Accuracy and Reproducibility of Contrast-Enhanced Mammography in the Assessment of Response to Neoadjuvant Chemotherapy in Breast Cancer Patients with Calcifications in the Tumor Bed
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Setting and Study Population
2.3. Clinicopathological Data
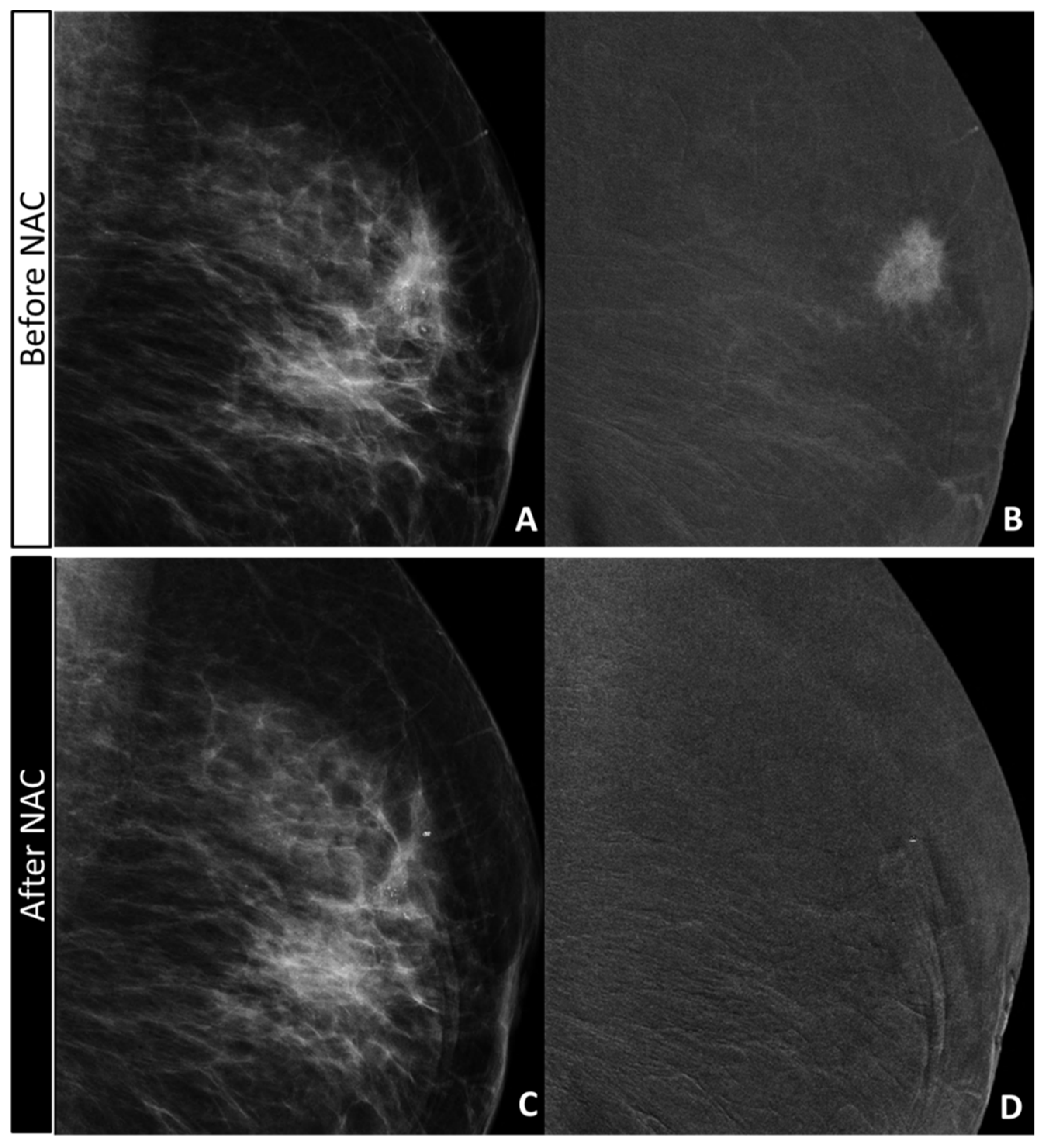
2.4. CEM Image Acquisition and Retrospective Review
- -
- the absence or presence of contrast enhancement (CE) on recombined images, defining the maximum dimension (mm) of CE before NAC (defining the tumor bed) and after NAC (defining the residual disease);
- -
- the maximum extension (mm) and characteristics (according to BIRADS lexicon) of calcifications on low-energy images before NAC (defining the tumor bed) and after NAC (defining the residual disease);
- -
- the maximum extension (mm) of the combined evaluation of pathological calcifications and enhancement before (defining the tumor bed) and after (defining the residual disease) NAC;
- -
- the radiological response after NAC according to both the combined evaluation and the evaluation of CE only:
- ○
- absence of residual disease (complete response (rCR)) or
- ○
- the persistence of residual disease (partial response (rPR), stable disease, and progressive disease compared to the initial tumor bed).
