Synovial Complement Factors in Patients with Periprosthetic Joint Infection after Undergoing Revision Arthroplasty of the Hip or Knee Joint
Abstract
1. Introduction
2. Materials and Methods
2.1. Synovial Fluid Samples
- (1)
- at least one of the following major criteria was fulfilled:
- The presence of a sinus tract with evidence of communication with the joint or visualization of the prosthesis
- Two positive cultures of the same organism
- (2)
- a score ≥6 with the following minor criteria was achieved (preoperative diagnosis):
- Elevated serum C-reactive protein (>1 mg/dL) or D-dimer level (>860 ng/mL; score: 2)
- Elevated serum erythrocyte sedimentation rate (>30 mm/h; score: 1)
- Elevated synovial white blood cell count (>3000 cells/µL) or leukocyte esterase (++; score: 3)
- Positive synovial alpha-defensin (signal-to-cutoff ratio > 1; score: 3)
- Elevated synovial polymorphonuclear percentage (>80%; score: 2)
- Elevated synovial C-reactive protein level (>6.9 mg/L; score: 1)
- (3)
- A score ≥6 with the following criteria is achieved (intraoperative diagnosis; ≤3, not infected; 4 or 5, inconclusive):
- Preoperative score
- Positive histology (score: 3)
- Positive purulence (score: 3)
- Single positive culture (score: 2)
2.2. Statistical Analyses
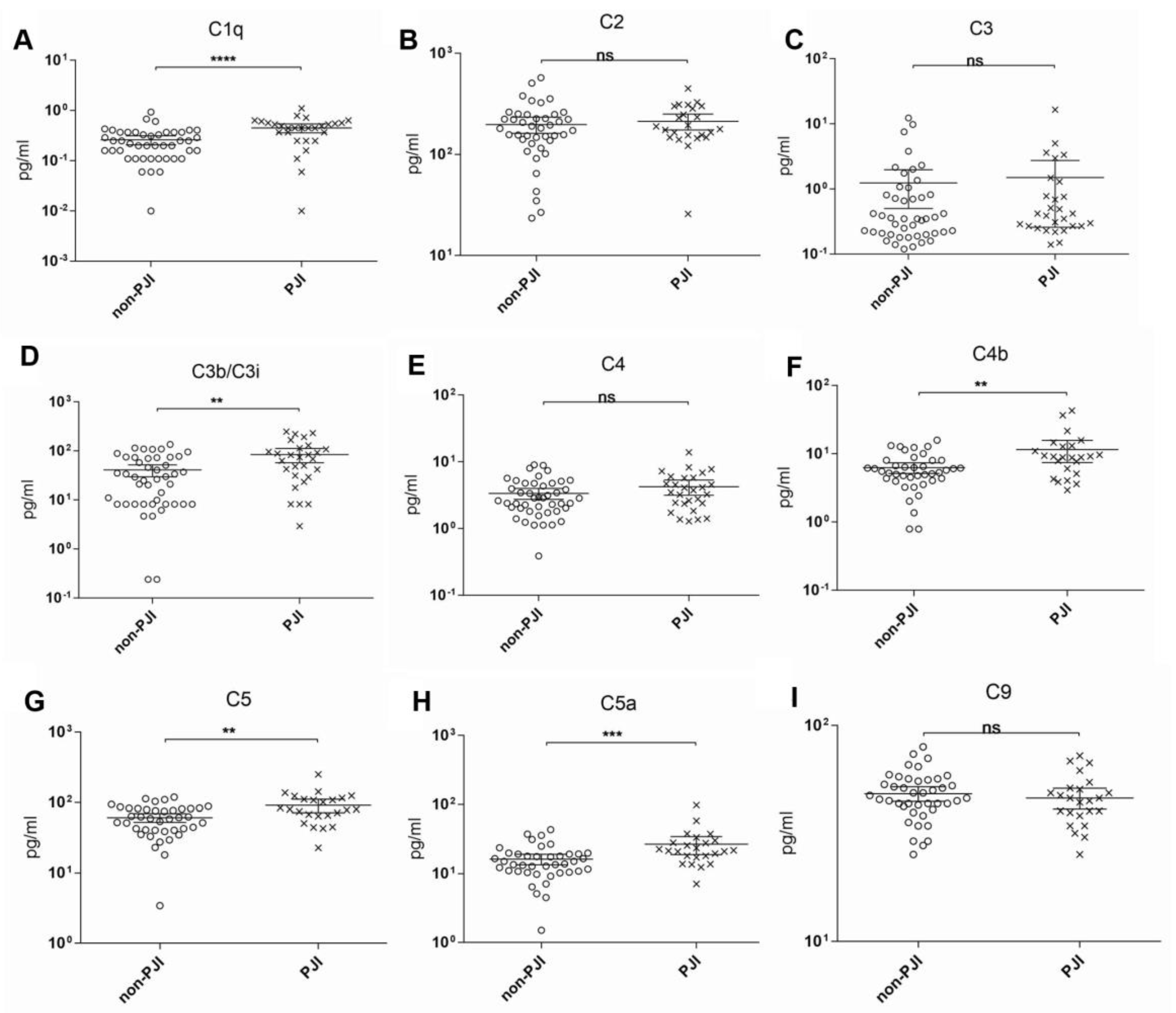
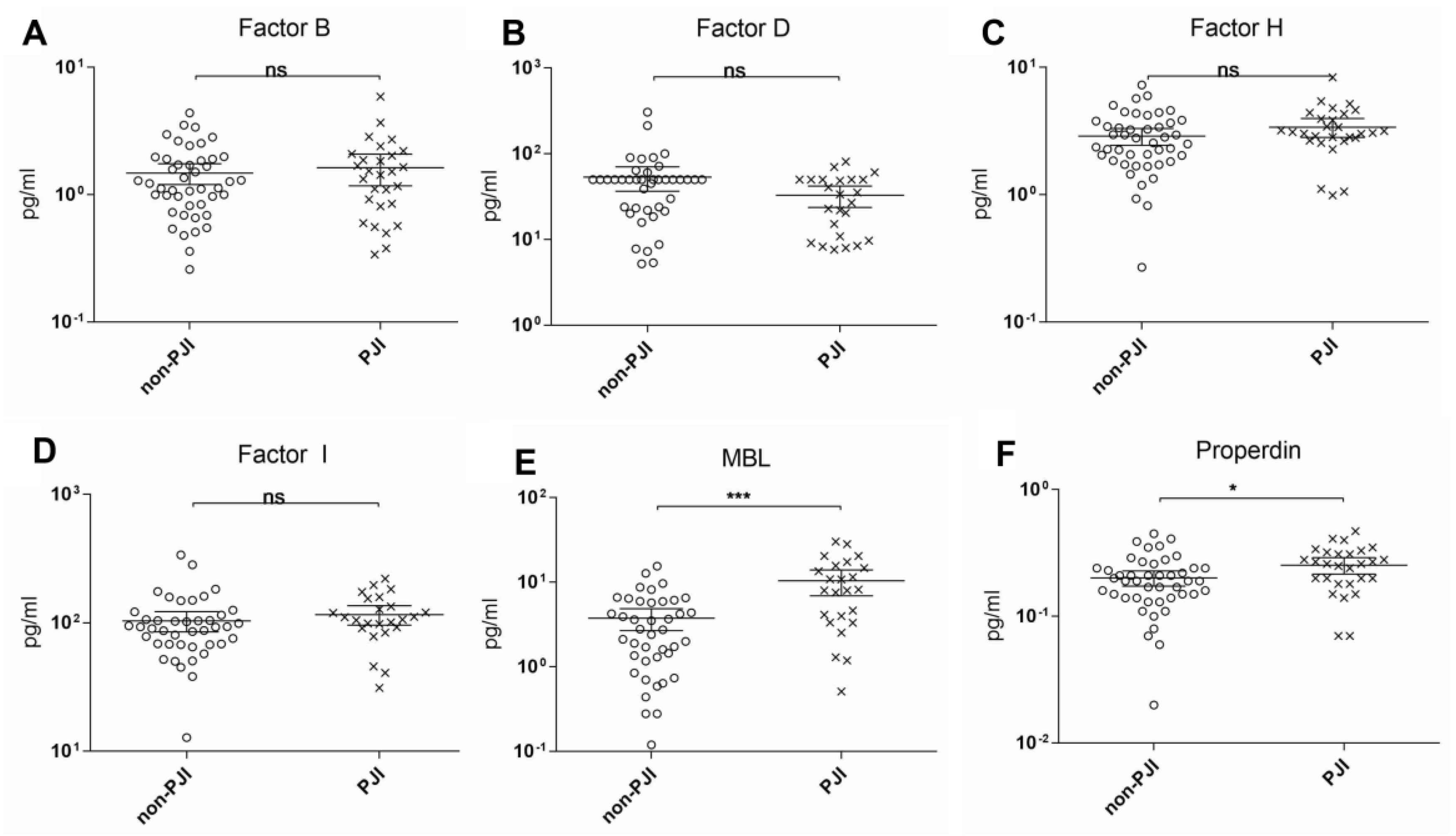
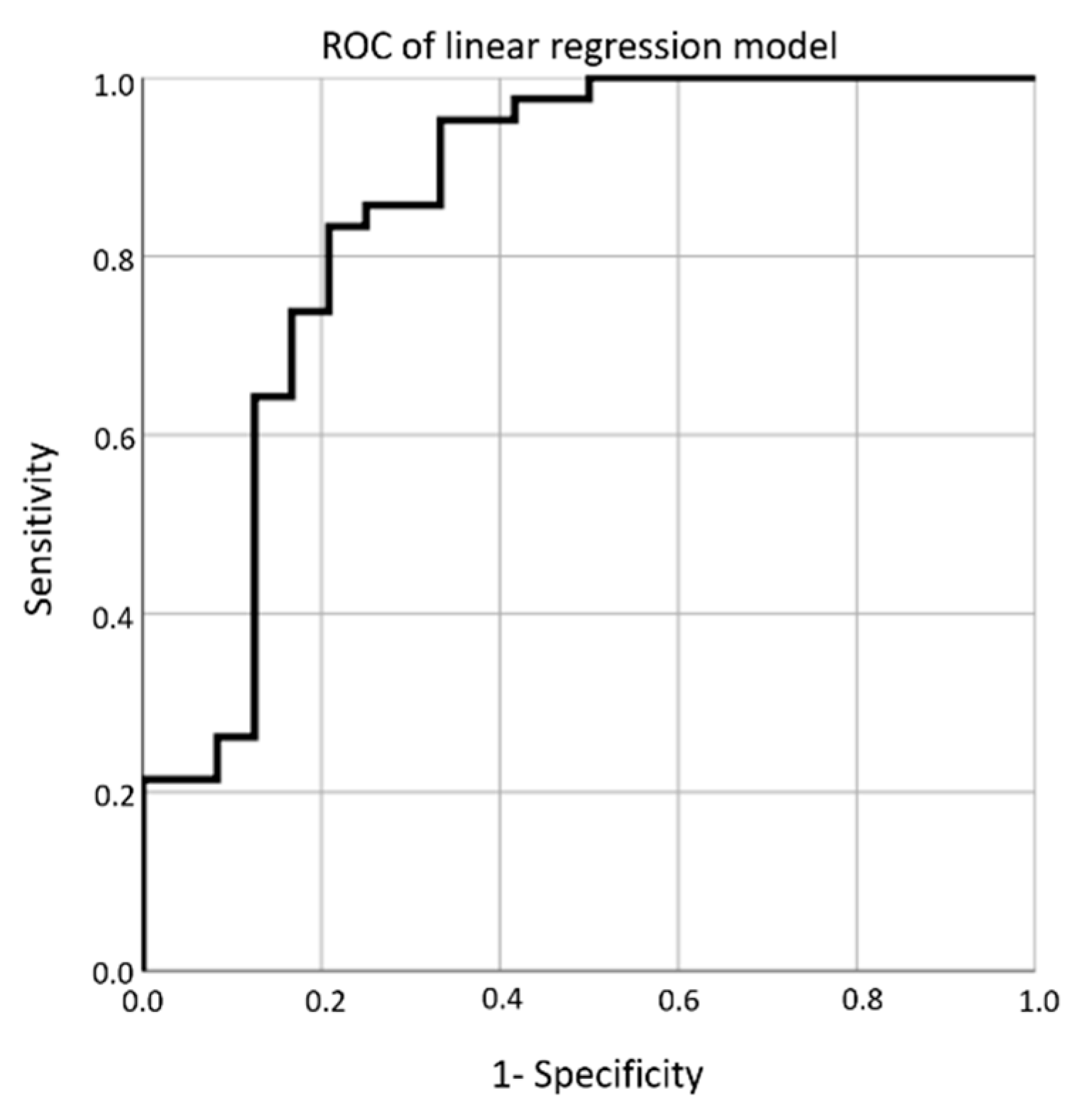
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AIS | adaptive immune system |
| AUC | area under the curve |
| CI | confidence interval |
| MSIS | Musculoskeletal Infection Society |
| PJI | periprosthetic joint infection |
| ROC | Receiver-operator characteristic |
References
