Role of Gelatinases MMP-2 and MMP-9 in Healthy and Complicated Pregnancy and Their Future Potential as Preeclampsia Biomarkers
Abstract
1. Introduction
2. General Concepts of Extracellular Matrix of Uterus and Vasculature
3. Type I and Type IV Collagen Characteristics
4. General Features of Matrix Metalloproteinases (MMPs)
5. Characteristics of Matrix Metalloproteinases-2 and -9
5.1. MMP-2 Structure and Function
5.2. MMP-9 Structure and Function
6. Types I and IV Collagen Turnover in Healthy Pregnancy
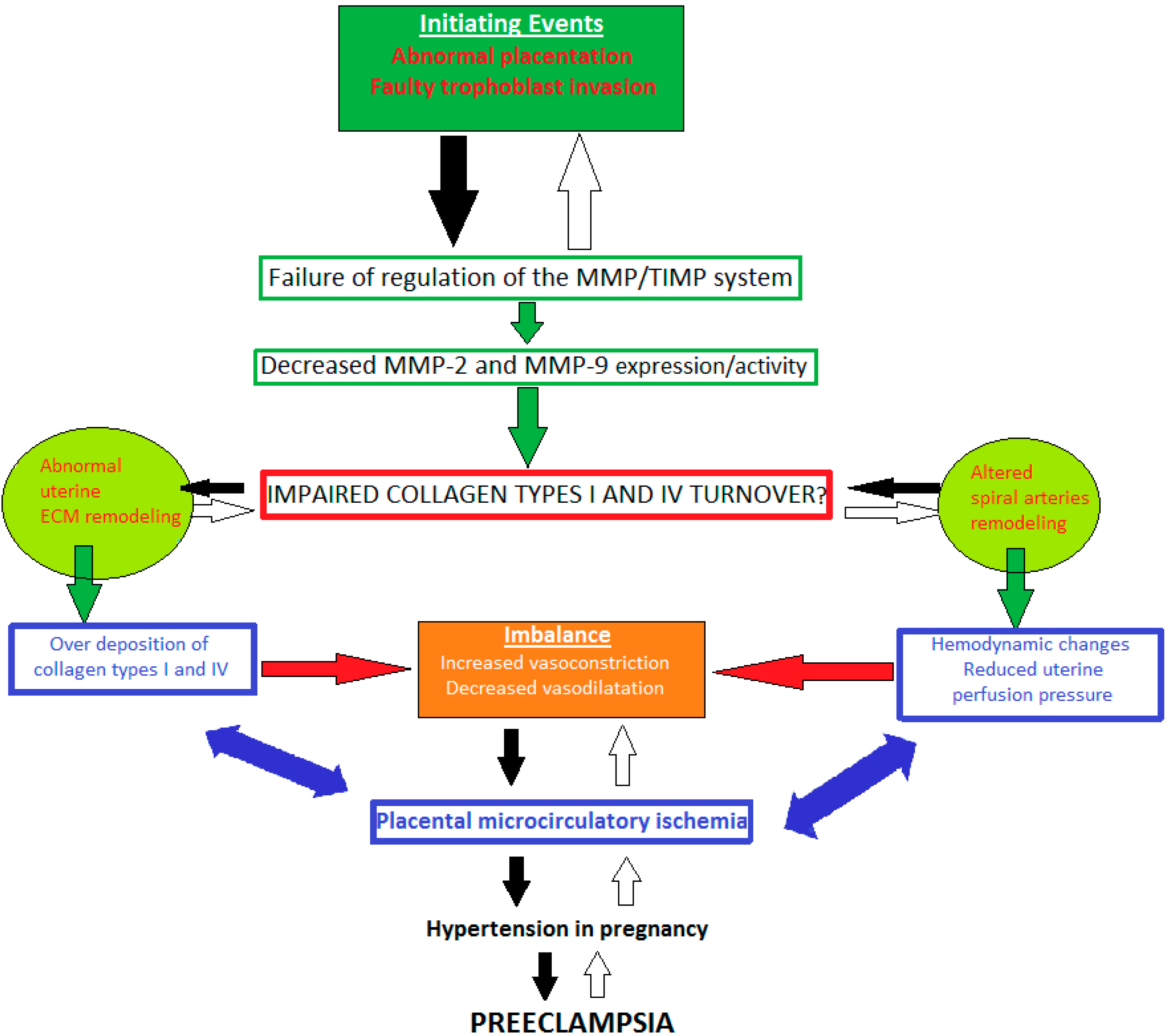
7. Impaired Types I and IV Collagen Turnover in Preeclampsia: The Role of MMP-2 and -9 Dysregulation
7.1. MMP-2 Dysregulation
7.2. MMP-9 Dysregulation
8. Limitations and Future Prospectus on the Application of MMP-2 and -9 as Preeclampsia Biomarkers
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Henriksen, K.; Karsdal, M.A. Type I Collagen. In Biochemistry of Collagens, Laminins and Elastin Structure, Function and Biomarkers, 1st ed.; Karsdal, M.A., Ed.; Academic Press: Cambridge, MA, USA, 2016; Chapter 1; pp. 1–11. [Google Scholar]
- Fleischmajer, R.; Macdonald, E.D.; Perlish, J.S.; Burgeson, R.E.; Fisher, L.W. Dermal collagen fibrils are hybrids of type I and type III collagen molecules. J. Struct. Biol. 1990, 105, 162–169. [Google Scholar] [CrossRef]
- Niyibizi, C.; Eyre, D.R. Bone type V collagen: Chain composition and location of a trypsin cleavage site. Connect. Tissue Res. 1989, 20, 247–250. [Google Scholar] [CrossRef]
- Gelse, K. Collagens—Structure, function, and biosynthesis. Adv. Drug Deliv. Rev. 2003, 55, 1531–1546. [Google Scholar] [CrossRef] [PubMed]
- Sand, J.M.B.; Genovese, F.; Gudmann, N.S.; Karsdal, M.A. Type IV collagen. In Biochemistry of Collagens, Laminins and Elastin, 2nd ed.; Karsdal, M.A., Ed.; Elsevier: Amsterdam, The Netherlands, 2019; Chapter 4; pp. 37–49. [Google Scholar]
- Mansell, J.P.; Bailey, A.J. Collagen Metabolism. In Encyclopedia of Endocrine Diseases; Martini, L., Ed.; Elsevier: Amsterdam, The Netherlands, 2004; pp. 520–529. [Google Scholar]
- Hudson, B.G.; Tryggvason, K.; Sundaramoorthy, M.; Neilson, E.G. Alport’s syndrome, Goodpasture’s syndrome, and type IV collagen. N. Engl. J. Med. 2003, 348, 2543–2556. [Google Scholar] [CrossRef] [PubMed]
- Tryggvason, L.; Patrakka, J. Alport’s Disease and Thin Basement Membrane Nephropathy. In Genetic Diseases of the Kidney; Lifton, R.P., Somlo, S., Giebisch, G.H., Eds.; Elsevier: Amsterdam, The Netherlands, 2009; Chapter 4; pp. 77–96. [Google Scholar]
- Visse, R.; Nagase, H. Matrix metalloproteinases and tissue inhibitors of metalloproteinases: Structure function and biochemistry. Circ. Res. 2003, 92, 827–839. [Google Scholar] [CrossRef]
- Somerville, R.; Oblander, S.; Apte, S. Matrix metalloproteinases: Old dogs with new tricks. Genome Biol. 2003, 4, 216. [Google Scholar] [CrossRef][Green Version]
- Stoneman, V.E.A.; Bennett, M.R. Role of apoptosis in atherosclerosis and its therapeutic implications. Clin. Sci. 2004, 107, 343–354. [Google Scholar] [CrossRef]
- Galis, Z.S.; Khatri, J.J. Matrix metalloproteinases in vascular remodeling and atherogenesis: The good, the bad, and the ugly. Circ. Res. 2002, 90, 251–262. [Google Scholar] [CrossRef]