2.5. Statistical Analyses
3. Results
3.1. Population
3.2. Accuracy of CEM in Detecting Residual Tumor
3.3. Analysis of Discordant Cases
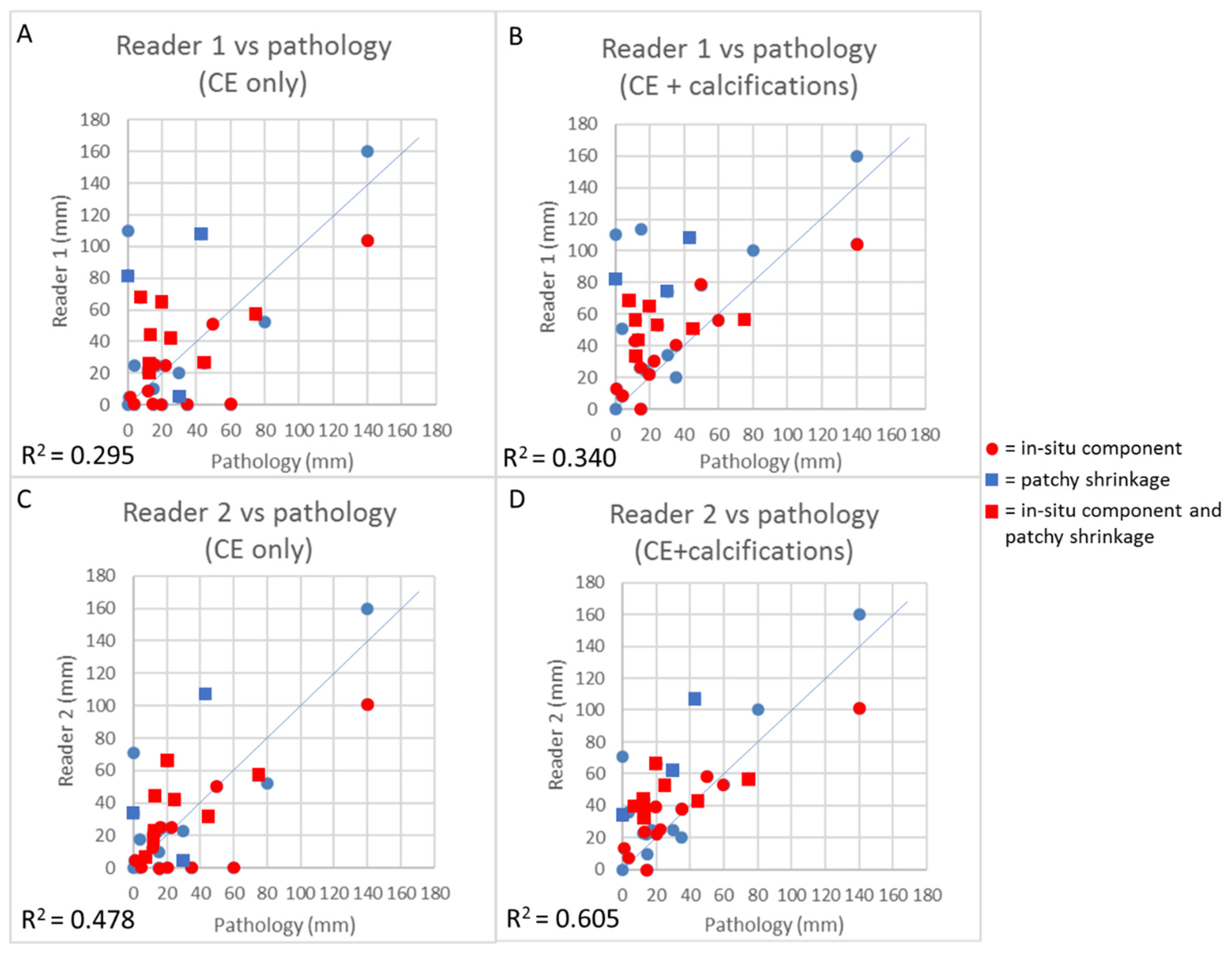
3.4. Concordance Between CEM and Pathology in the Measurement of Residual Tumor Size
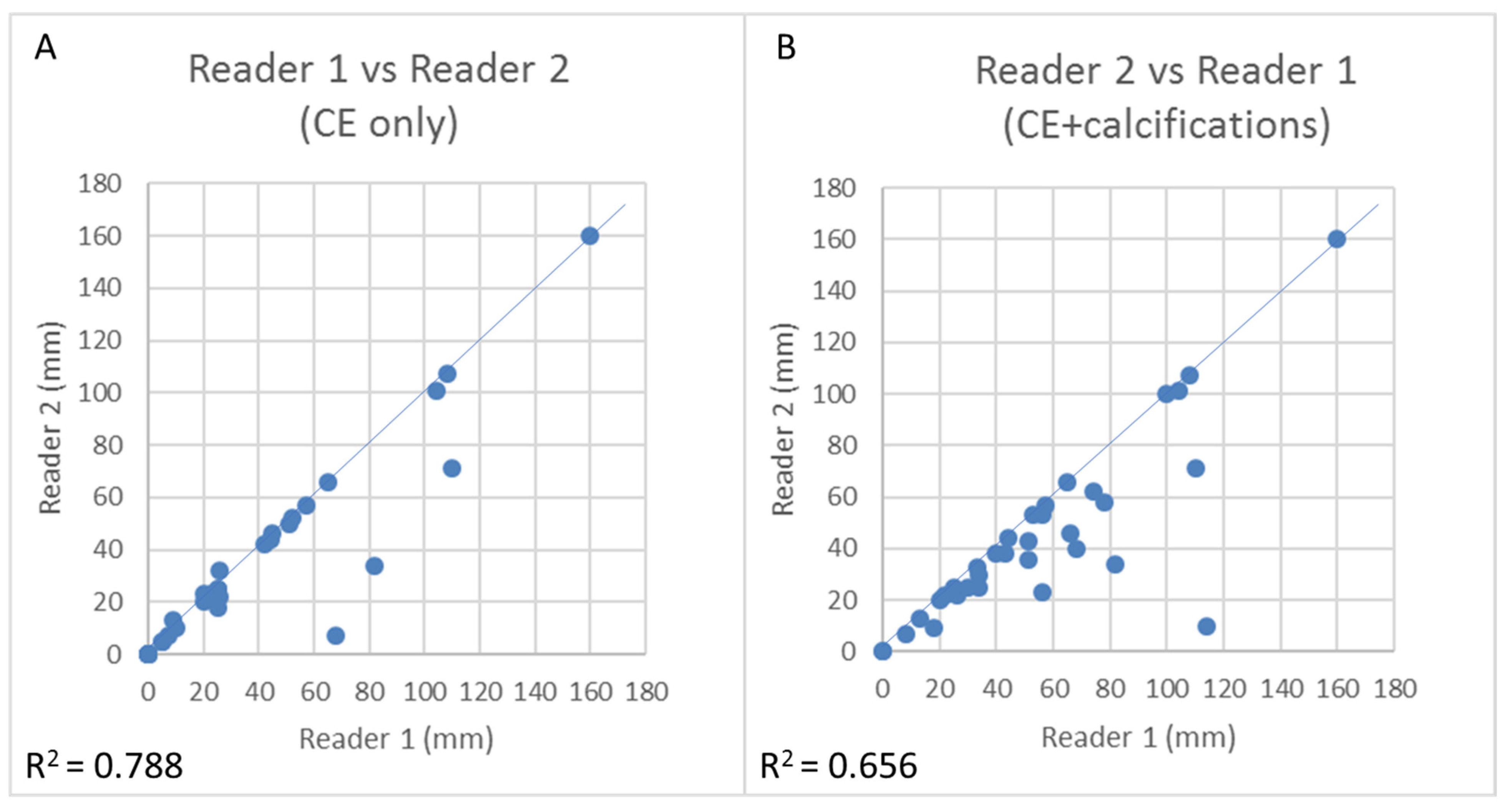
3.5. CEM Reproducibility
4. Discussion
4.1. Comparison with Previous Studies
4.2. Limitations
4.3. Implications for Practice and Research
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cardoso, F.; Senkus, E.; Costa, A.; Papadopoulos, E.; Aapro, M.; André, F.; Harbeck, N.; Aguilar Lopez, B.; Barrios, C.H.; Bergh, J.; et al. 4th ESO-ESMO international consensus guidelines for advanced breast cancer (ABC 4). Annu. Oncol. 2018, 29, 1634–1657. [Google Scholar] [CrossRef] [PubMed]
- Scheel, J.R.; Kim, E.; Partridge, S.C.; Lehman, C.D.; Rosen, M.A.; Bernreuter, W.K.; Pisano, E.D.; Marques, H.S.; Morris, E.A.; Weatherall, P.T.; et al. MRI, clinical examination, and mammography for preoperative assessment of residual disease and pathologic complete response after neoadjuvant chemotherapy for breast cancer: ACRIN 6657 trial. AJR Am. J. Roentgenol. 2018, 210, 1376–1385. [Google Scholar] [CrossRef]
- Senkus, E.; Kyriakides, S.; Ohno, S.; Penault-Llorca, F.; Poortmans, P.; Rutgers, E.; Zackrisson, S.; Cardoso, F.; ESMO guidelines committee. Primary breast cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Annu. Oncol. 2015, 26, 8–30. [Google Scholar] [CrossRef] [PubMed]
- Jones, E.F.; Hathi, D.K.; Freimanis, R.; Mukhtar, R.A.; Chien, A.J.; Esserman, L.J.; Van’t Veer, L.J.; Joe, B.N.; Hylton, N.M. Current landscape of breast cancer imaging and potential quantitative imaging markers of response in ER-positive breast cancers treated with Neoadjuvant therapy. Cancers Basel 2020, 12, 1511. [Google Scholar] [CrossRef]
- Namura, M.; Tsunoda, H.; Yagata, H.; Hayashi, N.; Yoshida, A.; Morishita, E.; Takei, J.; Suzuki, K.; Yamauchi, H. Discrepancies between pathological tumor responses and estimations of complete response by magnetic resonance imaging after Neoadjuvant Chemotherapy differ by breast cancer subtype. Clin. Breast Cancer 2018, 18, 128–134. [Google Scholar] [CrossRef]
