Diagnosis of SARS-Cov-2 Infection by RT-PCR Using Specimens Other Than Naso- and Oropharyngeal Swabs: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Protocol
2.2. Focused Question and Eligibility Criteria
- Observational studies (i.e., cross-sectional, case-control or cohort study types);
- Use of RT-PCR to detect the presence of SARS-CoV-2 in matched samples;
- Report SARS-CoV-2 positive and negative test results, and/or cycle threshold (CT) from index alternative specimens (saliva, DTS/POS, sputum, urine, feces, or tears/CS) evaluated against NPS and/or OPS;
- Studies with confirmed or suspected cases of SARS-CoV-2 infection.
2.3. Search Strategy and Study Selection
2.4. Data Extraction Process and Data Items
2.5. Risk of Bias Assessment
2.6. Quantitative Analyses
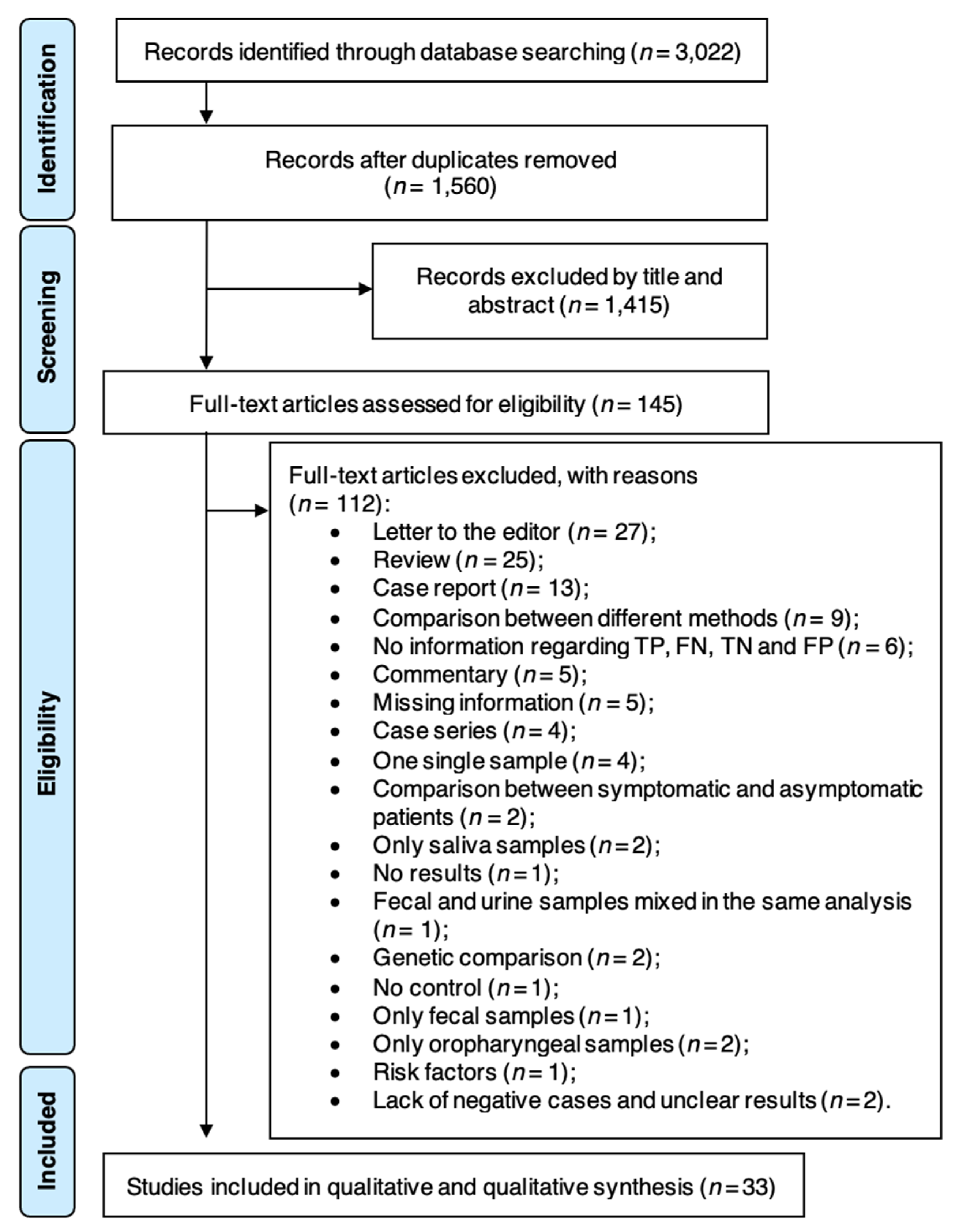
3. Results
3.1. Characterization of the Studies
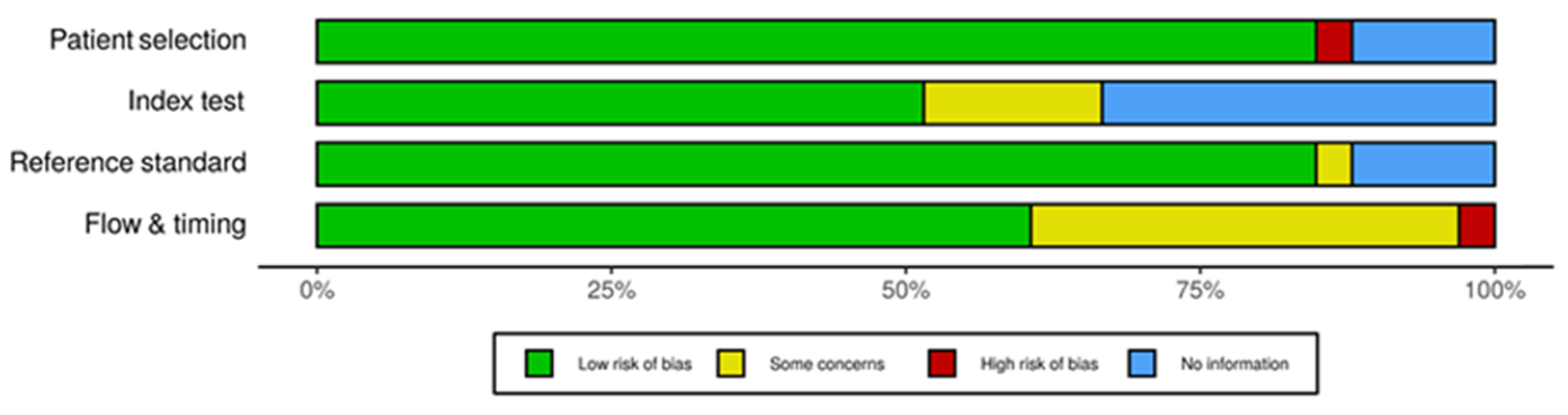
3.2. Quality Assessment
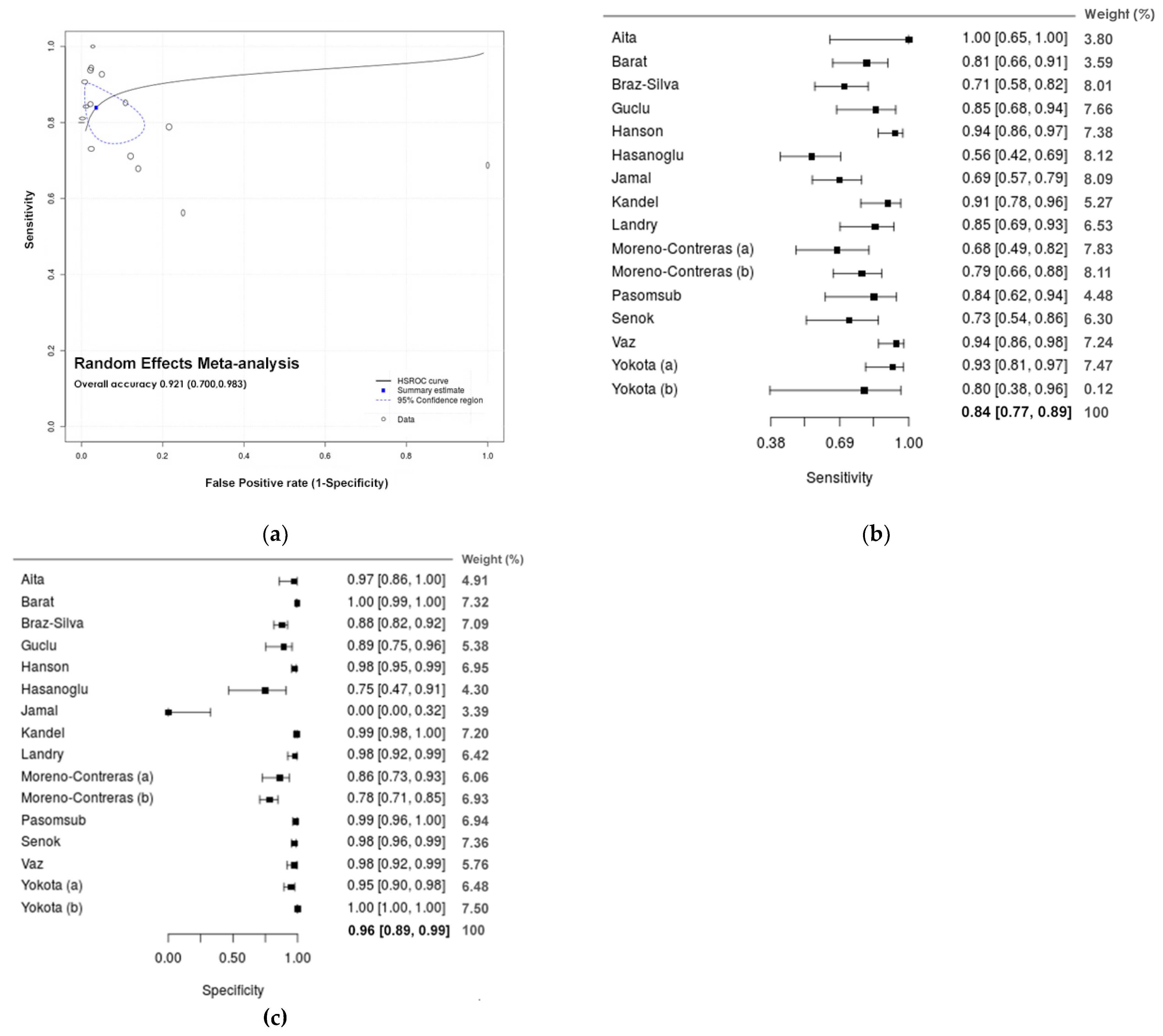
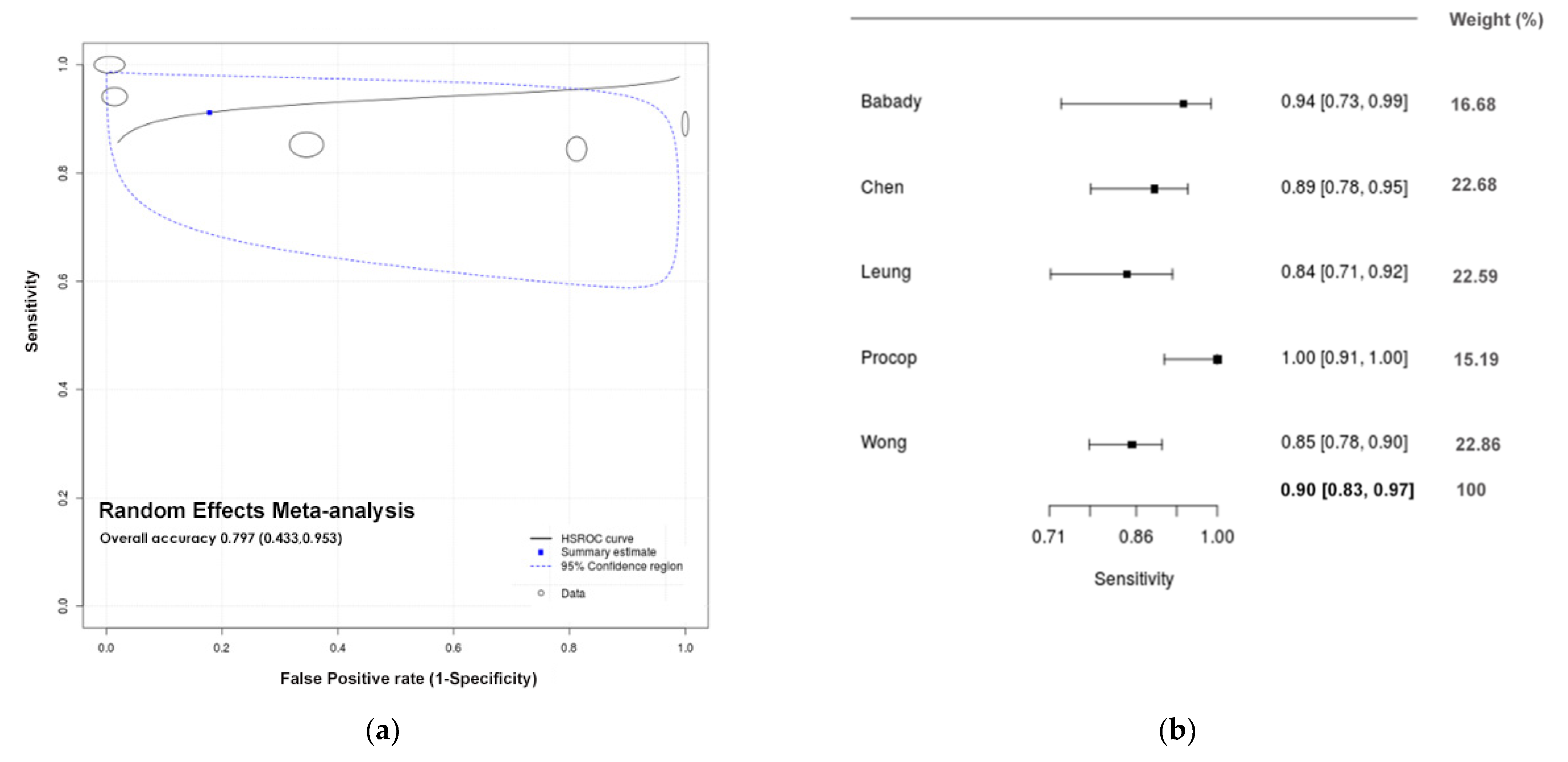
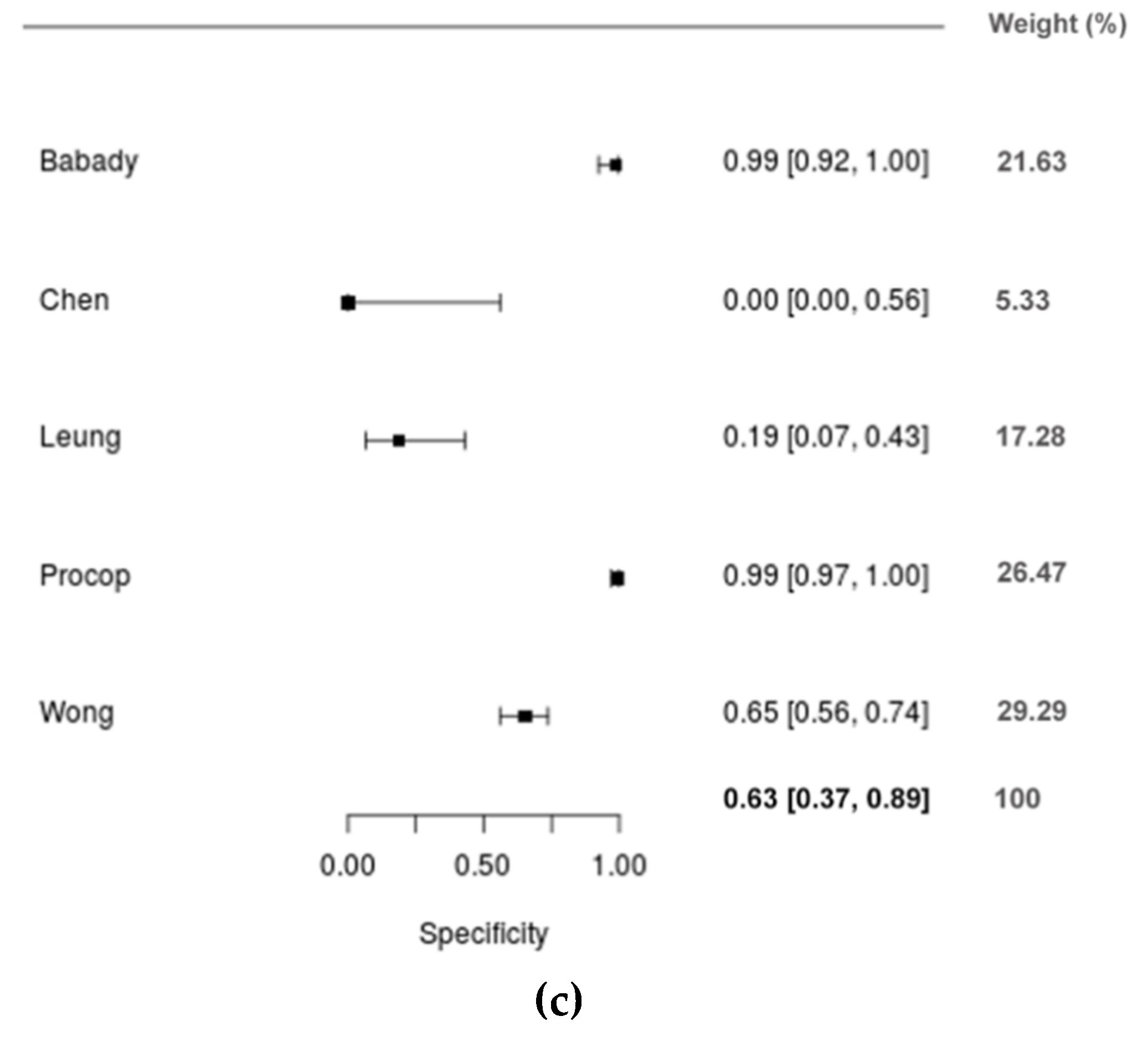
3.3. Quantitative Analysis (Meta-Analysis)
4. Discussion
Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- IHME Covid Projections. Available online: https://covid19.healthdata.org/global (accessed on 1 December 2020).
- Budhrani, A.B. A review: Coronavirus, its types, and impact of covid-19 on global wealth. Int. J. Res. Pharm. Sci. 2020, 11, 455–461. [Google Scholar] [CrossRef]
- Czumbel, L.M.; Kiss, S.; Farkas, N.; Mandel, I.; Hegyi, A.; Nagy, Á.; Lohinai, Z.; Szakács, Z.; Hegyi, P.; Steward, M.C.; et al. Saliva as a Candidate for COVID-19 Diagnostic Testing: A Meta-Analysis. Front. Med. 2020, 7, 465. [Google Scholar] [CrossRef]
- Mitacchione, G.; Schiavone, M.; Curnis, A.; Arca, M.; Antinori, S.; Gasperetti, A.; Mascioli, G.; Severino, P.; Sabato, F.; Caracciolo, M.M.; et al. Impact of prior statin use on clinical outcomes in COVID-19 patients: Data from tertiary referral hospitals during COVID-19 pandemic in Italy. J. Clin. Lipidol. 2020, 15, 68–78. [Google Scholar] [CrossRef] [PubMed]
- ECDC. Diagnostic Testing and Screening for SARS-CoV-2. Available online: https://www.ecdc.europa.eu/en/covid-19/latest-evidence/diagnostic-testing (accessed on 1 December 2020).
