or avoidant coping. |
| Holmes and Rahe Stress Inventory | Measures the amount of stress incurred within the past year. Participants select events that occurred in their life from the 43 life stress-related events. Each event has different scores. Participants accumulating a score greater than 300 are at higher risk of illness, while a score lower than 150 suggests a slight risk of illness. |
| State–Trait Anxiety Inventory (STAI) | Participants validate 20 questions that measure the state and trait anxiety. Participants respond to the questions on a scale of 1 to 4, where 1 denotes the least stress while 4 denotes a high-stress state. |
| Montreal Imaging Stress Task (MIST) | MIST consists of three stages, i.e., rest, control, and experiment. In the resting stage, the participant looks at the static screen of the computer. In the control stage, the participant is asked to solve a series of mathematical problems, while, in the experiment stage, some difficult and time-constrained arithmetic tasks are given to induce high stress. |
| Perceived Stress Questionnaire (PSQ) | Participants fill out two types of questionnaires consisting of 30 questions; the first questionnaire has questions about stressful experiences and feelings over the last 2 years while the second one has questions about stress during the last month. Participants have to score each question from 1 (no stress) to 4 (stressed). |
| S. No. | Bio-Signals | Units * |
|---|---|---|
| 1 | Skin conductance (also known as electrodermal Activity, EDA) [21,35,36,37,38] | µS |
| 2 | Electrocardiography (ECG) [19,37,39,40] | mV |
| 3 | Electroencephalograph (EEG) [41,42] | µV |
| 4 | Respiration (Resp) rate, blood pressure (BP), and blood volume pulse (BVP) using photoplethysmography (PPG) [35,43,44,45] | Breaths/min, mmHg and mV, respectively |
| 5 | Skin temperature (ST) [35,42,46] | °C |
| 7 | Electromyography (EMG) [39,40,43] | µV |
| 8 | Plasma catecholamines, copeptin and prolactin, steroids samples [47,48,49] | mcg/24-h, ng/mol, ng, respectively |
| 9 | α-amylase samples [47,49] | µL |
| 10 | Cortisol samples [50,51,52] | nmol/L |
| Ref | Year | Signals | No. of Subjects | Stressors | Conclusion | Features Extracting Methodology/Classifier |
|---|---|---|---|---|---|---|
| [39] | 2000 | ECG, EMG, EDA, and respiratory | 10 | Garage exit, city road, toll booth, highway driving, ramp turnaround, two-lane merge, bridge crossing, and entering the garage | The combination of all features from the four type of sensors had an overall accuracy of 86.6%. | K-nearest neighbors (k-NN) classifier |
| [40] | 2004 | ECG, EMG, EDA, and respiratory | 24 | Some audio, visual, and cognitive stimuli | Achieved a classification accuracy of 97.4% using data intervals of 5 min and found the highest correlation between heart rate and skin conductance metrics. | Artificial neural network (ANN) |
| [35] | 2004 | ECG, PPG, EDA, and ST | 50 | Rest, highway drive, and city drive | Achieved a recognition rate of 78.4% for three emotional and 61.8% for four emotional states. | Support vector machine (SVM) classifier |
| [59] | 2009 | ECG, ACC, GPS, EDA, and respiratory | 3 | Mental arithmetic test, Stroop color–word test | The PDM algorithm shows lower inter-subject variance and interestingly showed some comparable performance between and within subjects, where PSD performance decreased when used between subjects. | Principle dynamic modes (PDM) algorithm |
| [42] | 2010 | ECG, PPG, EDA, and ST | 22 | Public speaking, mathematics, mental, social, and physical challenges | SVM based model detects stress with high precision and recall rate (68% accuracy), especially when they used personalized information with SVM. | Support vector machine (SVM) classifier-based model |
| [62] | 2010 | EEG, EDA, PPG, and respiratory | 15 | International Affective Picture System (IAPS) | Characteristics of the EEG signal were extracted by wavelet coefficients and Higuchi’s algorithm, as well as correlation dimension. An accuracy of 82.7% using the Elman classifier was achieved. | Wavelet coefficients and Higuchi’s algorithm, as well as correlation dimension with Elman classifier |