- Buonomo, O.C.; Grasso, A.; Pistolese, C.A.; Anemona, L.; Portarena, I.; Meucci, R.; Morando, L.; Deiana, C.; Materazzo, M.; Vanni, G. Evaluation of Concordance between histopathological, radiological and biomolecular variables in breast cancer Neoadjuvant treatment. Anticancer Res. 2020, 40, 281–286. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, D.; Liu, Z.; Wang, Z.; Li, Q.; Xu, H.; Zhang, B.; Liu, T.; Jin, F. The diagnostic accuracy of magnetic resonance imaging in predicting pathologic complete response after neoadjuvant chemotherapy in patients with different molecular subtypes of breast cancer. Quant. Imaging Med. Surg. 2020, 10, 197–210. [Google Scholar] [CrossRef]
- Choi, W.J.; Kim, H.H.; Cha, J.H.; Shin, H.J.; Chae, E.Y.; Yoon, G.Y. Complete response on MR imaging after neoadjuvant chemotherapy in breast cancer patients: Factors of radiologic-pathologic discordance. Eur. J. Radiol. 2019, 118, 114–121. [Google Scholar] [CrossRef] [PubMed]
- Ko, E.S.; Han, B.K.; Kim, R.B.; Ko, E.Y.; Shin, J.H.; Hahn, S.Y.; Nam, S.J.; Lee, J.E.; Lee, S.K.; Im, Y.H.; et al. Analysis of factors that influence the accuracy of magnetic resonance imaging for predicting response after neoadjuvant chemotherapy in locally advanced breast cancer. Annu. Surg. Oncol. 2013, 20, 2562–2568. [Google Scholar] [CrossRef]
- Sun, S.; van la Parra, R.F.D.; Rauch, G.M.; Checka, C.; Tadros, A.B.; Lucci, A., Jr.; Teshome, M.; Black, D.; Hwang, R.F.; Smith, B.D.; et al. Patient selection for clinical trials eliminating surgery for HER2-positive breast cancer treated with Neoadjuvant systemic therapy. Annu. Surg. Oncol. 2019, 26, 3071–3079. [Google Scholar] [CrossRef] [PubMed]
- Van la Parra, R.F.D.; Kuerer, H.M. Selective elimination of breast cancer surgery in exceptional responders: Historical perspective and current trials. Breast Cancer Res. 2016, 18, 28. [Google Scholar] [CrossRef]
- Marinovich, M.L.; Macaskill, P.; Irwig, L.; Sardanelli, F.; Mamounas, E.; von Minckwitz, G.; Guarneri, V.; Partridge, S.C.; Wright, F.C.; Choi, J.H.; et al. Agreement between MRI and pathologic breast tumor size after neoadjuvant chemotherapy, and comparison with alternative tests: Individual patient data meta-analysis. BMC Cancer 2015, 15, 662. [Google Scholar] [CrossRef]