- Aimes, R.T.; Quigley, J.P. Matrix metalloproteinase-2 is an interstitial collagenase. Inhibitor-free enzyme catalyzes the cleavage of collagen fibrils and soluble native type I collagen generating the specific 3/4- and 1/4-length fragments. J. Biol. Chem. 1995, 270, 5872–5876. [Google Scholar] [CrossRef]
- Patterson, M.L.; Atkinson, S.J.; Knäuper, V.; Murphy, G. Specific collagenolysis by gelatinase A, MMP-2, is determined by the hemopexin domain and not the fibronectin-like domain. FEBS Lett. 2001, 503, 158–162. [Google Scholar] [CrossRef]
- Nagase, H.; Visse, R.; Murphy, G. Structure and function of matrix metalloproteinases and TIMPs—Review. Cardiovasc. Res. 2006, 69, 562–573. [Google Scholar] [CrossRef]
- Klein, T.; Bischoff, R. Physiology and pathophysiology of matrix metalloproteases—Review. Amino Acids 2011, 41, 271–290. [Google Scholar] [CrossRef] [PubMed]
- Templeton, N.S.; Stetler-Stevenson, W.G. Identification of a basal promoter for the human Mr 72,000 type IV collagenase gene and enhanced expression in a highly metastatic cell line. Cancer Res. 1991, 51, 6190–6193. [Google Scholar] [PubMed]
- Murphy, G.; Nguyen, Q.; Cockett, M.I.; Atkinson, S.J.; Allan, J.A.; Knight, C.G.; Willenbrock, F.; Docherty, A.J. Assessment of the role of the fibronectin-like domain of gelatinase A by analysis of a deletion mutant. J. Biol. Chem. 1994, 269, 6632–6636. [Google Scholar] [CrossRef]
- Chakrabarti, S.; Patel, K.D. Matrix metalloproteinase-2 (MMP-2) and MMP-9 in pulmonary pathology. Exp. Lung Res. 2005, 31, 599–621. [Google Scholar] [CrossRef] [PubMed]
- Nagase, H. Cell surface activation of progelatinaseA (proMMP-2) and cell migration. Cell Res. 1998, 8, 179–186. [Google Scholar] [CrossRef]
- Sopata, I.; Dancewicz, A.M. Presence of a gelatin-specific proteinase and its latent form in human leucocytes. Biochim. Biophys. Acta 1974, 370, 510–523. [Google Scholar] [CrossRef]
- Mattu, T.S.; Royle, L.; Langridge, J.; Wormald, M.R.; Van den Steen, P.E.; Van Damme, J.; Opdenakker, G.; Harvey, D.J.; Dwek, R.A.; Rudd, P.M. O-glycan analysis of natural human neutrophil gelatinase B using a combination of normal phase-HPLC and online tandem mass spectrometry: Implications for the domain organization of the enzyme. Biochemistry 2000, 39, 15695–15704. [Google Scholar] [CrossRef] [PubMed]
- Ogata, Y.; Enghild, J.J.; Nagase, H. Matrix metalloproteinase 3 (stromelysin) activates the precursor for the human matrix metalloproteinase 9. J. Biol. Chem. 1992, 267, 3581–3584. [Google Scholar] [CrossRef]
- Maeda, H.; Okamoto, T.; Akaike, T. Human matrix metalloprotease activation by insults of bacterial infection involving proteases and free radicals. Biol. Chem. 1998, 379, 193–200. [Google Scholar] [CrossRef]
- Bergers, G.; Brekken, R.; McMahon, G.; Vu, T.H.; Itoh, T.; Tamaki, K.; Tanzawa, K.; Thorpe, P.; Itohara, S.; Werb, Z.; et al. Matrix metalloproteinase-9 triggers the angiogenic switch during carcinogenesis. Nat. Cell Biol. 2000, 2, 737–744. [Google Scholar] [CrossRef] [PubMed]