- Evans, R.W. Diagnostic Testing for SARS-CoV-2. WHO: Geneva, Switzerland, 2020. [Google Scholar]
- Ravi, N.; Cortade, D.L.; Ng, E.; Wang, S.X. Diagnostics for SARS-CoV-2 detection: A comprehensive review of the FDA-EUA COVID-19 testing landscape. Biosens. Bioelectron. 2020, 165, 112454. [Google Scholar] [CrossRef]
- See, A.; Toh, S.T. Respiratory sampling for severe acute respiratory syndrome coronavirus 2: An Overview. Head Neck 2020, 42, 1652–1656. [Google Scholar] [CrossRef] [PubMed]
- Sapkota, D.; Søland, T.M.; Galtung, H.K.; Sand, L.P.; Giannecchini, S.; To, K.K.W.; Mendes-Correa, M.C.; Giglio, D.; Hasséus, B.; Braz-Silva, P.H. COVID-19 salivary signature: Diagnostic and research opportunities. J. Clin. Pathol. 2020. [Google Scholar] [CrossRef]
- Marty, F.M.; Chen, K.; Verrill, K.A. How to Obtain a Nasopharyngeal Swab Specimen. N. Engl. J. Med. 2020, 382, e76. [Google Scholar] [CrossRef]
- Fernandes, L.L.; Pacheco, V.B.; Borges, L.; Athwal, H.K.; de Paula Eduardo, F.; Bezinelli, L.; Correa, L.; Jimenez, M.; Dame-Teixeira, N.; Lombaert, I.M.A.; et al. Saliva in the Diagnosis of COVID-19: A Review and New Research Directions. J. Dent. Res. 2020, 99, 1435–1443. [Google Scholar] [CrossRef] [PubMed]
- Barat, B.; Das, S.; De Giorgi, V.; Henderson, D.K.; Kopka, S.; Lau, A.F.; Miller, T.; Moriarty, T.; Palmore, T.N.; Sawney, S.; et al. Pooled Saliva Specimens for SARS-CoV-2 Testing. J. Clin. Microbiol. 2020, 59. [Google Scholar] [CrossRef]
- Braz-Silva, P.H.; Mamana, A.C.; Romano, C.M.; Felix, A.C.; de Paula, A.V.; Fereira, N.E.; Buss, L.F.; Tozetto-Mendoza, T.R.; Caixeta, R.A.V.; Leal, F.E.; et al. Performance of at-home self-collected saliva and nasal-oropharyngeal swabs in the surveillance of COVID-19. medRxiv 2020, 13, 1858002. [Google Scholar] [CrossRef]
- Mesoraca, A.; Margiotti, K.; Viola, A.; Cima, A.; Sparacino, D.; Giorlandino, C. Evaluation of SARS-CoV-2 viral RNA in fecal samples. Virol. J. 2020, 17, 86. [Google Scholar] [CrossRef]
- Moreno-Contreras, J.; Espinoza, M.A.; Sandoval-Jaime, C.; Cantú-Cuevas, M.A.; Barón-Olivares, H.; Ortiz-Orozco, O.D.; Muñoz-Rangel, A.V.; Hernández-De la Cruz, M.; Eroza-Osorio, C.M.; Arias, C.F.; et al. Saliva sampling and its direct lysis, an excellent option to increase the number of SARS-CoV-2 diagnostic tests in settings with supply shortages. J. Clin. Microbiol. 2020, 58, 10. [Google Scholar] [CrossRef] [PubMed]
- Pasomsub, E.; Watcharananan, S.P.; Boonyawat, K.; Janchompoo, P.; Wongtabtim, G.; Suksuwan, W.; Sungkanuparph, S.; Phuphuakrat, A. Saliva sample as a non-invasive specimen for the diagnosis of coronavirus disease 2019: A cross-sectional study. Clin. Microbiol. Infect. 2020, 27, 285.e1–285.e4. [Google Scholar] [CrossRef] [PubMed]
- Peng, L.; Liu, J.; Xu, W.; Luo, Q.; Chen, D.; Lei, Z.; Huang, Z.; Li, X.; Deng, K.; Lin, B.; et al. SARS-CoV-2 can be detected in urine, blood, anal swabs, and oropharyngeal swabs specimens. J. Med. Virol. 2020, 92, 1676–1680. [Google Scholar] [CrossRef]
- Perchetti, G.A.; Nalla, A.K.; Huang, M.L.; Zhu, H.; Wei, Y.; Stensland, L.; Loprieno, M.A.; Jerome, K.R.; Greninger, A.L. Validation of SARS-CoV-2 detection across multiple specimen types. J. Clin. Virol. 2020, 128, 104438. [Google Scholar] [CrossRef] [PubMed]
- Rao, M.; Rashid, F.A.; Sabri, F.S.A.H.; Jamil, N.N.; Zain, R.; Hashim, R.; Amran, F.; Kok, H.T.; Samad, M.A.A.; Ahmad, N. Comparing Nasopharyngeal Swab and Early Morning Saliva for the Identification of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2). Clin. Infect. Dis. 2020. [Google Scholar] [CrossRef]
- Senok, A.; Alsuwaidi, H.; Atrah, Y.; Ayedi, O.A.; Zahid, J.A.; Han, A.; Marzooqi, A.A.; Heialy, S.A.; Altrabulsi, B.; Abdelwareth, L.; et al. Saliva as an alternative specimen for molecular COVID-19 testing in community settings and population-based screening. Infect. Drug Resist. 2020, 13, 3393–3399. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.; Li, L.; Tuersun, Y.; Zhao, X.; Feng, Q.; Zhang, T.; Tay, F.R.; Ma, J. Oropharyngeal Secretion as Alternative for SARS-CoV-2 Detection. J. Dent. Res. 2020, 99, 1199–1205. [Google Scholar] [CrossRef]
- Yokota, I.; Shane, P.Y.; Okada, K.; Unoki, Y.; Yang, Y.; Inao, T.; Sakamaki, K.; Iwasaki, S.; Hayasaka, K.; Sugita, J.; et al. Mass screening of asymptomatic persons for SARS-CoV-2 using saliva. Clin. Infect. Dis. 2020. [Google Scholar] [CrossRef]
- Lin, C.; Lin, C.; Xiang, J.; Yan, M.; Li, H.; Huang, S.; Huang, S.; Shen, C.; Shen, C. Comparison of throat swabs and sputum specimens for viral nucleic acid detection in 52 cases of novel coronavirus (SARS-Cov-2)-infected pneumonia (COVID-19). Clin. Chem. Lab. Med. 2020, 58, 1089–1094. [Google Scholar] [CrossRef]
- Dutescu, R.M.; Banasik, P.; Schildgen, O.; Schrage, N.; Uthoff, D. Detection of Coronavirus in Tear Samples of Hospitalized Patients With Confirmed SARS-CoV-2 From Oropharyngeal Swabs. Cornea 2021, 40, 348–350. [Google Scholar] [CrossRef]
- Wong, S.C.Y.; Tse, H.; Siu, H.K.; Kwong, T.S.; Chu, M.Y.; Yau, F.Y.S.; Cheung, I.Y.Y.; Tse, C.W.S.; Poon, K.C.; Cheung, K.C.; et al. Posterior Oropharyngeal Saliva for the Detection of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2). Clin. Infect. Dis. 2020, 71, 2939–2946. [Google Scholar] [CrossRef]
- Aita, A.; Basso, D.; Cattelan, A.M.; Fioretto, P.; Navaglia, F.; Barbaro, F.; Stoppa, A.; Coccorullo, E.; Farella, A.; Socal, A.; et al. SARS-CoV-2 identification and IgA antibodies in saliva: One sample two tests approach for diagnosis. Clin. Chim. Acta 2020, 510, 717–722. [Google Scholar] [CrossRef] [PubMed]