- Lobbes, M.B.I.; Prevos, R.; Smidt, M.; Tjan-Heijnen, V.C.G.; van Goethem, M.; Schipper, R.; Beets-Tan, R.G.H.; Wildberger, J.E. The role of magnetic resonance imaging in assessing residual disease and pathologic complete response in breast cancer patients receiving neoadjuvant chemotherapy: A systematic review. Insights Imaging 2013, 4, 163–175. [Google Scholar] [CrossRef]
- Fallenberg, E.M.; Dromain, C.; Diekmann, F.; Engelken, F.; Krohn, M.; Singh, J.M.; Ingold-Heppner, B.; Winzer, K.J.; Bick, U.; Renz, D.M. Contrast-enhanced spectral mammography versus MRI: Initial results in the detection of breast cancer and assessment of tumour size. Eur. Radiol. 2014, 24, 256–264. [Google Scholar] [CrossRef]
- Francescone, M.A.; Jochelson, M.S.; Dershaw, D.D.; Sung, J.S.; Hughes, M.C.; Zheng, J.; Moskowitz, C.; Morris, E.A. Low energy mammogram obtained in contrast-enhanced digital mammography (CEDM) is comparable to routine full-field digital mammography (FFDM). Eur. J. Radiol. 2014, 83, 1350–1355. [Google Scholar] [CrossRef] [PubMed]
- Lalji, U.C.; Jeukens, C.R.; Houben, I.; Nelemans, P.J.; van Engen, R.E.; van Wylick, E.; Beets-Tan, R.G.H.; Wildberger, J.E.; Paulis, L.E.; Lobbes, M.B.I. Evaluation of low-energy contrast-enhanced spectral mammography images by comparing them to full-field digital mammography using EUREF image quality criteria. Eur. Radiol. 2015, 25, 2813–2820. [Google Scholar] [CrossRef]
- Cheung, Y.C.; Tsai, H.P.; Lo, Y.F.; Ueng, S.H.; Huang, P.C.; Chen, S.C. Clinical utility of dual-energy contrast-enhanced spectral mammography for breast microcalcifications without associated mass: A preliminary analysis. Eur. Radiol. 2016, 26, 1082–1089. [Google Scholar] [CrossRef] [PubMed]
- Iotti, V.; Giorgi Rossi, P. Contrast-enhanced mammography in Neoadjuvant therapy response monitoring. In Contrast-Enhanced Mammography; Lobbes, M.B.I., Jochelson, M.S., Eds.; Springer: Cham, Switzerland, 2019; pp. 133–160. [Google Scholar] [CrossRef]