- Matsen Ko, L.; Parvizi, J. Diagnosis of Periprosthetic Infection. Orthop. Clin. N. Am. 2016, 47, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.S.; Koo, K.-H.; Kim, H.J.; Tian, S.; Kim, T.-Y.; Maltenfort, M.G.; Chen, A.F. Synovial Fluid Biomarkers for the Diagnosis of Periprosthetic Joint Infection: A Systematic Review and Meta-Analysis. J. Bone Jt. Surg. 2017, 99, 2077–2084. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.Z.; Saleh, A.; Klika, A.K.; Barsoum, W.K.; Higuera, C.A. Serum Inflammatory Markers for Periprosthetic Knee Infection in Obese Versus Non-Obese Patients. J. Arthroplast. 2014, 29, 1880–1883. [Google Scholar] [CrossRef]
- Deirmengian, C.; Lonner, J.H.; Booth, R.E. The Mark Coventry Award: White Blood Cell Gene Expression: A New Approach toward the Study and Diagnosis of Infection. Clin. Orthop. Relat. Res. 2005, 440, 38–44. [Google Scholar] [CrossRef]
- Fröschen, F.S.; Schell, S.; Schildberg, F.A.; Klausing, A.; Kohlhof, H.; Gravius, S.; Randau, T.M. Analysis of Synovial Biomarkers with a Multiplex Protein Microarray in Patients with PJI Undergoing Revision Arthroplasty of the Hip or Knee Joint. Arch. Orthop. Trauma Surg. 2020. [Google Scholar] [CrossRef]
- Fröschen, F.S.; Walter, S.G.; Randau, T.M.; Gravius, N.; Gravius, S.; Hischebeth, G.T.R. The Use of Negative Pressure Wound Therapy Increases Failure Rate in Debridement and Implant Retention for Acute Prosthetic Joint Infection. Technol. Health Care 2020, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Kleeman-Forsthuber, L.T.; Johnson, R.M.; Brady, A.C.; Pollet, A.K.; Dennis, D.A.; Jennings, J.M. Alpha-Defensin Offers Limited Utility in Routine Workup of Periprosthetic Joint Infection. J. Arthroplast. 2020, S0883540320312572. [Google Scholar] [CrossRef]
- Kleeman-Forsthuber, L.T.; Dennis, D.A.; Brady, A.C.; Pollet, A.K.; Johnson, R.M.; Jennings, J.M. Alpha-Defensin Is Not Superior to Traditional Diagnostic Methods for Detection of Periprosthetic Joint Infection in Total Hip Arthroplasty and Total Knee Arthroplasty Patients. J. Arthroplast. 2021, S0883540321000760. [Google Scholar] [CrossRef]