| [43] | 2011 | ECG, respiratory, EDA, and EMG | 18 | Perceived stress scale (PSS) questionnaire | 80% accuracy indicates the suitability of used features for the detection of stress in a subject. | Principal component analysis (PCA) technique |
| [36] | 2012 | ECG, EDA, and ACC | 20 | Stroop color test and mental arithmetic problems based on Montreal Imaging Stress Task (MIST) | The inclusion of accelerometer data improved the stress detection process (92.4%) in a mobile environment. | Decision tree classifier, 10-fold validation, and least complex classifier |
| [64] | 2013 | ECG, EMG, GSR, and ST (only concern on ECG and HRV) | 60 | Stroop word–color test | Optimum features of ECG were extracted through fast Fourier transform (FFT). Accuracy of 91.66% and 94.66% was achieved using probabilistic neural network (PNN) and k-nearest neighbor (kNN) classifier, respectively. | Fourier transform (FFT) along with PNN and kNN classifier |
| [65] | 2014 | ECG, respiratory, body temp, GSR | 10 | Hajj pilgrimage | During sleep, the activity of the upper body and the duration of sleep contributed most to the detection of stress. The body temperature can be neglected as it did not contribute anything. Stress state was classified with an accuracy of 73% using SVM as a classifier. | Support vector machine (SVM) classifier |
| [44] | 2015 | EDA and PPG | 5 | Trier Scope Stress Test (TSST) | Detected stress of each participant with an average accuracy of 78.98%, i.e., combining all five participants’ stress detection accuracies using SVM. | Support vector machine (SVM) classifier |
| [57] | 2015 | ECG and TEB measurements | 40 | Films, game based on the addition | The measurement showed high potential in the use of ECG and skin activation (TEB) signals for the detection of long periods of stress or sudden increment in mental work overload or emotional responses of the people. With MLP classifier, authors achieved an error rate of 21.23%, 4.77%, and 32.33% for activity identification, emotional state, and mental activity, respectively. | Low-pass filtering and decimated intermediate frequency-based algorithms along with multilayer perceptron classifier |
| [66] | 2016 | EDA, PPG, and sociometric badge for recording | 18 | STAI (State–Trait Anxiety Inventory) and TSST | Achieved higher accuracy, i.e., 92%, with SVM (RBF kernel) classifier, as compared to linear kernel SVM (80%), AdaBoost (67%) and KNN (62%), when using selected set of features. | SVM (RBF kernel) classifier, linear kernel SVM, AdaBoost, and KNN |
| [37] | 2017 | ECG, EDA, and respiratory | 14 | Real driving environment | Using a full feature set, SVM with linear kernel gave the highest inter-drive classification precision. For cross-drive level, SVM with RBF kernel gave a precision score of 89.7%. | SVM with linear kernel, SVM with RBF kernel |
| [21] | 2017 | EDA, ST, ACC, and PPG | 5 | Randomly generated equations (solved verbally) | Without contextual information, the stress detection was not in the range of acceptable accuracy, while, when they included the context information, the detection F-score jumped to 0.9 from 0.47 and precision increased to 95% from 7%. | Time-domain features such as mean inter-beat interval (IBI) of a sample, standard deviation, square root of the mean of the squares of the differences between adjacent IBI samples, and the percentage of the differences between adjacent IBI samples that are greater than x ms (x = 20, 50, 70) along with SVM classifier |
| [19] | 2017 | ECG and respiratory | 39 | Montreal Imaging Stress Task (MIST) | An accuracy of 84% using random forest features and SVM classifier in discriminating three stages of stress, while, for binary classification, i.e., rest and stress, they achieved an accuracy of 94%. | Random forest features and SVM classifier |