- Iotti, V.; Ravaioli, S.; Vacondio, R.; Coriani, C.; Caffarri, S.; Sghedoni, R.; Nitrosi, A.; Ragazzi, M.; Gasparini, E.; Masini, C.; et al. Contrast-enhanced spectral mammography in neoadjuvant chemotherapy monitoring: A comparison with breast magnetic resonance imaging. Breast Cancer Res. 2017, 19, 106. [Google Scholar] [CrossRef]
- Patel, B.K.; Hilal, T.; Covington, M.; Zhang, N.; Kosiorek, H.E.; Lobbes, M.B.I.; Northfelt, D.W.; Pockaj, B.A. Contrast-enhanced spectral mammography is comparable to MRI in the assessment of residual breast cancer following Neoadjuvant systemic therapy. Annu. Surg. Oncol. 2018, 25, 1350–1356. [Google Scholar] [CrossRef] [PubMed]
- Barra, F.R.; de Souza, F.F.; Camelo, R.E.F.A.; Ribeiro, A.C.O.; Farage, L. Accuracy of contrast-enhanced spectral mammography for estimating residual tumor size after neoadjuvant chemotherapy in patients with breast cancer: A feasibility study. Radiol. Bras. 2017, 50, 224–230. [Google Scholar] [CrossRef][Green Version]
- Barra, F.R.; Sobrinho, A.B.; Barra, R.R.; Magalhães, M.T.; Aguiar, L.R.; de Albuquerque, G.F.L.; Costa, R.P.; Farage, L.; Pratesi, R. Contrast-Enhanced Mammography (CEM) for detecting residual disease after Neoadjuvant chemotherapy: A comparison with breast Magnetic Resonance Imaging (MRI). Biomed. Res. Int. 2018, 2018, 8531916. [Google Scholar] [CrossRef]
- El Said, N.A.E.; Mahmoud, H.G.M.; Salama, A.; Nabil, M.; El Desouky, E.D. Role of contrast enhanced spectral mammography in predicting pathological response of locally advanced breast cancer post neo-adjuvant chemotherapy. Egypt J. Radiol. Nucl. Med. 2017, 48, 519–527. [Google Scholar] [CrossRef]
- Kim, Y.S.; Chang, J.M.; Moon, H.G.; Lee, J.; Shin, S.U.; Moon, W.K. Residual mammographic microcalcifications and enhancing lesions on MRI After Neoadjuvant systemic chemotherapy for locally advanced breast cancer: Correlation with histopathologic residual tumor size. Annu. Surg. Oncol. 2016, 23, 1135–1142. [Google Scholar] [CrossRef] [PubMed]
