Use of Contrast-Enhanced Ultrasound with Sonazoid for Evaluating the Radiotherapy Efficacy for Hepatocellular Carcinoma
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Enrollment
2.2. Imaging Method
2.2.1. US Imaging
2.2.2. SCEUS Procedures
2.2.3. CT Imaging
2.2.4. MR Imaging
2.3. Radiotherapy (RT)
2.4. Evaluation of the Therapeutic Efficacy of RT by SCEUS
2.5. Evaluation of the Therapeutic Efficacy of RT by CECT/CEMRI
2.6. Follow-Up of Alpha-Fetoprotein (AFP)
2.7. Diagnosis of Presence or Absence of Local Recurrence
2.8. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Achievement of Each Imaging Modality
3.3. Efficacy of RT
3.4. Changes in SCEUS and CECT/CEMRI Findings during Follow-Up Periods
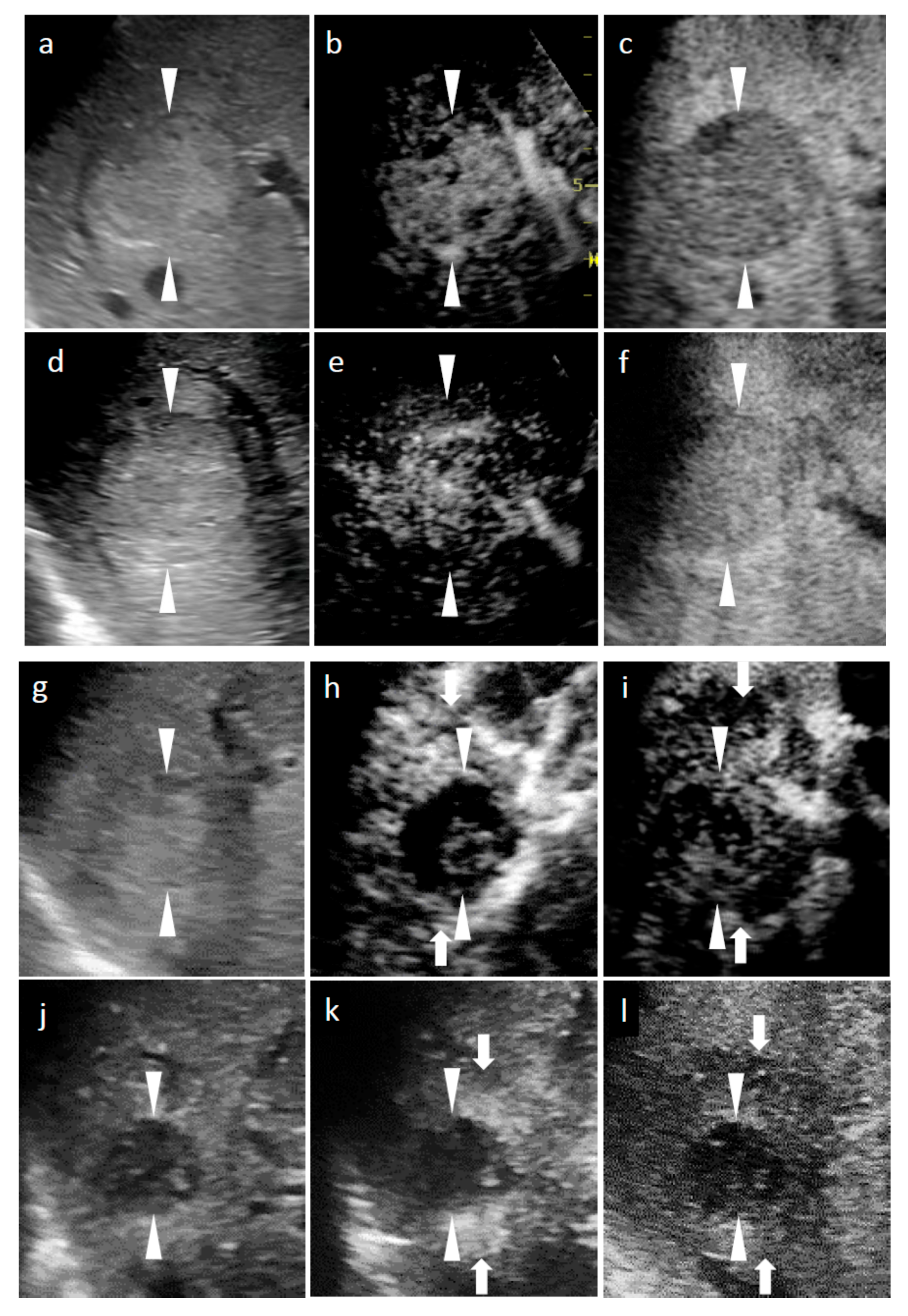
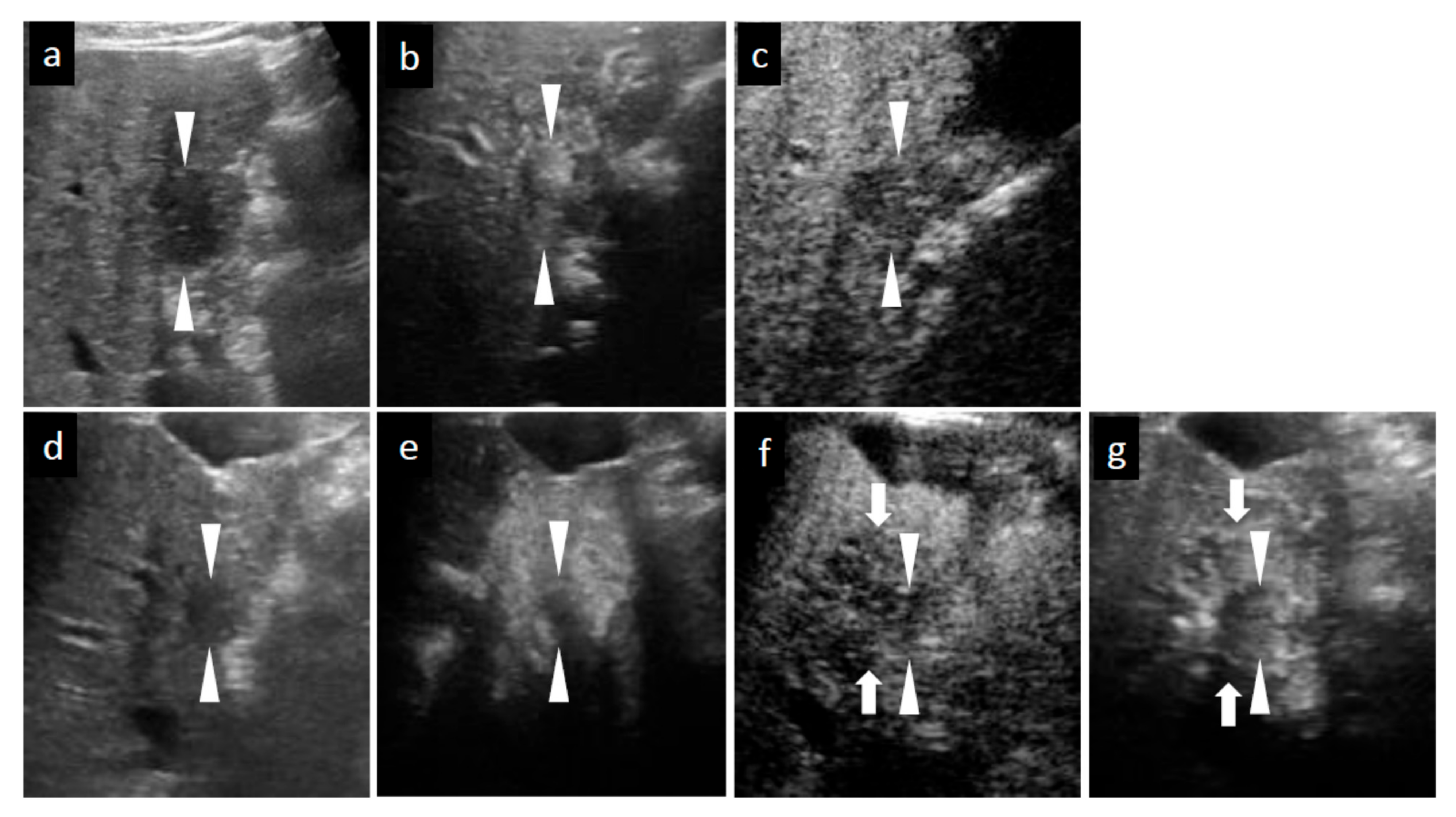
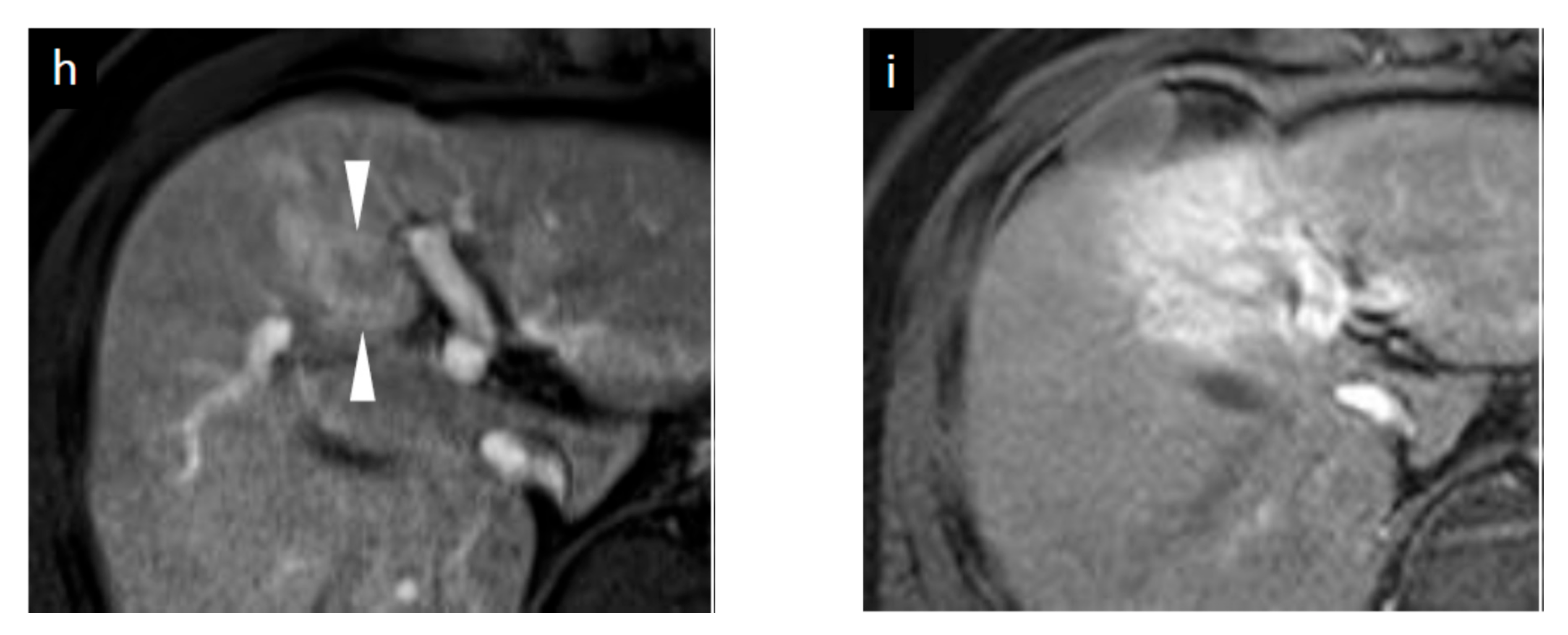
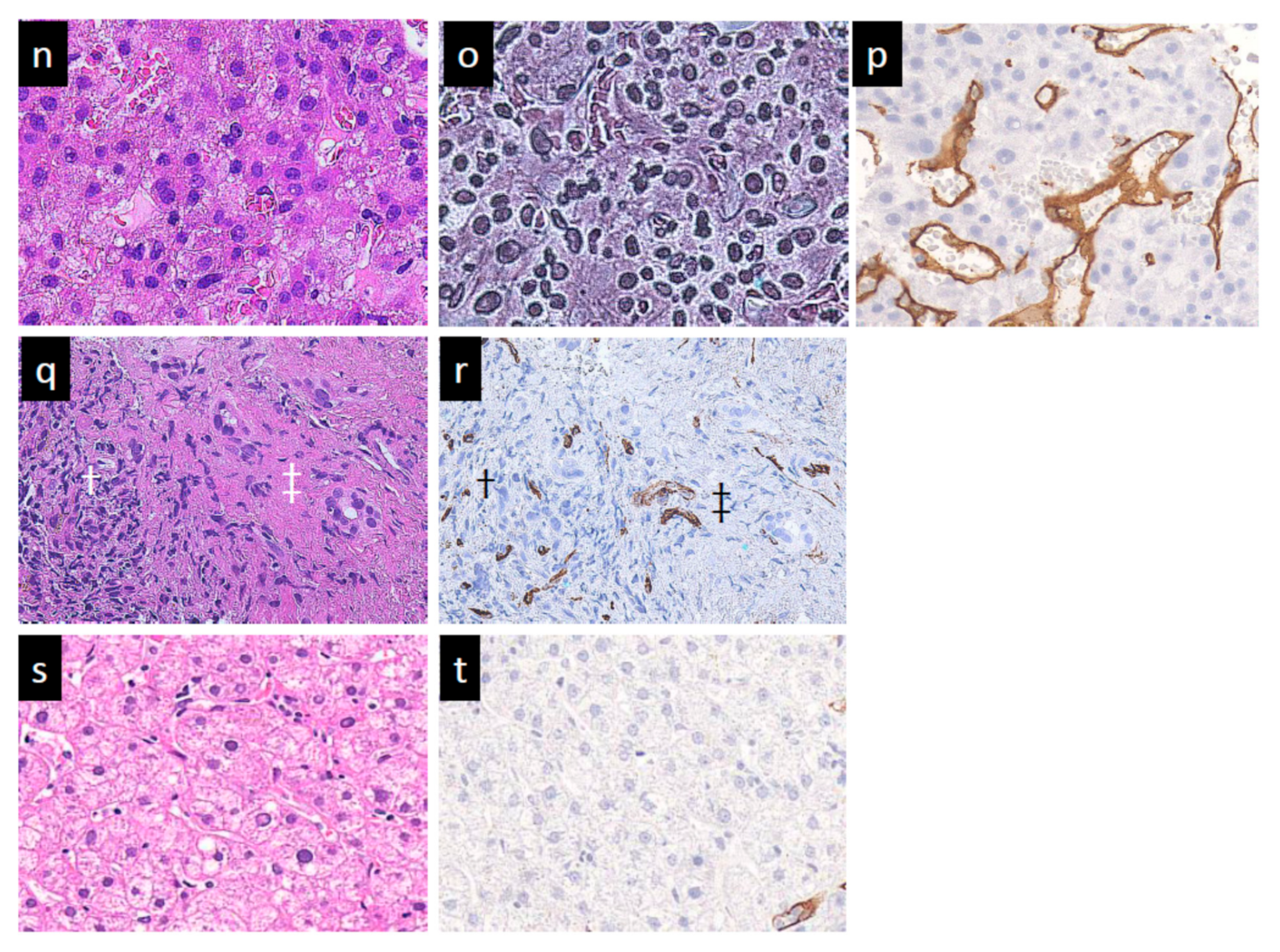
3.4.1. SCEUS Findings of the Cases with No Local Recurrence
3.4.2. SCEUS Findings of the Local Recurrence Cases
3.5. Comparison SCEUS Findings with CECT/CEMRI Findings 13 Months after RT
3.6. Comparison of SCEUS Findings for Cases with and without Local Recurrence 13 Months after RT
3.7. Changes in AFP after RT
3.8. Additional Treatment for Local Recurrence
3.9. Complications
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global Cancer Statistics 2018: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef]
- Villanueva, A. Hepatocellular Carcinoma. N. Engl. J. Med. 2019, 380, 1450–1462. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2020. CA Cancer J. Clin. 2020, 70, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Forner, A.; Reig, M.E.; de Lope, C.R.; Bruix, J. Current strategy for staging and treatment: The BCLC update and future prospects. Semin. Liver Dis. 2010, 30, 61–74. [Google Scholar] [CrossRef]
- Ayuso, C.; Rimola, J.; Vilana, R.; Burrel, M.; Darnell, A.; Garcia-Criado, A.; Bianchi, L.; Belmonte, E.; Caparroz, C.; Barrufet, M.; et al. Diagnosis and staging of hepatocellular carcinoma (HCC): Current guidelines. Eur. J. Radiol. 2018, 101, 72–81. [Google Scholar] [CrossRef] [PubMed]