| [67] | 2017 | PPG and inertial motion and driver behavior | 28 | Euro truck driving simulator version 2 | Sequential feature selection with RBF kernel SVM classifier was able to achieve a classification accuracy of 95%, which shows the suitability of their glove as the driver’s stress detection device. | RBF kernel-SVM classifier |
| [55] | 2018 | ECG | 1 | Daily life stress | The variation of stress index shows high concordance with the work schedule of the subject and, thus, can provide an acceptable solution for comparison of stress levels of different individuals. | By combining time- and frequency-domain nonlinear features |
| [45] | 2018 | PPG, Temp, ACC, and EDA | 28 | City Car Driving simulator | An accuracy of 68.31% for four states (i.e., normal, stressed, drowsiness, and fatigue) and accuracy of 84.46% for three states (i.e., normal, stressed, and drowsiness or fatigue) classification. | Used pulse intervals and compared their values with standard pulse interval values. Winner-takes-all (WTA) and max-wins voting (MWV) methods were used along with SVM classifier for classification |
| [56] | 2018 | EDA only (ECG, EMG, and respiratory) | 11 | Driving on the highway, in the city | After using Fisher projection and linear discriminant analysis (LDA) on the data collected from the dataset, the authors claimed to achieve a classification accuracy of 81.82%. | Fisher projection and linear discriminant analysis (LDA) |
| [69] | 2019 | PPG, EDA, GSR, and ACC | 21 | Summer camp (training, the contest, and free day) | If individual data of each person are enough to design a person-based model, it should be developed; otherwise, each person should be clustered in accordance to their behavior in stress and then a clustering model should be developed to increase the classification accuracy of the general model. | Clustering model like kNN |
| [54] | 2019 | EDA | 1 | Driving on the highway, in the city | From the experiment, the authors concluded that their classification results indicate that road type and traffic conditions are important features related to driving stress. The authors reported an accuracy of 80.3% with a sensitivity of 85%, a specificity of 78%, and positive predictivity of 70% while using logistic regression as a classifier. | EDA from Empatica E4 watch and logistic regression-based classifier |
| [38] | 2020 | Galvanic skin response (GSR) | 10 | Predefined PYSIONET dataset and driving on the highway, in the city | For the classification, the authors designed a binary logistic regression model and achieved an overall accuracy of 85.3% on data from PYSIONET, while they achieved 83.2% accuracy on the validation data, analyzed through cross-validation. The authors also proposed that their developed model can be embedded in existing wearable GSR sensor devices and, thus, can enable detecting and monitoring the driving stress in real time. | GSR from Empatica E4 watch and binary logistic regression-based classifier along with cross-validation technique |
| Ref | Year | Signals | No. of Subjects | Stressors | Conclusion |
|---|---|---|---|---|---|
| [50] | 2003 | Total testosterone, free testosterone, estradiol, androstenedione, and cortisol | 30 | Early follicular phase | Women with low levels of testosterone and androstenedione presented less competitive feelings. Moreover, estradiol levels were unrelated to any competitive feeling. |
| [51] | 2004 | Salivary cortisol (in the blood) | 12 | The psychosocial stress test, Trier social test (TSST), and reading test | The level of salivary alpha-amylase was significantly lower in smoking females than non-smokers, while it was higher in smoking males than in non-smokers. Identified that the production of salivary cortisol affected the association of norepinephrine and amylases. Activation of parasympathetic nervous systems decreased the overall saliva production and volume. Therefore, the volume of saliva and amylase levels should be measured relative to the saliva produced. |
| [84] | 2004 | BP, pulse rate (PPG), and saliva | 58 | Vertical Visual Analogue Scale (V-VAS) and State–Trait Anxiety Inventory | There was a positive correlation between salivary cortisol and 24 h blood pressure. |