- Yim, H.; Ha, T.; Kang, D.K.; Park, S.Y.; Jung, Y.; Kim, T.H. Change in microcalcifications on mammography after neoadjuvant chemotherapy in breast cancer patients: Correlation with tumor response grade and comparison with lesion extent. Acta Radiol. 2019, 60, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Um, E.; Kang, J.W.; Lee, S.; Kim, H.J.; Yoon, T.I.; Sohn, G.; Chung, I.Y.; Kim, J.; Lee, J.W.; Son, B.H.; et al. Comparing accuracy of mammography and Magnetic Resonance Imaging for residual calcified lesions in breast cancer patients undergoing Neoadjuvant systemic therapy. Clin. Breast Cancer 2018, 18, e1087–e1091. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Yoon, J.H.; Sohn, J.; Park, H.S.; Moon, H.J.; Kim, M.J.; Kim, E.K.; Kim, S.I.; Park, B.W. Magnetic Resonance Imaging after completion of Neoadjuvant chemotherapy can accurately discriminate between no residual carcinoma and residual ductal carcinoma in situ in patients with triple-negative breast cancer. PLoS ONE 2016, 11, e0149347. [Google Scholar] [CrossRef][Green Version]
- Feliciano, Y.; Mamtani, A.; Morrow, M.; Stempel, M.M.; Patil, S.; Jochelson, M.S. Do calcifications seen on mammography after Neoadjuvant chemotherapy for breast cancer always need to be excised? Annu. Surg. Oncol. 2017, 24, 1492–1498. [Google Scholar] [CrossRef] [PubMed]
- An, Y.Y.; Kim, S.H.; Kang, B.J. Residual microcalcifications after neoadjuvant chemotherapy for locally advanced breast cancer: Comparison of the accuracies of mammography and MRI in predicting pathological residual tumor. World J. Surg. Oncol. 2017, 15, 198. [Google Scholar] [CrossRef]
- Fushimi, A.; Kudo, R.; Takeyama, H. Do decreased breast microcalcifications after Neoadjuvant chemotherapy predict pathologic complete response? Clin. Breast Cancer 2020, 20, e82–e88. [Google Scholar] [CrossRef]
- Jochelson, M.S.; Lampen-Sachar, K.; Gibbons, G.; Dang, C.; Lake, D.; Morris, E.A.; Morrow, M. Do MRI and mammography reliably identify candidates for breast conservation after neoadjuvant chemotherapy? Annu. Surg. Oncol. 2015, 22, 1490–1495. [Google Scholar] [CrossRef]
- Hobbs, M.M.; Taylor, D.B.; Buzynski, S.; Peake, R.E. Contrast-enhanced spectral mammography (CESM) and contrast enhanced MRI (CEMRI): Patient preferences and tolerance. J. Med. Imaging Radiat. Oncol. 2015, 59, 300–305. [Google Scholar] [CrossRef] [PubMed]