- Nault, J.C.; Sutter, O.; Nahon, P.; Ganne-Carrie, N.; Seror, O. Percutaneous treatment of hepatocellular carcinoma: State of the art and innovations. J. Hepatol. 2018, 68, 783–797. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.K.; Kim, M.; Cho, C.K.; Yang, K.M.; Yoo, H.J.; Kim, J.H.; Bae, S.H.; Jung, D.H.; Kim, K.B.; Lee, D.H.; et al. Stereotactic body radiation therapy for inoperable hepatocellular carcinoma as a local salvage treatment after incomplete transarterial chemoembolization. Cancer 2012, 118, 5424–5431. [Google Scholar] [CrossRef] [PubMed]
- Bujold, A.; Massey, C.A.; Kim, J.J.; Brierley, J.; Cho, C.; Wong, R.K.; Dinniwell, R.E.; Kassam, Z.; Ringash, J.; Cummings, B.; et al. Sequential phase I and II trials of stereotactic body radiotherapy for locally advanced hepatocellular carcinoma. J. Clin. Oncol. 2013, 31, 1631–1639. [Google Scholar] [CrossRef] [PubMed]
- Moore, A.; Cohen-Naftaly, M.; Tobar, A.; Kundel, Y.; Benjaminov, O.; Braun, M.; Issachar, A.; Mor, E.; Sarfaty, M.; Bragilovski, D.; et al. Stereotactic body radiation therapy (SBRT) for definitive treatment and as a bridge to liver transplantation in early stage inoperable Hepatocellular carcinoma. Radiat. Oncol. 2017, 12, 163. [Google Scholar] [CrossRef]
- Hara, K.; Takeda, A.; Tsurugai, Y.; Saigusa, Y.; Sanuki, N.; Eriguchi, T.; Maeda, S.; Tanaka, K.; Numata, K. Radiotherapy for Hepatocellular Carcinoma Results in Comparable Survival to Radiofrequency Ablation: A Propensity Score Analysis. Hepatology 2019, 69, 2533–2545. [Google Scholar] [CrossRef]
- Kim, N.; Cheng, J.; Jung, I.; Liang, J.D.; Shih, Y.L.; Huang, W.; Kimura, T.; Lee, V.H.F.; Zeng, Z.C.; Zhenggan, R.; et al. Stereotactic body radiation therapy vs. radiofrequency ablation in Asian patients with hepatocellular carcinoma. J. Hepatol. 2020, 73, 121–129. [Google Scholar] [CrossRef]
- Takamatsu, S.; Kozaka, K.; Kobayashi, S.; Yoneda, N.; Yoshida, K.; Inoue, D.; Kitao, A.; Ogi, T.; Minami, T.; Kouda, W.; et al. Pathology and images of radiation-induced hepatitis: A review article. Jpn. J. Radiol. 2018, 36, 241–256. [Google Scholar] [CrossRef]
- Kimura, T.; Takahashi, S.; Takahashi, I.; Nishibuchi, I.; Doi, Y.; Kenjo, M.; Murakami, Y.; Honda, Y.; Aikata, H.; Chayama, K.; et al. The Time Course of Dynamic Computed Tomographic Appearance of Radiation Injury to the Cirrhotic Liver Following Stereotactic Body Radiation Therapy for Hepatocellular Carcinoma. PLoS ONE 2015, 10, e0125231. [Google Scholar] [CrossRef]