- Dunkelberger, J.R.; Song, W.-C. Complement and Its Role in Innate and Adaptive Immune Responses. Cell Res. 2010, 20, 34–50. [Google Scholar] [CrossRef]
- Struglics, A.; Okroj, M.; Swärd, P.; Frobell, R.; Saxne, T.; Lohmander, L.S.; Blom, A.M. The Complement System Is Activated in Synovial Fluid from Subjects with Knee Injury and from Patients with Osteoarthritis. Arthritis Res. Ther. 2016, 18, 223. [Google Scholar] [CrossRef]
- Sena, L.; Oliveira-Toré, C.F.; Skare, T.; de Messias-Reason, I.J.; Andrade, F.A. C3 Gene Functional Polymorphisms and C3 Serum Levels in Patients with Rheumatoid Arthritis. Immunol. Investig. 2020, 1–15. [Google Scholar] [CrossRef]
- Wimmer, M.D.; Randau, T.M.; Petersdorf, S.; Pagenstert, G.I.; Weißkopf, M.; Wirtz, D.C.; Gravius, S. Evaluation of an Interdisciplinary Therapy Algorithm in Patients with Prosthetic Joint Infections. Int. Orthop. 2013, 37, 2271–2278. [Google Scholar] [CrossRef]
- Parvizi, J.; Tan, T.L.; Goswami, K.; Higuera, C.; Della Valle, C.; Chen, A.F.; Shohat, N. The 2018 Definition of Periprosthetic Hip and Knee Infection: An Evidence-Based and Validated Criteria. J. Arthroplast. 2018, 33, 1309–1314. [Google Scholar] [CrossRef] [PubMed]
- Deirmengian, C.; Kardos, K.; Kilmartin, P.; Cameron, A.; Schiller, K.; Parvizi, J. Diagnosing Periprosthetic Joint Infection: Has the Era of the Biomarker Arrived? Clin. Orthop. Relat. Res. 2014, 472, 3254–3262. [Google Scholar] [CrossRef]
- Fleming, S.D.; Shea-Donohue, T.; Guthridge, J.M.; Kulik, L.; Waldschmidt, T.J.; Gipson, M.G.; Tsokos, G.C.; Holers, V.M. Mice Deficient in Complement Receptors 1 and 2 Lack a Tissue Injury-Inducing Subset of the Natural Antibody Repertoire. J. Immunol. 2002, 169, 2126–2133. [Google Scholar] [CrossRef]