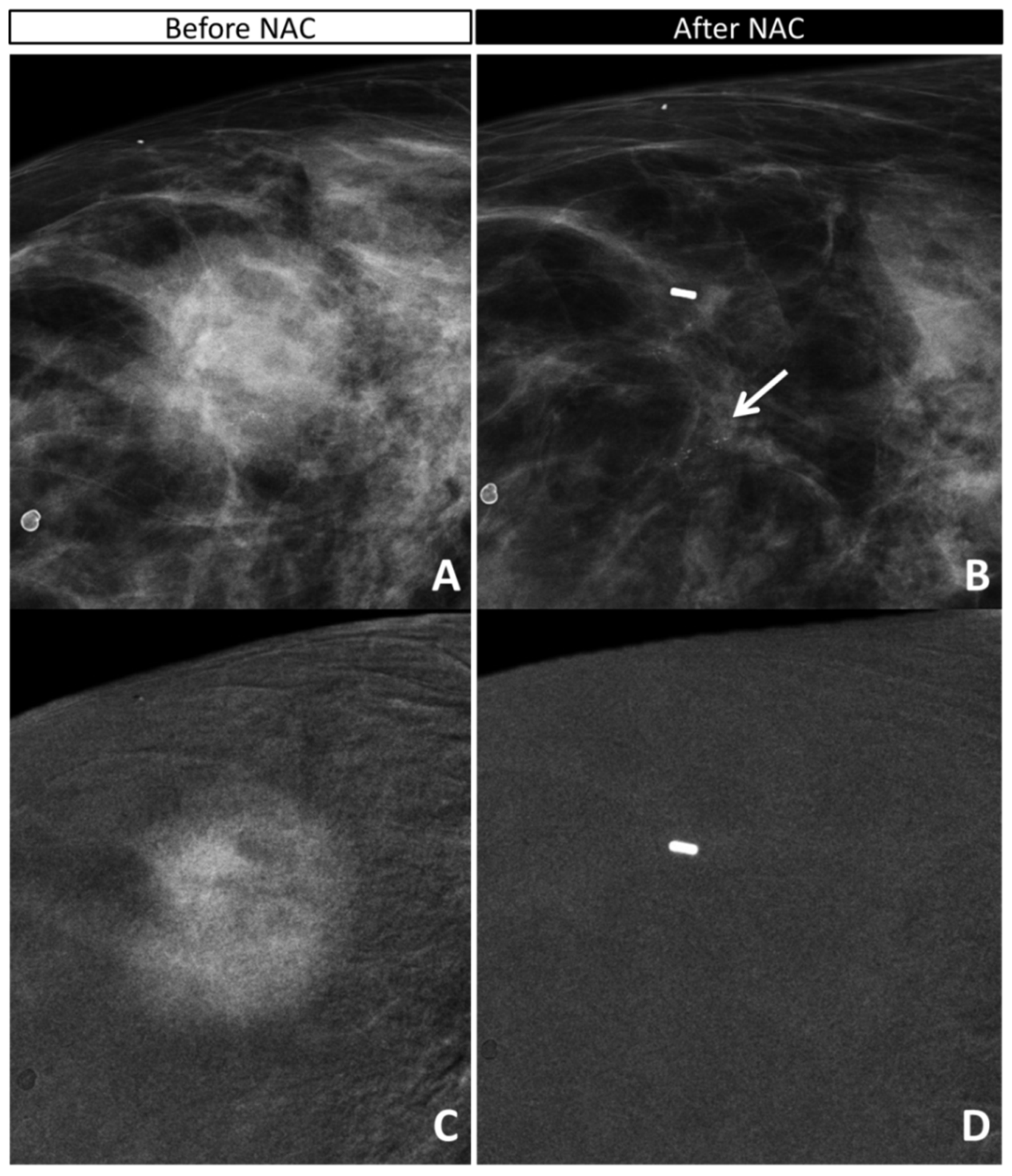
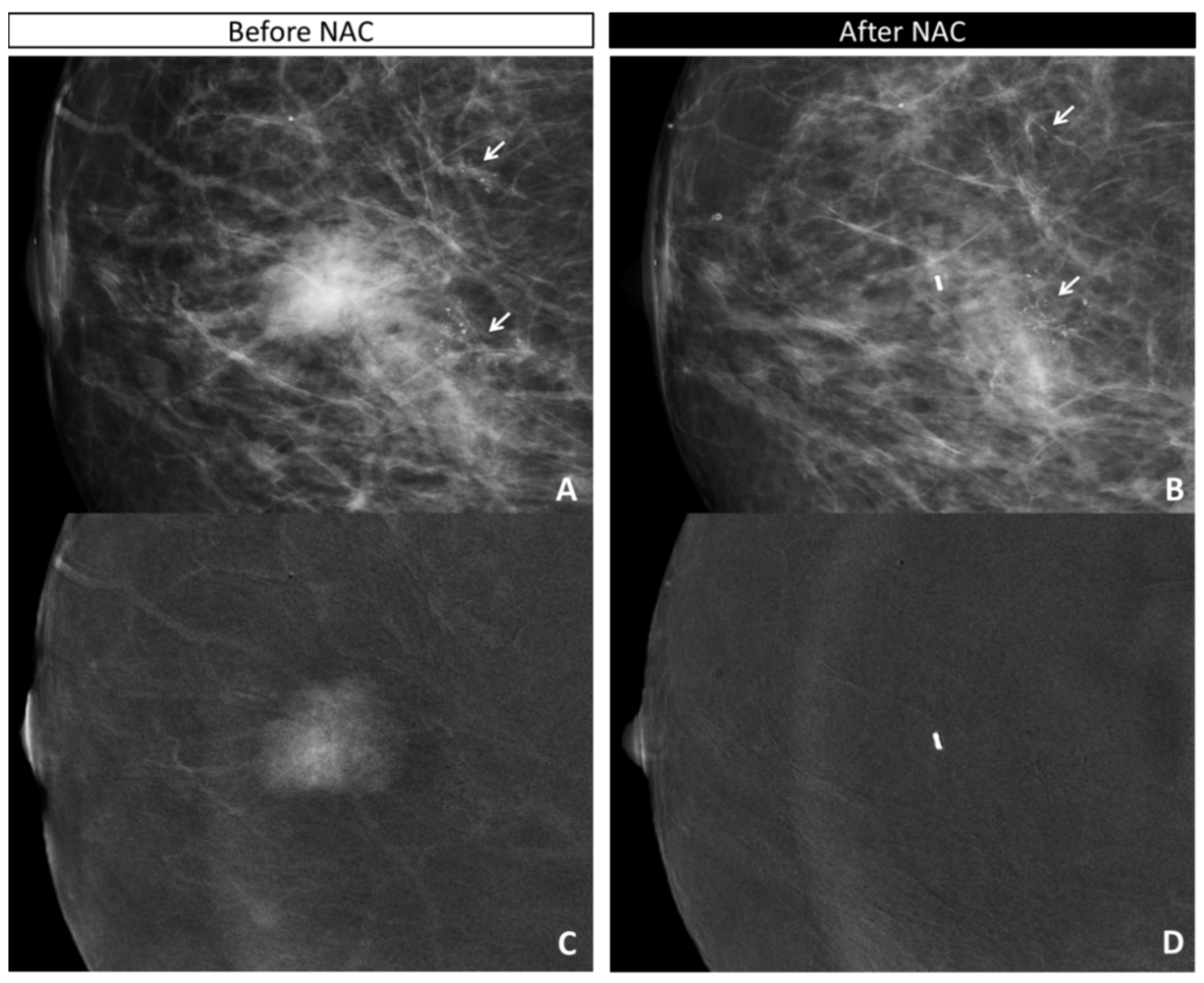
| Overall (n = 36) | Invasive Residue (n = 28) | In Situ Residue (n = 5) | |
|---|---|---|---|
| Mean age in years (IQR) | 52 (44;61) | 52 (43;58) | 52 (44;60) |
| Pre-NAC tumor size; median (IQR) (mm) | 50 (40;86.5) | 50 (40;86.5) | 46 (40;80.3) |
| cT2 | 23 (64%) | 18 (64%) | 4 (80%) |
| cT3 | 13 (36%) | 10 (36%) | 1 (20%) |
| Histological Subtypes | |||
| IDC | 34 (94%) | 26 (92.8%) | 5 (100%) |
| ILC | 1 (3%) | 1 (3.6%) | 0 |
| Metaplastic Carcinoma | 1 (3%) | 1 (3.6%) | 0 |
| Ductal in situ component (from pathology report) | |||
| DCIS at diagnosis | 16 (44%) | 10 (36%) | 4 (80%) |
| DCIS in the surgical specimen * | 24 (67%) | 18 (64%) | 5 (100%) |
| DCIS disappeared after NAC | 2 (/16 = 12%) | 1 (/10 = 10%) | 0 |
| Molecular Subtypes | |||
| Luminal B | 10 (28%) | 9 (32%) | 1 (20%) |
| Luminal B HER2+ | 7 (19%) | 6 (21%) | 0 |
| HER2+ | 13 (36%) | 8 (29%) | 3 (60%) |
| Triple negative | 6 (17%) | 5 (18%) | 1 (20%) |
| Ki67, median (IQR) | 30 (23.5–40) | 30 (23.5–42.5) | 30 (25–40) |
| Calcifications pre-NAC | |||
| Indistinct | 8 (22%) | 7 (25%) | 0 |
| Pleomorphic | 26 (72%) | 19 (68%) | 5 (100%) |
| Linear / branching | 2 (6%) | 2 (7%) | 0 |
| Variation of calcifications after NAC | |||
| Decreased | 12 (33%) | 8 (29%) | 3 (60%) |
| Stable | 18 (50%) | 14 (50%) | 2 (40%) |
| Increased | 6 (17%) | 6 (21%) | 0 |
| Enhancement after NAC | |||
| Concentric shrinkage | 14 (39%) | 14 (50%) | 0 |
| Patchy shrinkage | 13 (36%) | 10 (36%) | 2 (20%) |
| No residual enhancement | 9 (25%) | 4 (14%) | 3 (60%) |
| ypT | |||
| 0 | 3 (9%) | ||
| is | 5 (14%) | ||
| 1mic | 2 (5%) | ||
| 1a | 6 (16%) | ||
| 1a (m) | 5 (/6=83%) | ||
| 1b | 3 (9%) | ||
| 1b (m) | 2 (/3 = 67%) | ||
| 1c | 7 (19%) | ||
| 2 | 7 (19%) | ||
| 2 (m) | 1 (/7 = 14%) | ||
| 3 | 3 (9%) |
| Detection of Invasive Residual Tumor (n = 36) | ||||||||
| TP | FP | TN | FN | Sensitivity (95% CI) | Specificity (95% CI) | PPV (95% CI) | NPV (95% CI) | |
| CE only | 24 | 3 | 5 | 4 | 85.7% (67.3–96%) | 62.5% (24.5–91.5%) | 88.9% (76.4–95.2%) | 55.6% (30.3–78.2%) |