- Mendiratta-Lala, M.; Gu, E.; Owen, D.; Cuneo, K.C.; Bazzi, L.; Lawrence, T.S.; Hussain, H.K.; Davenport, M.S. Imaging Findings Within the First 12 Months of Hepatocellular Carcinoma Treated With Stereotactic Body Radiation Therapy. Int. J. Radiat. Oncol. Biol. Phys. 2018, 102, 1063–1069. [Google Scholar] [CrossRef] [PubMed]
- Oldrini, G.; Huertas, A.; Renard-Oldrini, S.; Taste-George, H.; Vogin, G.; Laurent, V.; Salleron, J.; Henrot, P. Tumor response assessment by MRI following stereotactic body radiation therapy for hepatocellular carcinoma. PLoS ONE 2017, 12, e0176118. [Google Scholar] [CrossRef]
- Mendiratta-Lala, M.; Masch, W.; Shankar, P.R.; Hartman, H.E.; Davenport, M.S.; Schipper, M.S.; Maurino, C.; Cuneo, K.C.; Lawrence, T.S.; Owen, D. Magnetic Resonance Imaging Evaluation of Hepatocellular Carcinoma Treated With Stereotactic Body Radiation Therapy: Long Term Imaging Follow-Up. Int. J. Radiat. Oncol. Biol. Phys. 2019, 103, 169–179. [Google Scholar] [CrossRef]
- Omata, M.; Cheng, A.; Kokudo, N.; Kudo, M.; Lee, J.M.; Jia, J.; Tateishi, R.; Han, K.H.; Chawla, Y.K.; Shiina, S.; et al. Asia-Pacific clinical guidelines on the management of hepatocellular carcinoma: 2017 update. Hepatol. Int. 2017, 11, 317–370. [Google Scholar] [CrossRef]
- Heimbach, J.K.; Kulik, L.M.; Finn, R.S.; Sirlin, C.B.; Abecassis, M.M.; Roberts, L.R.; Zhu, A.X.; Murad, M.H.; Marrero, J.A. AASLD Guidelines for the Treatment of Hepatocellular Carcinoma. Hepatology 2018, 67, 358–380. [Google Scholar] [CrossRef]
- European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J. Hepatol. 2018, 69, 182–236. [Google Scholar] [CrossRef] [PubMed]
- Lencioni, R.; Llovet, J.M. Modified RECIST (mRECIST) Assessment for Hepatocellular Carcinoma. Semin. Liver Dis. 2010, 30, 52–60. [Google Scholar] [CrossRef] [PubMed]
- Kibe, Y.; Takeda, A.; Tsurugai, Y.; Eriguchi, T. Local control by salvage stereotactic body radiotherapy for recurrent/residual hepatocellular carcinoma after other local therapies. Acta Oncol. 2020, 59, 888–894. [Google Scholar] [CrossRef]
- Mori, Y.; Hayakawa, A.; Abe, K.; Tanigawa, M.; Takahashi, M.; Naruse, H.; Yamaguchi, H. The safety and the efficacy of ultrasound contrast media Perflubutane microbubbles in clinical practice. Choonpa Igaku Jpn. Soc. Ultrason. Med. 2011, 38, 541–548. [Google Scholar] [CrossRef]