| [52] | 2008 | Cortisol level and EMG | 16 | Arithmetic problems, tasteless gum | Fast chewing had a greater effect on the stress release than a slow chewing rate, while the integration of EMG signals did not show any major difference in the 3 chewing rates. |
| [47] | 2010 | Salivary alpha-amylase, plasma catecholamines, BP, and HR | 33 | College academic final exam | The salivary alpha-amylase level changed significantly, but a partial correlation was found, statistically, between salivary alpha-amylase and blood pressure, heart rate, and plasma catecholamines. |
| [73] | 2012 | Hair cortisol | - | Daily life stress (3 months) | Identified some gaps in the currently available literature: firstly, to clarify the mechanism underlying cortisol incorporation into hair, and, secondly, to determine the factors that cause variation in hair cortisol such as the effect of hair washing. |
| [78] | 2013 | Sweat and saliva samples | 17 | Intense exercise | Intense exercise could increase the concentration of cortisol in hair, which was not decreased by hair washing. |
| [48] | 2016 | Steroid hormones in hair | 40 | Perceived Stress Questionnaire (PSQ) | The concentrations of steroids in the hair were a decisive predictor of the increased long-term HPA axis. Furthermore, this biomarker could capture stress even after burdening events or any physical activity was finished. |
| [81] | 2018 | Biochemical (salivary cortisol) and physiological (HRV measures) domains | 30 | Academic final examination, Psychological Stress Response Inventory | The salivary cortisol levels were negatively correlated with the HRV indicator of parasympathetic activity, while they were positively related with the HRV indicator of sympathetic activity. The results also showed that the value of the mental stress index (MSI) was very sensitive to acute stress and could predict stress with an accuracy of 97%. |
| [49] | 2019 | ST, HR, pulse wave (EDA, ECG, PPG), copeptin, prolactin (blood), cortisol, and alpha-amylase (saliva) | 40 | Trier Social Stress Test (TSST) | There was no clear correlation between physiological parameters and perceived stress levels. Moreover, alpha-amylase peak level time is 10 to 15 min after stress onset and, thus, should be measured within that time frame. Alpha-amylase and cortisol were measured in the morning (at that time, intra-individual variability is high). |
| [79] | 2019 | PPG and endocrine (salivary) cortisol | 32 | Childhood Trauma Questionnaire (CTQ) | No significant effect of early life stress on heart rate (autonomic indicator) and salivary cortisol (endocrine indicator), but the authors suggested that heart rate is a better indicator (of stress) than salivary cortisol as it is more sensitive to individual stress reactivity than salivary cortisol. |
| [76] | 2019 | Oxy-hemoglobin (oxy-Hb) | 4 | Decision-making and memory recall tests | Whenever high stress occurred, the average difference value of oxy-hemoglobin (oxy-Hb) increased. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iqbal, T.; Elahi, A.; Redon, P.; Vazquez, P.; Wijns, W.; Shahzad, A. A Review of Biophysiological and Biochemical Indicators of Stress for Connected and Preventive Healthcare. Diagnostics 2021, 11, 556. https://doi.org/10.3390/diagnostics11030556
Iqbal T, Elahi A, Redon P, Vazquez P, Wijns W, Shahzad A. A Review of Biophysiological and Biochemical Indicators of Stress for Connected and Preventive Healthcare. Diagnostics. 2021; 11(3):556. https://doi.org/10.3390/diagnostics11030556
Chicago/Turabian StyleIqbal, Talha, Adnan Elahi, Pau Redon, Patricia Vazquez, William Wijns, and Atif Shahzad. 2021. "A Review of Biophysiological and Biochemical Indicators of Stress for Connected and Preventive Healthcare" Diagnostics 11, no. 3: 556. https://doi.org/10.3390/diagnostics11030556
APA StyleIqbal, T., Elahi, A., Redon, P., Vazquez, P., Wijns, W., & Shahzad, A. (2021). A Review of Biophysiological and Biochemical Indicators of Stress for Connected and Preventive Healthcare. Diagnostics, 11(3), 556. https://doi.org/10.3390/diagnostics11030556