- Ezhilarasan, R.; Jadhav, U.; Mohanam, I.; Rao, J.S.; Gujrati, M.; Mohanam, S. The hemopexin domain of MMP-9 inhibits angiogenesis and retards the growth of intracranial glioblastoma xenograft in nude mice. Int. J. Cancer. 2009, 124, 306–315. [Google Scholar] [CrossRef] [PubMed]
- Lemjabbar, H.; Gosset, P.; Lamblin, C.; Tillie, I.; Hartmann, D.; Wallaert, B.; Tonnel, A.B.; Lafuma, C. Contribution of 92 kDa gelatinase/type IV collagenase in bronchial inflammation during status asthmaticus. Am. J. Respir. Crit. Care Med. 1999, 159, 1298–1307. [Google Scholar] [CrossRef] [PubMed]
- Van den Steen, P.E.; Dubois, B.; Nelissen, I.; Rudd, P.M.; Dwek, R.A.; Opdenakker, G. Biochemistry and molecular biology of gelatinase B or matrix metalloproteinase-9 (MMP-9). Crit. Rev. Biochem. Mol. Biol. 2002, 37, 375–536. [Google Scholar] [CrossRef] [PubMed]
- Lei, H.; Vadillo-Ortega, F.; Paavola, L.G.; Strauss, J.F. 92–kDa gelatinase (matrix metalloproteinase-9) is induced in rat amnion immediately prior to parturition. Biol. Reprod. 1995, 53, 339–344. [Google Scholar] [CrossRef]
- Vadillo-Ortega, F.; Hernandez, A.; Gonzalez-Avila, G.; Bermejo, L.; Iwata, K.; Strauss, J.F. Increased matrix metalloproteinase activity and reduced tissue inhibitor of metalloproteinases-1 levels in amniotic fluids from pregnancies complicated by premature rupture of membranes. Am. J. Obstet. Am. J. Obstet. Gynecol. 1996, 174, 1371–1376. [Google Scholar] [CrossRef]
- Vadillo-Ortega, F.; Gonzalez-Avila, G.; Furth, E.E.; Lei, H.; Muschel, R.J.; Stetler-Stevenson, W.G.; Strauss, J.F. 92-kd type IV collagenase (matrix metalloproteinase-9) activity in human amniochorion increases with labor. Am. J. Pathol. 1995, 146, 148–156. [Google Scholar] [PubMed]
- Crocker, S.J.; Pagenstecher, A.; Campbell, I.L. The TIMPs tango with MMPs and more in the central nervous system. J. Neurosci. Res. 2004, 75, 1–11. [Google Scholar] [CrossRef]
- Devarajan, P.; Johnston, J.J.; Ginsberg, S.S.; Van Wart, H.E.; Berliner, N. Structure and expression of neutrophil gelatinase cDNA. Identity with type IV collagenase from HT1080 cells. J. Biol. Chem. 1992, 267, 25228–25232. [Google Scholar] [CrossRef]
- Opdenakker, G.; Masure, S.; Grillet, B.; Van Damme, J. Cytokinemediated regulation of human leukocyte gelatinases and role in arthritis. Lymphokine Cytokine Res. 1991, 10, 317–324. [Google Scholar]
- Baruch, R.R.; Melinscak, H.; Lo, J.; Liu, Y.; Yeung, O.; Hurta, R.A. Altered matrix metalloproteinase expression associated with oncogene-mediated cellular transformation and metastasis formation. Cell Biol. Int. 2001, 25, 411–420. [Google Scholar] [CrossRef]
- Kjeldsen, L.; Johnsen, A.H.; Sengelov, H.; Borregaard, N. Isolation and primary structure of NGAL, a novel protein associated with human neutrophil gelatinase. J. Biol. Chem. 1993, 268, 10425–10432. [Google Scholar] [CrossRef]
- Pereira, R.D.; De Long, N.E.; Wang, R.C.; Yazdi, F.T.; Holloway, A.C.; Raha, S. Angiogenesis in the placenta: The role of reactive oxygen species signaling. BioMed Res. Int. 2015, 2015, 814543. [Google Scholar] [CrossRef] [PubMed]
- Burton, G.J.; Woods, A.W.; Jauniaux, E.; Kingdom, J.C. Rheological and physiological consequences of conversion of the maternal spiral arteries for uteroplacental blood flow during human pregnancy. Placenta 2009, 30, 473–482. [Google Scholar] [CrossRef] [PubMed]