- Claudon, M.; Dietrich, C.F.; Choi, B.I.; Cosgrove, D.O.; Kudo, M.; Nolsøe, C.P.; Piscaglia, F.; Wilson, S.R.; Barr, R.G.; Chammas, M.C.; et al. Guidelines and good clinical practice recommendations for contrast enhanced ultrasound (CEUS) in the liver-update 2012: A WFUMB-EFSUMB initiative in cooperation with representatives of AFSUMB, AIUM, ASUM, FLAUS and ICUS. Ultrason. Med. Biol. 2013, 34, 11–29. [Google Scholar] [CrossRef]
- Westwood, M.; Joore, M.; Grutters, J.; Redekop, K.; Armstrong, N.; Lee, K.; Gloy, V.; Raatz, H.; Misso, K.; Severens, J.; et al. Contrast enhanced ultrasound using SonoVue® (sulphur hexafluoride microbubbles) compared with contrast-enhanced computed tomography and contrastenhanced magnetic resonance imaging for the characterisation of focal liver lesions and detection of liver metastases: A systematic review and cost-effectiveness analysis. Health Technol. Assess. 2013, 17, 1–243. [Google Scholar] [CrossRef]
- Wiesinger, I.; Wiggermann, P.; Zausig, N.; Beyer, L.P.; Salzberger, B.; Stroszczynski, C.; Jung, E.M. Percutaneous treatment of malignant liver lesions: Evaluation of success using contrast- enhanced ultrasound (CEUS) and perfusion software. Ultraschall Med. 2018, 39, 440–447. [Google Scholar] [CrossRef]
- Nishigori, S.; Numata, K.; Irie, K.; Fukuda, H.; Chuma, M.; Maeda, S. Fusion imaging with contrast-enhanced ultrasonography for evaluating the early therapeutic efficacy of radiofrequency ablation for small hypervascular hepatocellular carcinomas with iso-echoic or unclear margins on conventional ultrasonography. J. Med. Ultrason. 2018, 45, 405–415. [Google Scholar] [CrossRef]
- Numata, K.; Fukuda, H.; Morimoto, M.; Kondo, M.; Nozaki, A.; Oshima, T.; Okada, M.; Takebayashi, S.; Maeda, S.; Tanaka, K. Use of fusion imaging combining contrast-enhanced ultrasonography with a perflubutane-based contrast agent and contrast-enhanced computed tomography for the evaluation of percutaneous radiofrequency ablation of hypervascular hepatocellular carcinoma. Eur. J. Radiol. 2012, 81, 2746–2753. [Google Scholar] [CrossRef] [PubMed]
- Sugimori, K.; Numata, K.; Okada, M.; Nihonmatsu, H.; Takebayashi, S.; Maeda, S.; Nakano, M.; Tanaka, K. Central vascular structures as a characteristic finding of regenerative nodules using hepatobiliary phase gadolinium ethoxybenzyl diethylenetriaminepentaacetic acid-enhanced MRI and arterial dominant phase contrastenhanced US. J. Med. Ultrason. 2017, 44, 89–100. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Numata, K.; Nihonmatsu, H.; Okada, M.; Maeda, S. Application of new ultrasound techniques for focal liver lesions. J. Med. Ultrason. 2020, 47, 215–237. [Google Scholar] [CrossRef] [PubMed]