- Reid, R.R.; Woodcock, S.; Shimabukuro-Vornhagen, A.; Austen, W.G.; Kobzik, L.; Zhang, M.; Hechtman, H.B.; Moore, F.D.; Carroll, M.C. Functional Activity of Natural Antibody is Altered in Cr2-Deficient Mice. J. Immunol. 2002, 169, 5433–5440. [Google Scholar] [CrossRef]
- Na, M.; Jarneborn, A.; Ali, A.; Welin, A.; Magnusson, M.; Stokowska, A.; Pekna, M.; Jin, T. Deficiency of the Complement Component 3 but Not Factor B Aggravates Staphylococcus Aureus Septic Arthritis in Mice. Infect. Immun. 2016, 84, 930–939. [Google Scholar] [CrossRef] [PubMed]
- Streit, G.; Alber, D.; Toubin, M.M.; Toussirot, E.; Wendling, D. Procalcitonin, C-Reactive Protein, and Complement-3a Assays in Synovial Fluid for Diagnosing Septic Arthritis: Preliminary Results. Jt. Bone Spine 2008, 75, 238–239. [Google Scholar] [CrossRef]
- Hokstad, I.; Deyab, G.; Wang Fagerland, M.; Lyberg, T.; Hjeltnes, G.; Førre, Ø.; Agewall, S.; Mollnes, T.E.; Hollan, I. Tumor Necrosis Factor Inhibitors Are Associated with Reduced Complement Activation in Spondylarthropathies: An Observational Study. PLoS ONE 2019, 14, e0220079. [Google Scholar] [CrossRef] [PubMed]
- AL-Ishaq, R.; Armstrong, J.; Gregory, M.; O’Hara, M.; Phiri, K.; Harris, L.G.; Rohde, H.; Siemssen, N.; Frommelt, L.; Mack, D.; et al. Effects of Polysaccharide Intercellular Adhesin (PIA) in an Ex Vivo Model of Whole Blood Killing and in Prosthetic Joint Infection (PJI): A Role for C5a. Int. J. Med Microbiol. 2015, 305, 948–956. [Google Scholar] [CrossRef] [PubMed]
- Giang, J.; Seelen, M.A.J.; van Doorn, M.B.A.; Rissmann, R.; Prens, E.P.; Damman, J. Complement Activation in Inflammatory Skin Diseases. Front. Immunol. 2018, 9, 639. [Google Scholar] [CrossRef]