- Espino, Y.; Sosa, S.; Flores-Pliego, A.; Espejel-Nuñez, A.; Medina-Bastidas, D.; Vadillo-Ortega, F.; Zaga-Clavellina, V.; Estrada-Gutierrez, G. New Insights into the Role of Matrix Metalloproteinases in Preeclampsia. Int. J. Mol. Sci. 2017, 18, 1448. [Google Scholar] [CrossRef]
- Pollheimer, J.; Fock, V.; Knöfler, M. Review: The ADAM metalloproteinases—Novel regulators of trophoblast invasion? Placenta 2014, 35, S57–S63. [Google Scholar] [CrossRef]
- Carter, A.M.; Enders, A.C.; Pijnenborg, R. The role of invasive trophoblast in implantation and placentation of primates. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2015, 370, 20140070. [Google Scholar] [CrossRef]
- Zhu, J.Y.; Pang, Z.J.; Yu, Y.H. Regulation of trophoblast invasion: The role of matrix metalloproteinases. Rev. Obstet. Gynecol. 2012, 5, e137–e143. [Google Scholar]
- Pulkkinen, M.; Lehto, M.; Jalkanen, M.; Näntö-Salonen, K. Collagen types and fibronectin in the uterine muscle of normal and hypertensive pregnant patients. Am. J. Obstet. Gynecol. 1984, 149, 711–717. [Google Scholar] [CrossRef]
- Sahay, A.S.; Sundrani, D.P.; Joshi, S.R. Regional changes of placental vascularization in preeclampsia: A review. IUBMB Life 2015, 67, 619–625. [Google Scholar] [CrossRef]
- Wallis, R.M.; Hillier, K. Regulation of collagen dissolution in the human cervix by oestradiol-17 beta and progesterone. J. Reprod. Fertil. 1981, 62, 55–61. [Google Scholar] [CrossRef]
- Sato, T.; Ito, A.; Mori, Y.; Yamashita, K.; Hayakawa, T.; Nagase, H. Hormonal regulation of collagenolysis in uterine cervical fibroblasts. Modulation of synthesis of procollagenase, prostromelysin and tissue inhibitor of metalloproteinases (TIMP) by progesterone and oestradiol-17 beta. Biochem. J. 1991, 275, 645–650. [Google Scholar] [CrossRef]
- Uldbjerg, N.; Forman, A.; Petersen, L. Biochemical changes of the uterus and cervix during pregnancy. In Medicine of the Fetus and Mother; Reece, E.A., Hobbins, J.C., Mahoney, M.J., Petrie, R.H., Eds.; JB Lippincott Co.: Philadelphia, PA, USA, 1992; pp. 849–868. [Google Scholar]
- Chen, J.; Khalil, R.A. MMP in Normal pregnancy and preeclampsia. Prog. Mol. Biol. Transl. Sci. 2017, 148, 87–165. [Google Scholar] [CrossRef]
- Montagnana, M.; Lippi, G.; Albiero, A.; Scevarolli, S.; Salvagno, G.L.; Franchi, M.; Guidi, G.C. Evaluation of metalloproteinases 2 and 9 and their inhibitors in physiologic and pre-eclamptic pregnancy. J. Clin. Lab. Anal. 2009, 23, 88–92. [Google Scholar] [CrossRef]
- Eleuterio, N.M.; Palei, A.C.; Rangel Machado, J.S.; Tanus-Santos, J.E.; Cavalli, R.C.; Sandrim, V.C. Positive correlations between circulating adiponectin and MMP2 in preeclampsia pregnant. Pregnancy Hypertens. 2015, 5, 205–208. [Google Scholar] [CrossRef]
- Khalil, R.A.; Granger, J.P. Vascular mechanisms of increased arterial pressure in preeclampsia: Lessons from animal models. Am. J. Phys. Regul. Integr. Comp. Phys. 2002, 283, R29–R45. [Google Scholar] [CrossRef]
- Gilbert, J.S.; Babcock, S.A.; Granger, J.P. Hypertension produced by reduced uterine perfusion in pregnant rats is associated with increased soluble fms-like tyrosine kinase-1 expression. Hypertension 2007, 50, 1142–1147. [Google Scholar] [CrossRef]