- Duisyenbi, Z.; Numata, K.; Nihonmatsu, H.; Fukuda, H.; Chuma, M.; Kondo, M.; Nozaki, A.; Tanaka, K.; Maeda, S. Comparison between low mechanical index and high mechanical index contrast modes of Contrast-Enhanced ultrasonography: Evaluation of perfusion defects of hypervascular hepatocellular carcinomas during the Post-Vascular phase. J. Ultrasound Med. 2019, 38, 2329–2338. [Google Scholar] [CrossRef]
- Takeda, A.; Sanuki, N.; Tsurugai, Y.; Iwabuchi, S.; Matsunaga, K.; Ebinuma, H.; Imajo, K.; Aoki, Y.; Saito, H.; Kunieda, E. Phase 2 study of stereotactic body radiotherapy and optional transarterial chemoembolization for solitary hepatocellular carcinoma not amenable to resection and radiofrequency ablation. Cancer 2016, 122, 2041–2049. [Google Scholar] [CrossRef] [PubMed]
- Kudo, M.; Hatanaka, K.; Maekawa, K. Newly developed Novel Ultrasound Technique, Defect Reperfusion Ultrasound Imaging, Using Sonazoid in the management of hepatocellular carcinoma. Oncology 2010, 78, 40–45. [Google Scholar] [CrossRef]
- Numata, K.; Morimoto, M.; Ogura, T.; Sugimori, K.; Takebayashi, S.; Okada, M.; Tanaka, K. Ablation Therapy Guided by Contrast-Enhanced Sonography with Sonazoid for Hepatocellular Carcinoma Lesions Not Detected by Conventional Sonography. J. Ultrasound Med. 2008, 27, 395–406. [Google Scholar] [CrossRef] [PubMed]
- Sugimoto, K.; Moriyasu, F.; Saito, K.; Taira, J.; Saguchi, T.; Yoshimura, N.; Oshiro, H.; Imai, Y.; Shiraishi, J. Comparison of Kuppfer-Phase Sonazoid-Enhanced Sonography and Hepatobiliary-Phase Gadoxetic Acid-Enhanced Magnetic Resonance Imaging of Hepatocellular Carcinoma and Correlation With Histologic Grading. J. Ultrasound Med. 2012, 31, 529–538. [Google Scholar] [CrossRef] [PubMed]
- Inoue, T.; Hyodo, T.; Korenaga, K.; Murakami, T.; Imai, Y.; Higashi, A.; Suda, T.; Takano, T.; Miyoshi, K.; Koda, M.; et al. Kuppfer phase image of Sonazoid-enhanced US is useful in predicting a hypervascularization of non-hypervascular hypointense hepatic lesions detected on Gd-EOB-DTPA-enhanced MRI: A multicenter retrospective study. J. Gastroenterol. 2016, 51, 144–152. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Numata, K.; Nakano, M.; Tanabe, M.; Chuma, M.; Nihonmatsu, H.; Nozaki, A.; Ogushi, K.; Luo, W.; Ruan, L.; et al. Diagnostic Value of Imaging Methods in the Histological Four Grading of Hepatocellular Carcinoma. Diagnostics 2020, 10, 321. [Google Scholar] [CrossRef] [PubMed]