- Babady, N.E.; McMillen, T.; Jani, K.; Viale, A.; Robilotti, E.V.; Aslam, A.; Diver, M.; Sokoli, D.; Mason, G.; Shah, M.K.; et al. Performance of Severe Acute Respiratory Syndrome Coronavirus 2 Real-Time RT-PCR Tests on Oral Rinses and Saliva Samples. J. Mol. Diagn. 2020, 23, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.H.K.; Yip, C.C.Y.; Poon, R.W.S.; Chan, K.H.; Cheng, V.C.C.; Hung, I.F.N.; Chan, J.F.W.; Yuen, K.Y.; To, K.K.W. Evaluating the use of posterior oropharyngeal saliva in a point-of-care assay for the detection of SARS-CoV-2. Emerg. Microbes Infect. 2020, 9, 1356–1359. [Google Scholar] [CrossRef]
- Chu, A.W.H.; Chan, W.M.; Ip, J.D.; Yip, C.C.Y.; Chan, J.F.W.; Yuen, K.Y.; To, K.K.W. Evaluation of simple nucleic acid extraction methods for the detection of SARS-CoV-2 in nasopharyngeal and saliva specimens during global shortage of extraction kits. J. Clin. Virol. 2020, 129, 104519. [Google Scholar] [CrossRef] [PubMed]
- Hanson, K.E.; Barker, A.P.; Hillyard, D.R.; Gilmore, N.; Barrett, J.W.; Orlandi, R.R.; Shakirb, S.M. Self-collected anterior nasal and saliva specimens versus health care worker-collected nasopharyngeal swabs for the molecular detection of SARS-CoV-2. J. Clin. Microbiol. 2020, 58. [Google Scholar] [CrossRef] [PubMed]
- Karimi, S.; Arabi, A.; Shahraki, T.; Safi, S. Detection of severe acute respiratory syndrome Coronavirus-2 in the tears of patients with Coronavirus disease 2019. Eye 2020, 34, 1220–1223. [Google Scholar] [CrossRef]
- Procop, G.W.; Shrestha, N.K.; Vogel, S.; van Sickle, K.; Harrington, S.; Rhoads, D.D.; Rubin, B.P.; Terpeluk, P. A Direct Comparison of Enhanced Saliva to Nasopharyngeal Swab for the Detection of SARS-CoV-2 in Symptomatic Patients. J. Clin. Microbiol. 2020, 58. [Google Scholar] [CrossRef]
- Sohn, Y.; Jeong, S.J.; Chung, W.S.; Hyun, J.H.; Baek, Y.J.; Cho, Y.; Kim, J.H.; Ahn, J.Y.; Choi, J.Y.; Yeom, J.-S. Assessing Viral Shedding and Infectivity of Asymptomatic or Mildly Symptomatic Patients with COVID-19 in a Later Phase. J. Clin. Med. 2020, 9, 2924. [Google Scholar] [CrossRef]
- Vaz, S.N.; Santana, D.S.D.; Netto, E.M.; Pedroso, C.; Wang, W.K.; Santos, F.D.A.; Brites, C. Saliva is a reliable, non-invasive specimen for SARS-CoV-2 detection. Braz. J. Infect. Dis. 2020, 24, 422–427. [Google Scholar] [CrossRef] [PubMed]
- Hasanoglu, I.; Korukluoglu, G.; Asilturk, D.; Cosgun, Y.; Kalem, A.K.; Altas, A.B.; Kayaaslan, B.; Eser, F.; Kuzucu, E.A.; Guner, R. Higher viral loads in asymptomatic COVID-19 patients might be the invisible part of the iceberg. Infection 2020, 49, 117–126. [Google Scholar] [CrossRef]
- Wu, P.; Duan, F.; Luo, C.; Liu, Q.; Qu, X.; Liang, L.; Wu, K. Characteristics of Ocular Findings of Patients with Coronavirus Disease 2019 (COVID-19) in Hubei Province, China. Jama Ophthalmol. 2020, 138, 575–578. [Google Scholar] [CrossRef] [PubMed]
- Güçlü, E.; Koroglu, M.; Yürümez, Y.; Toptan, H.; Kose, E.; Güneysu, F.; Karabay, O. Comparison of saliva and oro-nasopharyngeal swab sample in the molecular diagnosis of COVID-19. Rev. Assoc. Med. Bras. 2020, 66, 1116–1121. [Google Scholar] [CrossRef]
- Jamal, A.J.; Mozafarihashjin, M.; Coomes, E.; Powis, J.; Li, A.X.; Paterson, A.; Anceva-Sami, S.; Barati, S.; Crowl, G.; Faheem, A.; et al. Sensitivity of Nasopharyngeal Swabs and Saliva for the Detection of Severe Acute Respiratory Syndrome Coronavirus 2. Clin. Infect. Dis. 2020, 27, 9–11. [Google Scholar] [CrossRef] [PubMed]
- Kandel, C.; Zheng, J.; McCready, J.; Serbanescu, M.A.; Racher, H.; Desaulnier, M.; Powis, J.E.; Vojdani, K.; Finlay, L.; Sheldrake, E.; et al. Detection of SARS-CoV-2 from Saliva as Compared to Nasopharyngeal Swabs in Outpatients. Viruses 2020, 12, 1314. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.E.; Lee, J.Y.; Lee, A.; Kim, S.; Park, K.H.; Jung, S.I.; Kang, S.J.; Oh, T.H.; Kim, U.J.; Lee, S.Y.; et al. Viral Load Kinetics of SARS-CoV-2 Infection in Saliva in Korean Patients: A Prospective Multi-center Comparative Study. J. Korean Med. Sci. 2020, 35, e287. [Google Scholar] [CrossRef]
- Lai, C.K.C.; Chen, Z.; Lui, G.; Ling, L.; Li, T.; Wong, M.C.S.; Ng, R.W.Y.; Tso, E.Y.K.; Ho, T.; Fung, K.S.C.; et al. Prospective Study Comparing Deep Throat Saliva With Other Respiratory Tract Specimens in the Diagnosis of Novel Coronavirus Disease 2019. J. Infect. Dis. 2020, 222, 1612–1619. [Google Scholar] [CrossRef]
- Landry, M.L.; Criscuolo, J.; Peaper, D.R. Challenges in use of saliva for detection of SARS CoV-2 RNA in symptomatic outpatients. J. Clin. Virol. 2020, 130, 104567. [Google Scholar] [CrossRef]
- Leung, E.C.M.; Chow, V.C.Y.; Lee, M.K.P.; Lai, R.W.M. Deep throat saliva as an alternative diagnostic specimen type for the detection of SARS-CoV-2. J. Med. Virol. 2020. [Google Scholar] [CrossRef]
- FDA. Emergency Use Authorization Issued in August 2020. Available online: https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-issues-emergency-use-authorization-yale-school-public-health (accessed on 1 December 2020).
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; Altman, D.; Antes, G.; Atkins, D.; Barbour, V.; Barrowman, N.; Berlin, J.A.; et al. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Plos Med. 2009, 6, e1000097. [Google Scholar] [CrossRef]
- Bristol, M.S. QUADAS2: Background Document. 2014. Available online: https://www.bristol.ac.uk/media-library/sites/quadas/migrated/documents/background-doc.pdf (accessed on 1 December 2020).