- Alexander, B.T.; Kassab, S.E.; Miller, M.T.; Abram, S.R.; Reckelhoff, J.F.; Bennett, W.A.; Granger, J.P. Reduced uterine perfusion pressure during pregnancy in the rat is associated with increases in arterial pressure and changes in renal nitric oxide. Hypertension 2001, 37, 1191–1195. [Google Scholar] [CrossRef]
- Eiland, E.; Nzerue, C.; Faulkner, M. Preeclampsia. J. Pregnancy 2012, 2012, 1–7. [Google Scholar] [CrossRef]
- Palei, A.C.; Granger, J.P.; Tanus-Santos, J.E. Matrix metalloproteinases as drug targets in preeclampsia. Curr. Drug Targets 2013, 14, 325–334. [Google Scholar] [CrossRef]
- Lin, C.; He, H.; Cui, N.; Ren, Z.; Zhu, M.; Khalil, R.A. Decreased uterine vascularization and uterine arterial expansive remodeling with reduced matrix metalloproteinase-2 and -9 in hypertensive pregnancy. Am. J. Physiol. Heart Circ. Physiol. 2020, 318, H165–H180. [Google Scholar] [CrossRef]
- Lim, K.H.; Zhou, Y.; Janatpour, M.; McMaster, M.; Bass, K.; Chun, S.H.; Fisher, S.J. Human cytotrophoblast differentiation/invasion is abnormal in pre-eclampsia. Am. J. Pathol. 1997, 151, 1809–1818. [Google Scholar]
- Goldman-Wohl, D.S.; Yagel, S. Examination of distinct fetal and maternal molecular pathways suggests a mechanism for the development of preeclampsia. J. Reprod. Immunol. 2007, 76, 54–60. [Google Scholar] [CrossRef]
- Goldman-Wohl, D.; Yagel, S. Regulation of trophoblast invasion: From normal implantation to pre-eclampsia. Mol. Cell. Endocrinol. 2002, 187, 233–238. [Google Scholar] [CrossRef]
- Karthikeyan, V.J.; Lane, A.D.; Beevers, D.G.; Lip, G.Y.H.; Blann, A.D. Matrix metalloproteinases and their tissue inhibitors in hypertension-related pregnancy complications. J. Hum. Hypertens. 2012, 27, 72–78. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, Z.; Yao, W.; Wu, X.; Huang, J.; Huang, L.; Sun, Y. Anthropometric Indices Predict the Development of Hypertension in Normotensive and Pre-Hypertensive Middle-Aged Women in Tianjin, China: A Prospective Cohort Study. Med. Sci. Monit. 2018, 24, CLR1871–CLR1879. [Google Scholar] [CrossRef]
- Shimonovitz, S.; Hurwitz, A.; Dushnik, M.; Anteby, E.; Geva-Eldar, T.; Yagel, S. Developmental regulation of the expression of 72 and 92 kd type IV collagenases in human trophoblasts: A possible mechanism for control of trophoblast invasion. Am. J. Obstet. Gynecol. 1994, 171, 832–838. [Google Scholar] [CrossRef]
- Merchant, S.J.; Narumiya, H.; Zhang, Y.; Guilbert, L.J.; Davidge, S.T. The effects of preeclampsia and oxygen environment on endothelial release of matrix metalloproteinase. Hypertens. Pregnancy 2004, 23, 47–60. [Google Scholar] [CrossRef]
- Su, M.T.; Tsai, P.Y.; Tsai, H.L.; Chen, Y.C.; Kuo, P.L. miR-346 and miR-582-3p-regulated EG-VEGF expression and trophoblast invasion via matrix metalloproteinases 2 and 9. Biofactors 2017, 43, 210–219. [Google Scholar] [CrossRef]
- Isaka, K.; Usuda, S.; Ito, H.; Sagawa, Y.; Nakamura, H.; Nishi, H.; Suzuki, Y.; Li, Y.F.; Takayama, M. Expression and activity of matrix metalloproteinase 2 and 9 in human trophoblasts. Placenta 2003, 24, 53–64. [Google Scholar] [CrossRef]