- Kudo, M.; Matsui, O.; Izumi, N.; Iijima, H.; Kadoya, M.; Imai, Y.; Okusaka, T.; Miyayama, S.; Tsuchiya, K.; Ueshima, K.; et al. JSH Concensus-Based Clinical Practice Guidelines for the Management of Hepatocellular Carcinoma: 2014 Update by the Liver Cancer Study Group of Japan. Liver Cancer 2014, 3, 458–468. [Google Scholar] [CrossRef]
- Shiozawa, K.; Watanabe, M.; Ikehara, T.; Kobayashi, K.; Ochi, Y.; Suzuki, Y.; Fuchinoue, K.; Yoneda, M.; Kenmochi, T.; Okubo, Y.; et al. Evaluation of contrast-enhanced ultrasonography for hepatocellular carcinoma prior to and following stereotactic body radiation therapy using the CyberKnife system: A preliminary report. Oncol. Lett. 2016, 11, 208–212. [Google Scholar] [CrossRef] [PubMed]
- Bae, S.H.; Park, H.C.; Lim, D.H.; Lee, J.A.; Gwak, G.Y.; Choi, M.S.; Lee, J.H.; Koh, K.C.; Palik, S.W.; Yoo, B.C. Salvage Treatment With Hypofractionated Radiotherapy in Patients With Recurrent Small Hepatocellular Carcinoma. Int. J. Radiat. Oncol. Biol. Phys. 2012, 82, 603–607. [Google Scholar] [CrossRef]
- Herfarth, K.K.; Hof, H.; Bahner, M.L.; Lohr, F.; Hoss, A.; van Kaick, G.; Wannenmacher, M.; Debus, J. Assessment of focal liver reaction by multiphasic CT after stereotactic single-dose radiotherapy of liver tumors. Int. J. Radiat. Oncol. Biol. Phys. 2003, 57, 444–451. [Google Scholar] [CrossRef]
- Sanuki-Fujimoto, N.; Takeda, A.; Ohashi, T.; Kunieda, E.; Iwabuchi, S.; Takatsuka, K.; Koike, N.; Shigematsu, N. CT evaluations of focal liver reactions following stereotactic body radiotherapy for small hepatocellular carcinoma with cirrhosis: Relationship between imaging appearance and baseline liver function. Br. J. Radiol. 2010, 83, 1063–1071. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Jung, Y. Radiation-induced liver disease: Current understanding and future perspectives. Exp. Mol. Med. 2017, 49, 359. [Google Scholar] [CrossRef] [PubMed]
- Deleve, L.D.; Shulman, H.M.; McDonald, G.B. Toxic injury to hepatic sinusoids: Sinusoidal obstruction syndrome (veno-occlusive disease). Semin. Liver Dis. 2002, 22, 27–42. [Google Scholar] [CrossRef] [PubMed]