- McGuinness, L.A.; Higgins, J.P.T. Risk-of-bias VISualization (robvis): An R package and Shiny web app for visualizing risk-of-bias assessments. Res. Synth. Methods 2021, 12, 55–61. [Google Scholar] [CrossRef]
- Freeman, S.C.; Kerby, C.R.; Patel, A.; Cooper, N.J.; Quinn, T.; Sutton, A.J. Development of an interactive web-based tool to conduct and interrogate meta-analysis of diagnostic test accuracy studies: MetaDTA. Bmc Med. Res. Methodol. 2019, 19, 81. [Google Scholar] [CrossRef]
- Leeflang, M.M.G. Systematic reviews and meta-analyses of diagnostic test accuracy. Clin. Microbiol. Infect. 2014, 20, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Harbord, R.M.; Deeks, J.J.; Egger, M.; Whiting, P.; Sterne, J.A.C. A unification of models for meta-analysis of diagnostic accuracy studies. Biostatistics 2007, 8, 239–251. [Google Scholar] [CrossRef]
- Wallace, B.C.; Dahabreh, I.J.; Trikalinos, T.A.; Lau, J.; Trow, P.; Schmid, C.H. End-Users: R as a Computational Back-End. J. Stat. Softw. 2012, 49, 1–15. [Google Scholar] [CrossRef]
- Jeong, H.W.; Kim, S.M.; Kim, H.S.; Kim, Y.I.; Kim, J.H.; Cho, J.Y.; Kim, S.H.; Kang, H.; Kim, S.G.; Park, S.J.; et al. Viable SARS-CoV-2 in various specimens from COVID-19 patients. Clin. Microbiol. Infect. 2020, 26, 1520–1524. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Hu, Y.; Yu, Y.; Zhang, X.; Li, B.; Wu, J.; Li, J.; Wu, Y.; Xia, X.; Tang, H.; et al. Positive result of Sars-Cov-2 in faeces and sputum from discharged patients with COVID-19 in Yiwu, China. J. Med. Virol. 2020, 92, 1938–1947. [Google Scholar] [CrossRef]
- Yu, X.; Sun, S.; Shi, Y.; Wang, H.; Zhao, R.; Sheng, J. SARS-CoV-2 viral load in sputum correlates with risk of COVID-19 progression. Crit. Care 2020, 24, 170. [Google Scholar] [CrossRef]
- Ceron, J.; Lamy, E.; Martinez-Subiela, S.; Lopez-Jornet, P.; Capela-Silva, F.; Eckersall, P.; Tvarijonaviciute, A. Use of Saliva for Diagnosis and Monitoring the SARS-CoV-2: A General Perspective. J. Clin. Med. 2020, 9, 1491. [Google Scholar] [CrossRef]
- Li, Y.; Yao, L.; Li, J.; Chen, L.; Song, Y.; Cai, Z.; Yang, C. Stability issues of RT-PCR testing of SARS-CoV-2 for hospitalized patients clinically diagnosed with COVID-19. J. Med. Virol. 2020, 92, 903–908. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef]
- Yoon, J.G.; Yoon, J.; Song, J.Y.; Yoon, S.Y.; Lim, C.S.; Seong, H.; Noh, J.Y.; Cheong, H.J.; Kim, W.J. Clinical significance of a high SARS-CoV-2 viral load in the Saliva. J. Korean Med. Sci. 2020, 35, e195. [Google Scholar] [CrossRef] [PubMed]
- Broughton, J.P.; Deng, X.; Yu, G.; Fasching, C.L.; Servellita, V.; Singh, J.; Miao, X.; Streithorst, J.A.; Granados, A.; Sotomayor-Gonzalez, A.; et al. CRISPR–Cas12-based detection of SARS-CoV-2. Nat. Biotechnol. 2020, 38, 870–874. [Google Scholar] [CrossRef] [PubMed]
| Study | Type of Study | Date -Month (year) | Test | Information | Specimens | Main Findings | Funding | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Method (Device) | Kit (Targets) | N | Positive | Mean Age (Median) | Ratio M/F | Continent (Country) | Control | Index Specimen | |||||
| Aita et al. [26] | Cross-sectional | September (2020) | RT-PCR (QX200 AutoDG Droplet Digital PCR System) | One-Step RT-ddPCR Advanced Kit | 43 | 7 | 63.0 (NI) | 2.06 | Europe (Italy) | NPS | Saliva (Stimulated) | Saliva collection can be adopted to detect SARS-CoV-2 infection in alternative to NP-swabs | NI |
| Babady et al. [27] | Cross-sectional | January (2021) | RT-PCR (ABI 7500 Fast, QuantStudio 5) | (N) | 87 | 35 | NI | NI | Americas (USA) | NPS | DTS/POS | Saliva is an acceptable alternative to NPSs for SARS-CoV-2 RNA detection by RT-PCR | National Cancer Institute Cancer Center (grant P30 CA008748) |
| Barat et al. [12] | Cohort (prospective) | December (2020) | RT-PCR (Cobas 1246800 instrument) | NucliSENS®easyMAG®platform (ORF1ab, E) | 459 | 37 | NI (42.0) | 0.69 | Americas (USA) | NPS/MT | Saliva (Unstimulated) | Saliva is not sensitive as NP/MT testing | National Cancer Institute, National Institutes of Health, (75N910D00024 & 75N91019F00130) |
| Braz-Silva et al. [13] | Cohort (prospective) | December (2020) | RT-PCR (-) | Altona RealStar® SARS-CoV-2 RT-PCR Kit 1.0 (E, S) | 201 | 22 | 38.3 (NI) | 0.58 | Americas (Brazil) | NPS | Saliva (Unstimulated) | Self-collected samples are feasible adequate alternative for SARS-CoV-2 detection | Universidade de São Paulo |
| Chen et al. [28] | Cross-sectional | May (2020) | RT-PCR (Xpert Xpress SARS-CoV-2 assay) | - | 58 | 55 | NI (38.0) | 0.48 | Asia (Hong Kong) | NPS | DTS/POS | POS and NPS were found to have similar detection rates in the point-of-care test for SARS-CoV-2 detection | Consultancy Services for Enhancing Laboratory Surveillance of Emerging Infectious Diseases and Research Capability on Antimicrobial Resistance, and Research Grants Council (T11/707/15) |
| Chu et al. [29] | Cohort (retrospective) | June (2020) | RT-PCR (-) | - | 50 | NI | NI | NI | Asia (Hong Kong) | NPS | DTS/POS | PKH pre-processing is an alternative method for nucleic acid extraction when commercial extraction kits are not available. | Public and private funding (fully disclosed in the article) |
| Dutescu et al. [24] | Cohort (prospective) | November (2020) | RT-PCR (Real-Time PCR Cycler iwith LightMix SarbecoV) | Superscript III one-step RT-PCR system (E) | 18 | 13 | 66.3 (NI) | 1 | Europe (Germany) | OPS | Tears | Tear fluid and OPS lavage present a higher percentage of SARS-CoV-2 | None |