- Lee, J.-H.; Jung, J.H.; Kim, J.; Baek, W.-K.; Rhee, J.; Kim, T.-H.; Kim, S.-H.; Kim, K.P.; Son, C.-N.; Kim, J.-S. Proteomic Analysis of Human Synovial Fluid Reveals Potential Diagnostic Biomarkers for Ankylosing Spondylitis. Clin. Proteom. 2020, 17, 20. [Google Scholar] [CrossRef]
- Thordardottir, S.; Vikingsdottir, T.; Bjarnadottir, H.; Jonsson, H.; Gudbjornsson, B. Activation of Complement Following Total Hip Replacement. Scand. J. Immunol. 2016, 83, 219–224. [Google Scholar] [CrossRef] [PubMed]
- Sigmund, I.K.; Holinka, J.; Gamper, J.; Staats, K.; Böhler, C.; Kubista, B.; Windhager, R. Qualitative α-Defensin Test (Synovasure) for the Diagnosis of Periprosthetic Infection in Revision Total Joint Arthroplasty. Bone Jt. J. 2017, 99-B, 66–72. [Google Scholar] [CrossRef]
- Renz, N.; Yermak, K.; Perka, C.; Trampuz, A. Alpha Defensin Lateral Flow Test for Diagnosis of Periprosthetic Joint Infection: Not a Screening but a Confirmatory Test. J. Bone Jt. Surg. 2018, 100, 742–750. [Google Scholar] [CrossRef] [PubMed]
- Grzelecki, D.; Walczak, P.; Szostek, M.; Grajek, A.; Rak, S.; Kowalczewski, J. Blood and Synovial Fluid Calprotectin as Biomarkers to Diagnose Chronic Hip and Knee Periprosthetic Joint Infections. Bone Jt. J. 2021, 103-B, 46–55. [Google Scholar] [CrossRef] [PubMed]
- Qin, L.; Li, X.; Wang, J.; Gong, X.; Hu, N.; Huang, W. Improved Diagnosis of Chronic Hip and Knee Prosthetic Joint Infection Using Combined Serum and Synovial IL-6 Tests. Bone Jt. Res. 2020, 9, 587–592. [Google Scholar] [CrossRef]

| PJI | Non-PJI | p Value 1 | |
|---|---|---|---|
| Total (n) | 28 | 46 | |
| F: M | 17:11 | 29:17 | 0.841 |
| Hip: knee | 11:17 | 10:36 | 0.104 |
| Age (years) | 72.9 ± 11.7 | 67.6 ± 10.5 | 0.018 |
| BMI (kg/m2) | 33.5 ± 11.7 | 29.7 ± 5.75 | 0.183 |
| Target | ROC Area 1 | ROC 95% CI | ROC p Value | Cutoff (pg/mL) | Sensitivity (%) | Specificity (%) | Likelihood Ratio |