| CE+ calcs | 27 | 7 | 1 | 1 | 96.4% (81.7–99.9%) | 12.5% (0.3–52.7%) | 79.4% (74.6–83.5%) | |
| Detection of Overall Residual Tumor (in situ or invasive) (n = 36) | ||||||||
| TP | FP | TN | FN | Sensitivity (95% CI) | Specificity (95% CI) | PPV (95% CI) | NPV (95% CI) | |
| CE only | 26 | 1 | 2 | 7 | 78.8% (61.1–91%) | 66.7% (9.4–99.2%) | 96.3% (83.9–99.2%) | 22.2% (9.21–44.6%) |
| CE+ calcs | 32 | 2 | 1 | 1 | 96.8% (84.2–99.9%) | 94.1% (87.8–97.2%) | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iotti, V.; Ragazzi, M.; Besutti, G.; Marchesi, V.; Ravaioli, S.; Falco, G.; Coiro, S.; Bisagni, A.; Gasparini, E.; Giorgi Rossi, P.; et al. Accuracy and Reproducibility of Contrast-Enhanced Mammography in the Assessment of Response to Neoadjuvant Chemotherapy in Breast Cancer Patients with Calcifications in the Tumor Bed. Diagnostics 2021, 11, 435. https://doi.org/10.3390/diagnostics11030435
Iotti V, Ragazzi M, Besutti G, Marchesi V, Ravaioli S, Falco G, Coiro S, Bisagni A, Gasparini E, Giorgi Rossi P, et al. Accuracy and Reproducibility of Contrast-Enhanced Mammography in the Assessment of Response to Neoadjuvant Chemotherapy in Breast Cancer Patients with Calcifications in the Tumor Bed. Diagnostics. 2021; 11(3):435. https://doi.org/10.3390/diagnostics11030435
Chicago/Turabian StyleIotti, Valentina, Moira Ragazzi, Giulia Besutti, Vanessa Marchesi, Sara Ravaioli, Giuseppe Falco, Saverio Coiro, Alessandra Bisagni, Elisa Gasparini, Paolo Giorgi Rossi, and et al. 2021. "Accuracy and Reproducibility of Contrast-Enhanced Mammography in the Assessment of Response to Neoadjuvant Chemotherapy in Breast Cancer Patients with Calcifications in the Tumor Bed" Diagnostics 11, no. 3: 435. https://doi.org/10.3390/diagnostics11030435
APA StyleIotti, V., Ragazzi, M., Besutti, G., Marchesi, V., Ravaioli, S., Falco, G., Coiro, S., Bisagni, A., Gasparini, E., Giorgi Rossi, P., Vacondio, R., & Pattacini, P. (2021). Accuracy and Reproducibility of Contrast-Enhanced Mammography in the Assessment of Response to Neoadjuvant Chemotherapy in Breast Cancer Patients with Calcifications in the Tumor Bed. Diagnostics, 11(3), 435. https://doi.org/10.3390/diagnostics11030435