| Baseline Characteristics | All | No Local Recurrence | Local Recurrence |
|---|---|---|---|
| Number of patients/lesions | 59/59 | 56/56 | 3/3 |
| Age (mean ± SD, years) | 72.59 ± 8.768 | 72.43 ± 8.571 | 75.67 ± 11.441 |
| Sex (female/male) | 19/40 | 19/37 | 0/3 |
| Etiology of HCC (hepatitis B/hepatitis C/NBNC/alcohol abuse) | 4/46/5/4 | 4/43/5/4 | 0/3/0/0 |
| Child-Pugh classification (class A/B) | 50/9 | 47/9 | 3/0 |
| Diameters of lesion (median, range; mm) | 19.6 (8–40) | 16.5 (8–40) | 20.0 (20–34) |
| Liver segment (1/2/3/4/5/6/7/8) | 6/3/3/12/1/0/11/23 | 6/2/3/11/1/0/11/22 | 0/1/0/1/0/0/0/1 |
| RT (SBRT/HFRT) | 48/11 | 47/9 | 1/2 |
| Follow-up period a (median (range) in months) | 44.5 (13–82) | 48 (16–82) | 21 (20–22) |
| Observed Parameters | Follow-Up Period Number (%) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 Month | 4 Months | 7 Months | 10 Months | 13 Months | ||||||
| Modality | US | C/M | US | C/M e | US | C/M e | US | C/M f | US | C/M f |
| Tumor size | ||||||||||
| Reduction a | 4 (7.1) | 9 (17.6) | 17 (30.4) | 15 (33.3) | 35 (62.5) | 29 (64.4) | 41 (73.2) | 37 (84.1) | 46 (82.1) | 37 (84.1) |
| Increase b | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| No change c | 52 (92.9) | 42 (82.4) | 39 (69.6) | 30 (66.7) | 21 (37.5) | 16 (35.6) | 15 (26.8) | 7 (15.9) | 10 (17.9) | 7 (15.9) |
| Tumor vascularity | ||||||||||
| Reduction d | 12 (21.4) | 9 (17.6) | 49 (87.5) | 30 (66.7) | 53 (94.6) | 37 (82.2) | 55 (98.2) | 42 (95.5) | 56 (100) | 44 (100) |
| No change | 44 (78.6) | 42 (82.4) | 7 (12.5) | 15 (33.3) | 3 (5.4) | 8 (17.8) | 1 (1.8) | 2 (4.5) | 0 (0) | 0 (0) |
| Hypervascularity of surrounding liver parenchyma during AP | ||||||||||
| Presence | 22 (39.3) | 50 (89.3) | 56 (100) | 56 (100) | 51 (91.1) | |||||
| Absence | 34 (60.7) | 6 (10.7) | 0 (0) | 0 (0) | 5 (8.9) | |||||
| Perfusion defect during PVP | ||||||||||
| Presence | 8 (14.3) | 49 (87.5) | 54 (96.4) | 56 (100) | 56 (100) | |||||
| Absence | 48 (85.7) | 7 (12.5) | 2 (3.6) | 0 (0) | 0 (0) | |||||
| Observed Parameters | Follow-Up Period Number (%) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 Month | 4 Months | 7 Months | 10 Months | 13 Months | ||||||
| Modality | US | C/M | US | C/M | US | C/M | US | C/M | US | C/M |