|---|---|---|---|---|---|---|---|
| C1q | 0.754 | 0.629–0.878 | 0.00026 | 0.32 | 75.0 | 70.0 | 2.5 |
| C2 | 0.560 | 0.418–0.702 | 0.424 | 147.57 | 79.5 | 31.0 | 1.15 |
| C3 | 0.567 | 0.434–0.699 | 0.372 | 0.27 | 67.9 | 47.8 | 0.97 |
| C3b/iC3b | 0.703 | 0.578–0.826 | 0.004 | 41.58 | 71.4 | 65.0 | 2.04 |
| C4 | 0.587 | 0.454–0.721 | 0.210 | 2.42 | 0.71 | 0.46 | 1.31 |
| C4b | 0.689 | 0.551-0.827 | 0.011 | 7.23 | 70.8 | 71.4 | 2.41 |
| C5 | 0.697 | 0.566–0.829 | 0.008 | 65.09 | 79.2 | 59.5 | 1.85 |
| C5a | 0.742 | 0.619–0.864 | 0.001 | 18.05 | 75.0 | 69.0 | 2.41 |
| C9 | 0.437 | 0.292–0.583 | 0.401 | 39.91 | 70.8 | 21.4 | 0.90 |
| Factor B | 0.519 | 0.381–0.658 | 0.07 | 1.09 | 69.9 | 50.0 | 1.39 |
| Factor D | 0.305 | 0.177–0.432 | 0.009 | 8.61 | 83.3 | 11.9 | 0.95 |
| Factor H | 0.609 | 0.478–0.741 | 0.067 | 2.62 | 82.1 | 50.0 | 1.64 |
| Factor I | 0.630 | 0.488–0.772 | 0.080 | 84.84 | 79.2 | 52.4 | 1.65 |
| MBL | 0.750 | 0.623–0.877 | 0.001 | 3.80 | 75.0 | 59.5 | 1.84 |
| Properdin | 0.652 | 0.522–0.782 | 0.029 | 0.20 | 75.0 | 54.3 | 1.63 |
| Cutoff | Sensitivity | Specificity | Likelihood Ratio |
|---|---|---|---|
| 0.000 | 1.000 | 0.000 | 1.000 |
| 0.023 | 1.000 | 0.083 | 1.091 |
| 0.041 | 1.000 | 0.167 | 1.200 |
| 0.087 | 1.000 | 0.250 | 1.334 |
| 0.114 | 1.000 | 0.333 | 1.500 |
| 0.178 | 1.000 | 0.417 | 1.713 |
| 0.251 | 1.000 | 0.500 | 2.000 |
| 0.364 | 0.976 | 0.542 | 2.129 |
| 0.420 | 0.952 | 0.583 | 2.286 |
| 0.460 | 0.952 | 0.667 | 2.856 |
| 0.491 | 0.905 | 0.667 | 2.713 |
| 0.508 | 0.881 | 0.667 | 2.643 |
| 0.531 | 0.857 | 0.667 | 2.571 |
| 0.574 | 0.857 | 0.750 | 3.429 |
| 0.592 | 0.833 | 0.750 | 3.332 |
| 0.624 | 0.833 | 0.792 | 4.000 |
| 0.656 | 0.810 | 0.792 | 3.886 |
| 0.678 | 0.786 | 0.792 | 3.771 |
| 0.707 | 0.738 | 0.792 | 3.543 |
| 0.742 | 0.714 | 0.833 | 4.286 |