| Güçlü et al. [37] | Cross-sectional | September (2020) | RT-PCR (-) | RT-PCR SARS-CoV-2 kit | 64 | 30 | 51.0 (NI) | 1.37 | Europe (Turkey) | NPS/OPS | Saliva (Unclear method) | Saliva samples can be used instead of ONS samples in detecting SARS-CoV-2 | NI |
| Hanson et al. [30] | Cohort (prospective) | October (2020) | RT-PCR (Panther Fusion system) | Hologic Aptima SARS-CoV-2 TMA (-) | 354 | 80 | 35.0 (NI) | NI | Americas (USA) | NPS | Saliva (Unclear method) | Saliva is an acceptable specimen type for symptomatic patients, especially if swab or PPE 144 supplies are limited. | ARUP Institute for Clinical and Experimental Pathology |
| Hasanoglu et al. [35] | Cross-sectional | October (2020) | RT-PCR (-) | Bio-Speedy® COVID-19 RT-qPCR Detection Kit, Bio-Rad CFX96 Touch™ (-) | 60 | 48 | 33.9 (NI) | 0.94 | Europe (Turkey) | NPS/OPS | Saliva (Unclear method), Rectal | Asymptomatic patients have higher SARSCoV-2 viral loads than symptomatic patients. Viral load of nasopharyngeal/ oropharyngeal samples decreases with increasing disease severity. | None |
| Jamal et al. [38] | Cross-sectional | June (2020) | RT-PCR (-) | Allplex 2019-nCoV Assay (-) | 72 | 64 | NI (66.0) | 0.85 | America (Canada) | NPS | Saliva (Stimulated) | NPS were more sensitive than saliva for SARS-CoV-2 detection | Canadian Institutes of Health Research (nº. 440359) and Vanier Canada Graduate Scholarship |
| Kandel et al. [39] | Cohort (prospective) | November (2020) | RT-PCR (CFX96 Touch Real-time PCR detection system) | (E-gene, 5′-UTR) | 429 | 42 | NI (42.0) | NI | America (Canada) | NPS | Saliva (Stimulated) | Saliva performs comparably to NPS for the detection of SARS-CoV-2 | University of Toronto |
| Karimi et al. [31] | Cross-sectional | May (2020) | RT-PCR (-) | NI | 43 | 30 | 56.6 (NI) | 2.07 | Asia (Iran) | NPS | Tears | Ocular transmission of SARS-CoV-2 should be considered even in the absence of ocular manifestations | NI |
| Kim et al. [40] | Cross-sectional | August (2020) | RT-PCR (CFX96™ Real-time PCR detection system) | PowerChek™ 2019-nCoV Real-time PCR Kit (E, RdRP) | 53 | NI | NI (59.0) | NI | Asia (Korea) | NPS/OPS | Saliva (Stimulated, Sputum | Saliva is not appropriate for initial diagnosis COVID-19 to replace NP/OP swabs | Fund at the Chonnam National University (No. CNU 2020-1967). |
| Lai et al. [41] | Cross-sectional | August (2020) | RT-PCR (StepOnePlus Real-Time PCR System) | (N) | 65 | NI | NI | 0.85 | Asia (Hong Kong) | NPS/OPS | DTS/POS, Sputum | DTS produced the lowest viral RNA concentration and RT-PCR-positive rate compared with conventional respiratory specimens in all phases of illness | Food and Health Bureau, Hong Kong SAR Government (nº. COVID190107) |
| Landry et al. [42] | Cohort (prospective) | July (2020) | RT-PCR (-) | (N2) | 124 | 33 | NI | NI | Americas (USA) | NPS | Saliva (Unstimulated) | Real-time RT-PCR of pure saliva had an overall sensitivity for SARS CoV-2 RNA detection of 85.7% when compared to simultaneously collected NPS | None |
| Leung et al. [43] | Cohort (retrospective) | July (2020) | RT-PCR (-) | LightMix Modular SARS-CoV (COVID19) | 95 | 45 | 42.0 (NI) | 0.72 | Asia (Hong Kong) | NPS | DTS/POS | SARS-CoV-2 detection by RT-PCR was equivalent in DTS and NPS specimens | NI |
| Li et al. [53] | Cross-sectional | April (2020) | RT-PCR (LightCycler 480 instrument II) | (E, N, RdRP) | 12 | 9 | 52.8 (NI) | 0.86 | Asia (China) | NPS | Sputum, Feces | Faecal virus nucleic acid should be tested as a routine monitoring index for COVID-19 | Jin hua Science and Technology Bureau (nº. 2020XG-32) and Zhejiang University special scientific research fund (nº. 2020XGZX064) |
| Lin et al. [23] | Cohort (retrospective) | April (2020) | RT-PCR (-) | 2019-nCoV nucleic acid detection kit (E, N, ORF1ab) | 52 | 40 | 57.3 (NI) | 1.08 | Asia (China) | TS | Sputum | The detection rates of 2019-nCoV from sputum specimens were significantly higher than those from throat swabs | Zhongnan Hospital of Wuhan University Science, Technology and Innovation Seed Fund (nº. znpy2017022) |
| Mesoraca et al. [14] | Cross-sectional | June (2020) | RT-PCR (iQ5 real-time PCR detection system) | Real Time Multi- plex RT-PCR kit (E, N, ORF1ab) | 15 | 15 | NI | 1.29 | Europe (Italy) | RT | FS | qRT-PCR assays of fecal specimens is an important step to control infection | None |
| Moreno-Contreras et al. [15] | Cross-sectional | September (2020) | RT-PCR (ABI Prism 7500 sequence detector system) | StarQ one-step RT-qPCR (E) | 71 | 28 | NI (41.0) | 0.85 | Americas (Mexico) | NPS | Saliva (Stimulated) | Saliva samples can serve as a suitable source for viral RNA detection of COVID-19 | CONACyT (nº. 314343) |
| 182 | 52 | NPS/OPS | Saliva (Stimulated) | ||||||||||
| Pasomsub et al. [16] | Cross-sectional | May (2020) | RT-PCR (CFX96 Real-Time Detection System) | SARS-CoV-2 Nucleic Acid Diagnostic Kit (ORF1ab, N) | 200 | 19 | NI (36.0) | 0.53 | Asia (Thailand) | NPS | Saliva (Unclear method) | Saliva might be an alternative specimen for the diagnosis of COVID-19 | Mahidol University |
| Peng et al. [17] | Cohort (retrospective) | April (2020) | RT-PCR (SLAN-96P Real-time PCR Detection System) | SARS-CoV-2 RNA Detection Kit (N) | 7 | NI | 38.9 (NI) | NI | Asia (China) | OPS | Blood, Urine, Anal Swab | SARS-CoV-2 can infect multiple systems, including the urinary tract. Testing different specimen types may be useful for monitoring disease changes and progression, and for establishing a prognosis | National Natural Science Foundation of China (nº. 81570539, 81873572) and Guangdong Province Science and Technology Project (nº. 2020B111105001) |