- Li, W.; Mata, K.M.; Mazzuca, M.Q.; Khalil, R.A. Altered matrix metalloproteinase-2 and -9 expression/activity links placental ischemia and anti-angiogenic sFlt-1 to uteroplacental and vascular remodeling and collagen deposition in hypertensive pregnancy. Biochem. Pharmacol. 2014, 89, 370–385. [Google Scholar] [CrossRef]
- Ren, Z.; Cui, N.; Zhu, M.; Khalil, R.A. Placental growth factor reverses decreased vascular and uteroplacental MMP-2 and MMP-9 and increased MMP-1 and MMP-7 and collagen types I and IV in hypertensive pregnancy. Am. J. Physiol. Heart Circ. Physiol. 2018, 315, H33–H47. [Google Scholar] [CrossRef]
- Hao, S.; You, J.; Lin, C.; Zhao, H.; Huang, Y.; Zheng, L.; Tian, L.; Maric, I.; Liu, X.; Li, T.; et al. Changes in pregnancy-related serum biomarkers early in gestation are associated with later development of preeclampsia. PLoS ONE 2020, 15, e0230000. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Ren, Z.; Zhu, M.; Khalil, R.A. Decreased homodimerization and increased TIMP-1 complexation of uteroplacental and uterine arterial matrix metalloproteinase-9 during hypertension-in-pregnancy. Biochem. Pharmacol. 2017, 138, 81–95. [Google Scholar] [CrossRef]
- Dias-Junior, C.A.; Chen, J.; Cui, N. Angiogenic imbalance and diminished matrix metalloproteinase-2 and -9 underlie regional decreases in uteroplacental vascularization and feto-placental growth in hypertensive pregnancy. Biochem. Pharmacol. 2017, 146, 101–116. [Google Scholar] [CrossRef] [PubMed]
- Laskowska, M. Altered maternal serum matrix metalloproteinases MMP-2, MMP-3, MMP-9, and MMP-13 in severe early-and late-onset preeclampsia. BioMed Res. Int. 2017, 2017, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Myers, J.E.; Merchant, S.J.; MacLeod, M.; Mires, G.J.; Baker, P.N.; Davidge, S.T. MMP-2 levels are elevated in the plasma of women who subsequently develop preeclampsia. Hypertens. Pregnancy 2005, 24, 103–115. [Google Scholar] [CrossRef]
- Palei, A.C.; Sandrim, V.C.; Cavalli, R.D.C.; Tanus-Santos, J.E. Comparative assessment of matrix metalloproteinase (MMP)-2 and MMP-9, and their inhibitors, tissue inhibitors of metalloproteinase (TIMP)-1 and TIMP-2 in preeclampsia and gestational hypertension. Clin. Biochem. 2008, 41, 875–880. [Google Scholar] [CrossRef]
- Palei, A.C.; Sandrim, V.C.; Amaral, L.M.; Machado, J.S.R.; Cavalli, R.D.C.; Duarte, G.; Tanus-Santos, J.E. Association between matrix metalloproteinase (MMP)-2 polymorphisms and MMP-2 levels in hypertensive disorders of pregnancy. Exp. Mol. Pathol. 2012, 92, 217–221. [Google Scholar] [CrossRef]
- Martinez-Fierro, M.L.; Perez-Favila, A.; Garza-Veloz, I.; Espinoza-Juarez, M.A.; Avila-Carrasco, L.; Delgado-Enciso, I.; Ortiz-Castro, Y.; Cardenas-Vargas, E.; Cid-Baez, M.A.; Ramirez-Santoyo, R.M.; et al. Matrix metalloproteinase multiplex screening identifies increased MMP-2 urine concentrations in women predicted to develop preeclampsia. Biomarkers 2018, 23, 18–24. [Google Scholar] [CrossRef]
- Lavee, M.; Goldman, S.; Daniel-Spiegel, E.; Shalev, E. Matrix metalloproteinase-2 is elevated in midtrimester amniotic fluid prior to the development of preeclampsia. Reprod. Biol. Endocrinol. 2009, 23, 85. [Google Scholar] [CrossRef] [PubMed]