| Tumor size | ||||||||||
| Reduction a | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Increase b | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| No change c | 3 (100) | 3 (100) | 3 (100) | 3 (100) | 3 (100) | 3 (100) | 3 (100) | 3 (100) | 3 (100) | 3 (100) |
| Tumor vascularity | ||||||||||
| Reduction d | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| No change | 3 (100) | 3 (100) | 3 (100) | 3 (100) | 3 (100) | 3 (100) | 3 (100) | 3 (100) | 3 (100) | 3 (100) |
| Hypervascularity of surrounding liver parenchyma during AP | ||||||||||
| Presence | 0 (0) | 3 (100) | 3 (100) | 3 (100) | 3 (100) | |||||
| Absence | 3 (100) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | |||||
| Perfusion defect during PVP | ||||||||||
| Presence | 0 (0) | 3 (100) | 3 (100) | 3 (100) | 3 (100) | |||||
| Absence | 3 (100) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | |||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Funaoka, A.; Numata, K.; Takeda, A.; Saigusa, Y.; Tsurugai, Y.; Nihonmatsu, H.; Chuma, M.; Fukuda, H.; Okada, M.; Nakano, M.; et al. Use of Contrast-Enhanced Ultrasound with Sonazoid for Evaluating the Radiotherapy Efficacy for Hepatocellular Carcinoma. Diagnostics 2021, 11, 486. https://doi.org/10.3390/diagnostics11030486
Funaoka A, Numata K, Takeda A, Saigusa Y, Tsurugai Y, Nihonmatsu H, Chuma M, Fukuda H, Okada M, Nakano M, et al. Use of Contrast-Enhanced Ultrasound with Sonazoid for Evaluating the Radiotherapy Efficacy for Hepatocellular Carcinoma. Diagnostics. 2021; 11(3):486. https://doi.org/10.3390/diagnostics11030486
Chicago/Turabian StyleFunaoka, Akihiro, Kazushi Numata, Atsuya Takeda, Yusuke Saigusa, Yuichirou Tsurugai, Hiromi Nihonmatsu, Makoto Chuma, Hiroyuki Fukuda, Masahiro Okada, Masayuki Nakano, and et al. 2021. "Use of Contrast-Enhanced Ultrasound with Sonazoid for Evaluating the Radiotherapy Efficacy for Hepatocellular Carcinoma" Diagnostics 11, no. 3: 486. https://doi.org/10.3390/diagnostics11030486
APA StyleFunaoka, A., Numata, K., Takeda, A., Saigusa, Y., Tsurugai, Y., Nihonmatsu, H., Chuma, M., Fukuda, H., Okada, M., Nakano, M., & Maeda, S. (2021). Use of Contrast-Enhanced Ultrasound with Sonazoid for Evaluating the Radiotherapy Efficacy for Hepatocellular Carcinoma. Diagnostics, 11(3), 486. https://doi.org/10.3390/diagnostics11030486