| 0.760 | 0.667 | 0.833 | 4.000 |
| 0.774 | 0.643 | 0.833 | 3.856 |
| 0.799 | 0.619 | 0.875 | 4.951 |
| 0.818 | 0.595 | 0.875 | 4.762 |
| 0.846 | 0.548 | 0.875 | 4.381 |
| 0.851 | 0.524 | 0.875 | 4.190 |
| 0.866 | 0.476 | 0.875 | 3.809 |
| 0.878 | 0.429 | 0.875 | 3.429 |
| 0.890 | 0.405 | 0.875 | 3.237 |
| 0.896 | 0.357 | 0.875 | 2.856 |
| 0.905 | 0.310 | 0.875 | 2.475 |
| 0.908 | 0.286 | 0.875 | 2.286 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fröschen, F.S.; Schell, S.; Wimmer, M.D.; Hischebeth, G.T.R.; Kohlhof, H.; Gravius, S.; Randau, T.M. Synovial Complement Factors in Patients with Periprosthetic Joint Infection after Undergoing Revision Arthroplasty of the Hip or Knee Joint. Diagnostics 2021, 11, 434. https://doi.org/10.3390/diagnostics11030434
Fröschen FS, Schell S, Wimmer MD, Hischebeth GTR, Kohlhof H, Gravius S, Randau TM. Synovial Complement Factors in Patients with Periprosthetic Joint Infection after Undergoing Revision Arthroplasty of the Hip or Knee Joint. Diagnostics. 2021; 11(3):434. https://doi.org/10.3390/diagnostics11030434
Chicago/Turabian StyleFröschen, Frank Sebastian, Sophia Schell, Matthias Dominik Wimmer, Gunnar Thorben Rembert Hischebeth, Hendrik Kohlhof, Sascha Gravius, and Thomas Martin Randau. 2021. "Synovial Complement Factors in Patients with Periprosthetic Joint Infection after Undergoing Revision Arthroplasty of the Hip or Knee Joint" Diagnostics 11, no. 3: 434. https://doi.org/10.3390/diagnostics11030434
APA StyleFröschen, F. S., Schell, S., Wimmer, M. D., Hischebeth, G. T. R., Kohlhof, H., Gravius, S., & Randau, T. M. (2021). Synovial Complement Factors in Patients with Periprosthetic Joint Infection after Undergoing Revision Arthroplasty of the Hip or Knee Joint. Diagnostics, 11(3), 434. https://doi.org/10.3390/diagnostics11030434