- Narumiya, H.; Zhang, Y.; Fernandez-Patron, C.; Guilbert, L.J.; Davidge, S.T. Matrix metalloproteinase-2 is elevated in the plasma of women with preeclampsia. Hypertens. Pregnancy 2001, 20, 185–194. [Google Scholar] [CrossRef]
- Luizon, M.R.; Palei, A.C.T.; Sandrim, V.C.; Amaral, L.M.; Machado, J.S.R.; Lacchini, R. Tissue inhibitor of matrix metalloproteinase-1 polymorphism, plasma TIMP-1 levels, and antihypertensive therapy responsiveness in hypertensive disorders of pregnancy. Pharmacogenom. J. 2014, 14, 535–541. [Google Scholar] [CrossRef] [PubMed]
- Tayebjee, M.; Karalis, I.; Nadar, S.; Beevers, D.J.; MacFadyen, R.; Lip, G.Y.H. Circulating matrix metalloproteinase–9 and tissue inhibitors of metalloproteinases-1 and -2 levels in gestational hypertension. AJH 2005, 18, 325–329. [Google Scholar] [CrossRef] [PubMed]
- Ab Hamid, J.; Mohtarrudin, N.; Osman, M.; Asri, A.A.; Hassan, W.H.W.; Aziz, R. Matrix metalloproteinase-9 and tissue inhibitors of metalloproteinases 1 and 2 as potential biomarkers for gestational hypertension. Singap. Med. J. 2012, 53, 681–683. [Google Scholar]
- Early pregnancy prediction of preeclampsia in nulliparous women, combining clinical risk and biomarkers the screening for pregnancy endpoints (SCOPE) international cohort study. Hypertension 2014, 64, 644–652. [CrossRef]
- Wang, Z.; Lu, S.; Liu, C.; Zhao, B.; Pei, K.; Tian, L.; Ma, X. Expressional and epigenetic alterations of placental matrix metalloproteinase 9 in preeclampsia. Gynecol. Endocrinol. 2010, 26, 96–102. [Google Scholar] [CrossRef]
- Meng, H.; Qi, M.; Liu, Y.; Xu, L.; Li, Q. Research on correlation between MMP-9 and early-onset preeclampsia. Int. J. Clin. Exp. Med. 2016, 9, 17442–17448. [Google Scholar]
- Zhang, Y.; Li, P.; Guo, Y.; Liu, X.; Zhang, Y. MMP-9 and TIMP-1 in placenta of hypertensive disorder complicating pregnancy. Exp. Ther. Med. 2019, 18, 637–641. [Google Scholar] [CrossRef]
| Authors | Hypertensive Disorder | Sample | Method | Findings |
|---|---|---|---|---|
| Li et al., 2014 [66] | Normal pregnant vs. RUPP rats | Uterus, placenta, aorta | Zymography | Decreased MMP-2 activity |
| Ren et al., 2018 [67] | Normal pregnant vs. RUPP rats | Uterus, placenta, aorta | Zymography | Decreased MMP-2 activity |
| Lin et al., 2020 [56] | Normal pregnant vs. RUPP rats | Uterus, uterine spiral arteries | Zymography Immunohistochemistry Uterine histology | Decreased MMP-2 activity/expression and concentration |
| Chen et al., 2017 [69] | Normal pregnant vs. RUPP rats | Uterus, placenta, uterine artery | Zymography | Decreased MMP-2 activity |
| Dias-Junior et al., 2017 [70] | Normal pregnant vs. RUPP rats | Uterus | Zymography | Decreased MMP-2 activity/levels |
| Laskowska, 2017 [71] | Early onset PE vs. Late onset PE | Serum | ELISA | Increased MMP-2 levels |
| Myers Jenny et al., 2005 [72] | PE | Plasma | Zymography | Increased MMP-2 activity at 22 and at diagnosis, no difference at 26 week |
| Palei et al., 2008 and 2012 [73,74] | PE/GH | Plasma | Zymography ELISA | Increased MMP-2 and MMP-2/TIMP-2 ratio in GH |
| Montegranna et al., 2009 [49] | PE | Serum | ELISA | Increased MMP-2 levels in PE vs. both non-pregnant and physiologic pregnant women |