| Perchetti et al. [18] | Cross-sectional | May (2020) | RT-PCR (ABI 7500 Real-Time PCR System) | AgPath-ID One-Step RT-PCR kit (N1, N2) | NI | NI | NI | NI | Americas (USA) | NPS | BAL, Sputum, Plasma, CSF, Stool | A modified CDC-based laboratory developed test is able to detect SARSCoV- 2 accurately with similar sensitivity across all sample types tested | University of Washington Medical Center |
| Procop et al. [32] | Cross-sectional | September (2020) | RT-PCR (ABI 7500 Fast Dx instruments) | (N, RdRP) | 216 | 38 | NI | 0.58 | Americas (USA) | NPS | DTS/POS | Saliva specimen performed as well as NPS for the qualitative detection of SARS-CoV-2 in symptomatic patients | NI |
| Rao et al. [19] | Cross-sectional | August (2020) | RT-PCR (-) | (E, RdRP) | 217 | 217 | NI (27.0) | NI | Asia (Malaysia) | NPS | DTS/POS | Saliva is a better alternative specimen for detection of SARS-CoV-2 | National Institute of Malaysia, Ministry of Health, Malaysia (NMRR-20-860-54884) |
| Senok et al. [20] | Cross-sectional | August (2020) | RT-PCR (-) | NeoPlex COVID-19 kit (RdRp, N) | 401 | 26 | 35.5 (NI) | 4.57 | Asia (United Arab Emirates) | NPS | Saliva (Unstimulated) | Saliva is a specimen with good diagnostic accuracy for SARS-CoV-2 RT-PCR | None |
| Sohn et al. [33] | Cross-sectional | September (2020) | RT-PCR (-) | Allplex™ 2019-nCoV Assay (E, N, RdRP) | 48 | 48 | 32.6 (NI) | 0.41 | Asia (Korea) | NPS | Saliva (Unclear method) | Saliva can be used as a reliable specimen for the diagnosis of SARS-CoV-2 infection | None |
| Vaz et al. [34] | Cross-sectional | October (2020) | RT-PCR (-) | BIOMOL OneStep/ COVID-19 Kit (E, RdRP) | 155 | 71 | NI (40.0) | 0.45 | America (Brazil) | NPS/OPS | Saliva (Stimulated) | Use of self-collected saliva samples is an easy, convenient, and low-cost alternative to conventional NP swab-based molecular tests | NI |
| Wong et al. [25] | Cohort (retrospective) | June (2020) | RT-PCR (-) | LightMix® Modular SARS and Wuhan CoV E-gene kit with (E) | 229 | 122 | 39.0 (36.0) | NI | Asia (Hong Kong) | NPS | DTS/POS | POS is an acceptable alternative specimen to nasopharyngeal specimen for the detection of SARS-CoV-2 | NI |
| Wu et al. [36] | Cross-sectional | March (2020) | RT-PCR (-) | - | 38 | 28 | 65.8 (NI) | 1.92 | Asia (China) | NPS | CS | Although there is a low prevalence of SARS-CoV-2 in tears, it is possible to transmit via the eyes | National Natural Science Foundation of China (nº. 81770896 and nº. 81770920) |
| Yokota et al. [22] | Cross-sectional | September (2020) | RT-PCR (7500 Real-time PCR systems) | THUNDERBIRD Probe One-Step qRT-PCR kit (N2) | 161 | 41 | NI (44.9) | 1.69 | Asia (Japan) | NPS | Saliva (Unclear method) | Both nasopharyngeal and self-collected saliva specimens had high sensitivity and specificity | Health, Labour and Welfare Policy Research Grants 20HA2002 |
| RT-LAMP | Loopamp 2019-SARS-CoV-2 Detection Reagent Kit (N2) | 1763 | 5 | NI (33.5) | 1.11 | NPS | Saliva (Unclear method) | ||||||
| Yu et al. [54] | Cross-sectional | March (2020) | RT-PCR (-) | (ORF1ab, N) | 76 | NI | 40.0 (NI) | 1 | Asia (China) | NPS | Sputum | Sputum is a better indicator of viral replication in the body than throat and nasal swabs, and the viral load of sputum samples tends to increase and then decrease during the course of the disease | Beijing Ditan Hospital, Capital Medical University, and the Beijing Key Laboratory of Emerging Infectious Diseases |
| Specimen | N | Sensitivity (95% CI) | Specificity (95% CI CI) | duct (95% CI CI) | FPR (95% CI CI) | dOR (95% CI CI) |
|---|---|---|---|---|---|---|
| Saliva | 16 | 0.839 (0.774;0.888) | 0.964 (0.895;0.988) | 2.792 (−1.457;7.041) | 0.036 (0.012;0.105) | 138.757 (34.059;565.290) |
| DTS/POS | 5 | 0.901 (0.833;0.969) | 0.631 (0.368;0.893) | −1.808 (−3.189;−0.427) | 0.178 (0.014;0.763) | 47.821 (1.723;1327.016) |
| Sputum | 2 | 0.875 (0.711;0.952) | 0.250 (0.130;0.426) | 1.531 (0.301;2.762) | 0.750 (0.574;0.870) | 2.333 (0.624;8.719) |
| Tears/CS | 3 | 0.174 (0.078;0.342) | 0.961 (0.127;1.000) | −1.500 (−4.328;1.328) | 0.039 (0.000;0.873) | 5.155 (0.039;680.590) |
| Feces | 3 | 0.460 (0.131;0.827) | 0.914 (0.064;0.999) | - | 0.086 (0.001;0.936) | 9.016 (0.092;885.010) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moreira, V.M.; Mascarenhas, P.; Machado, V.; Botelho, J.; Mendes, J.J.; Taveira, N.; Almeida, M.G. Diagnosis of SARS-Cov-2 Infection by RT-PCR Using Specimens Other Than Naso- and Oropharyngeal Swabs: A Systematic Review and Meta-Analysis. Diagnostics 2021, 11, 363. https://doi.org/10.3390/diagnostics11020363
Moreira VM, Mascarenhas P, Machado V, Botelho J, Mendes JJ, Taveira N, Almeida MG. Diagnosis of SARS-Cov-2 Infection by RT-PCR Using Specimens Other Than Naso- and Oropharyngeal Swabs: A Systematic Review and Meta-Analysis. Diagnostics. 2021; 11(2):363. https://doi.org/10.3390/diagnostics11020363
Chicago/Turabian StyleMoreira, Vânia M., Paulo Mascarenhas, Vanessa Machado, João Botelho, José João Mendes, Nuno Taveira, and M. Gabriela Almeida. 2021. "Diagnosis of SARS-Cov-2 Infection by RT-PCR Using Specimens Other Than Naso- and Oropharyngeal Swabs: A Systematic Review and Meta-Analysis" Diagnostics 11, no. 2: 363. https://doi.org/10.3390/diagnostics11020363
APA StyleMoreira, V. M., Mascarenhas, P., Machado, V., Botelho, J., Mendes, J. J., Taveira, N., & Almeida, M. G. (2021). Diagnosis of SARS-Cov-2 Infection by RT-PCR Using Specimens Other Than Naso- and Oropharyngeal Swabs: A Systematic Review and Meta-Analysis. Diagnostics, 11(2), 363. https://doi.org/10.3390/diagnostics11020363