| Martinez-Fierro et al., 2018 [75] | Early-pregnancy | Urine | Quantitive evaluation | Increased MMP-2 levels |
| Lavee et al., 2009 [76] | PE | Amniotic fluid | ELISA | Increased MMP-2 levels in amniotic fluid |
| Narumiya et al., 2001 [77] | PE | Plasma | Zymography | Increased MMP-2 activity |
| Authors | Hypertensive Disorder | Sample | Method | Findings |
|---|---|---|---|---|
| Laskowska, 2017 [71] | Early onset PE vs. Late onset PE | Serum | ELISA | Decreased MMP-9 levels |
| Li et al., 2014 [66] | Normal pregnant vs. RUPP rats | Uterus, placenta, aorta | Zymography | Decreased MMP-9 activity |
| Ren et al., 2018 [67] | Normal pregnant vs. RUPP rats | Uterus, placenta, aorta | Zymography | Decreased MMP-9 activity |
| Lin et al., 2020 [56] | Normal pregnant vs. RUPP rats | Uterus, uterine spiral arteries | Zymography Immunohistochemistry Uterine histology | Decreased MMP-9 activity/expression and concentration |
| Chen et al., 2017 [69] | Normal pregnant vs. RUPP rats | Uterus, placenta, uterine artery | Zymography | Decreased MMP-9 activity |
| Dias-Junior et al., 2017 [70] | Normal pregnant vs. RUPP rats | Uterus | Zymography | Decreased MMP-9 activity/levels |
| Luizon et al., 2014 [78] | PE, GH | Plasma | ELISA | Decreased MMP-9 levels andMMP-9/TIMP-1 ratio in GH patients with GG genotype |
| Taybjee et al., 2004 [79] | GH | Plasma | ELISA | Decreased MMP-9 levels |
| SCOPE [81] | PE | Plasma | Decreased MMP-9 levels | |
| Montegranna et al., 2009 [49] | PE | Serum | ELISA | Decreased MMP-9 levels in PE vs. normal pregnant women (Non-significantly) |
| Zhamg et al., 2019 [84] | PE | Placenta | Immunohistochemistry | Decreased MMP-9 expression with aggraviation of pregnancy induced hypertension |
| Palei et al., 2008, 2012 [73,74] | PE/GH | Plasma | Zymography ELISA | Increased MMP-9 activity in GH |
| Ab Hamid et al., 2012 [80] | GH | Serum | ELISA | Increased MMP-9 levels |
| Wang et al., 2010 [82] | PE | Placenta | MSR, PCR | Increased MMP-9 expression levels |
| Meng et al., 2016 [83] | PE | Placenta, Serum | ELISA Western blot | Increased serum MMP-9 levels Decreased MMP-9 expression in placenta |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nikolov, A.; Popovski, N. Role of Gelatinases MMP-2 and MMP-9 in Healthy and Complicated Pregnancy and Their Future Potential as Preeclampsia Biomarkers. Diagnostics 2021, 11, 480. https://doi.org/10.3390/diagnostics11030480
Nikolov A, Popovski N. Role of Gelatinases MMP-2 and MMP-9 in Healthy and Complicated Pregnancy and Their Future Potential as Preeclampsia Biomarkers. Diagnostics. 2021; 11(3):480. https://doi.org/10.3390/diagnostics11030480
Chicago/Turabian StyleNikolov, Asparuh, and Nikola Popovski. 2021. "Role of Gelatinases MMP-2 and MMP-9 in Healthy and Complicated Pregnancy and Their Future Potential as Preeclampsia Biomarkers" Diagnostics 11, no. 3: 480. https://doi.org/10.3390/diagnostics11030480
APA StyleNikolov, A., & Popovski, N. (2021). Role of Gelatinases MMP-2 and MMP-9 in Healthy and Complicated Pregnancy and Their Future Potential as Preeclampsia Biomarkers. Diagnostics, 11(3), 480. https://doi.org/10.3390/diagnostics11030480







