Pyonephrosis Ultrasound and Computed Tomography Features: A Pictorial Review
Abstract
1. Introduction
2. MeSH Terms
- adult;
- contrast media;
- diagnosis, differential;
- female/male;
- humans;
- hydronephrosis/diagnosis;
- image enhancement/methods;
- kidney/pathology;
- pyelonephritis/diagnosis;
- reproducibility of results;
- sensitivity and specificity;
- genito urinary disease;
- kidney disease;
- genito urinary infection complications;
- hydronephrosis, infected;
- infected hydronephrosis;
- male urogenital diseases;
- urologic diseases;
- kidney diseases;
- hydronephrosis;
- female urogenital diseases and pregnancy complications;
- female urogenital diseases;
- urologic diseases;
- kidney diseases;
- hydronephrosis
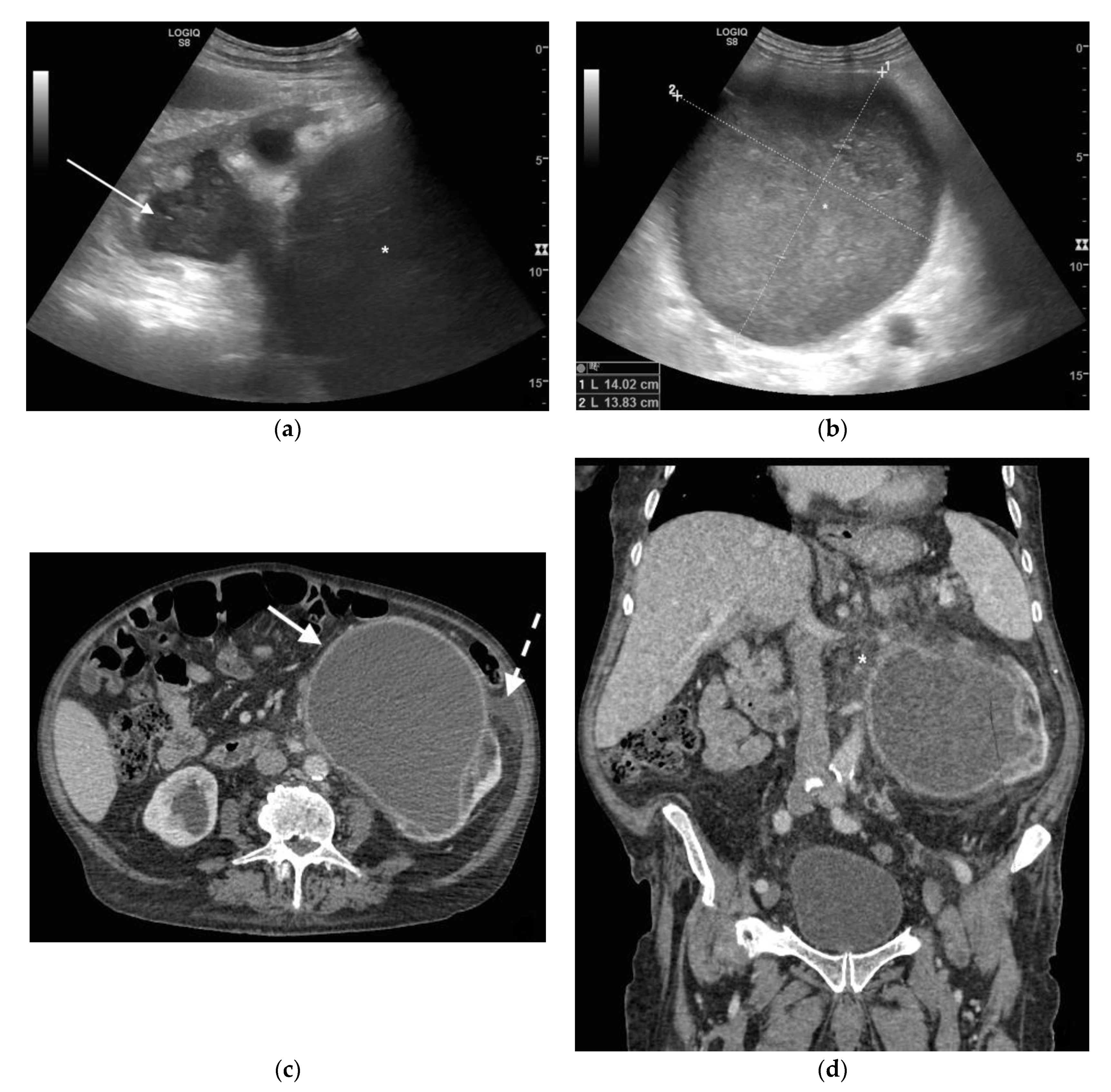
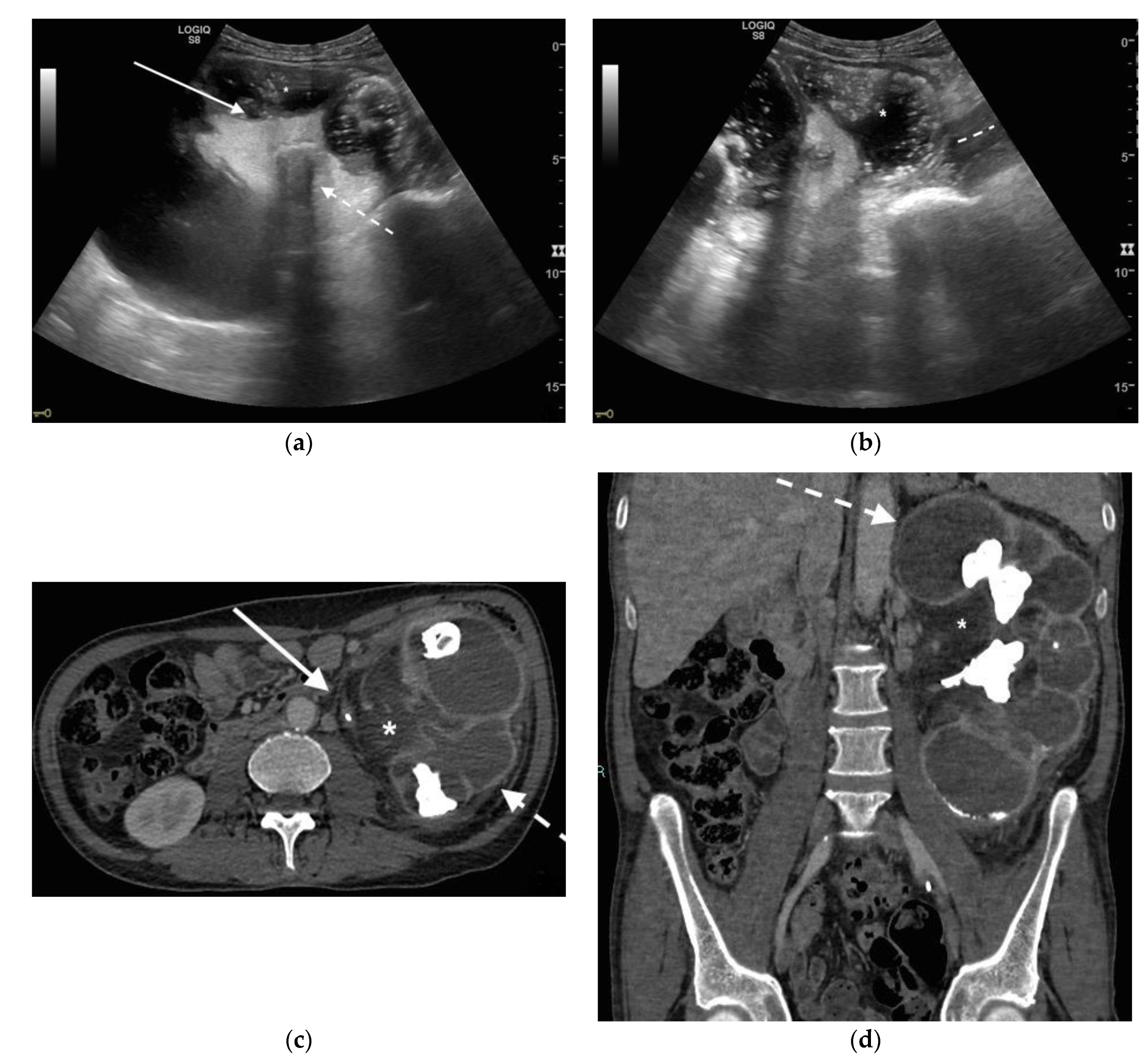
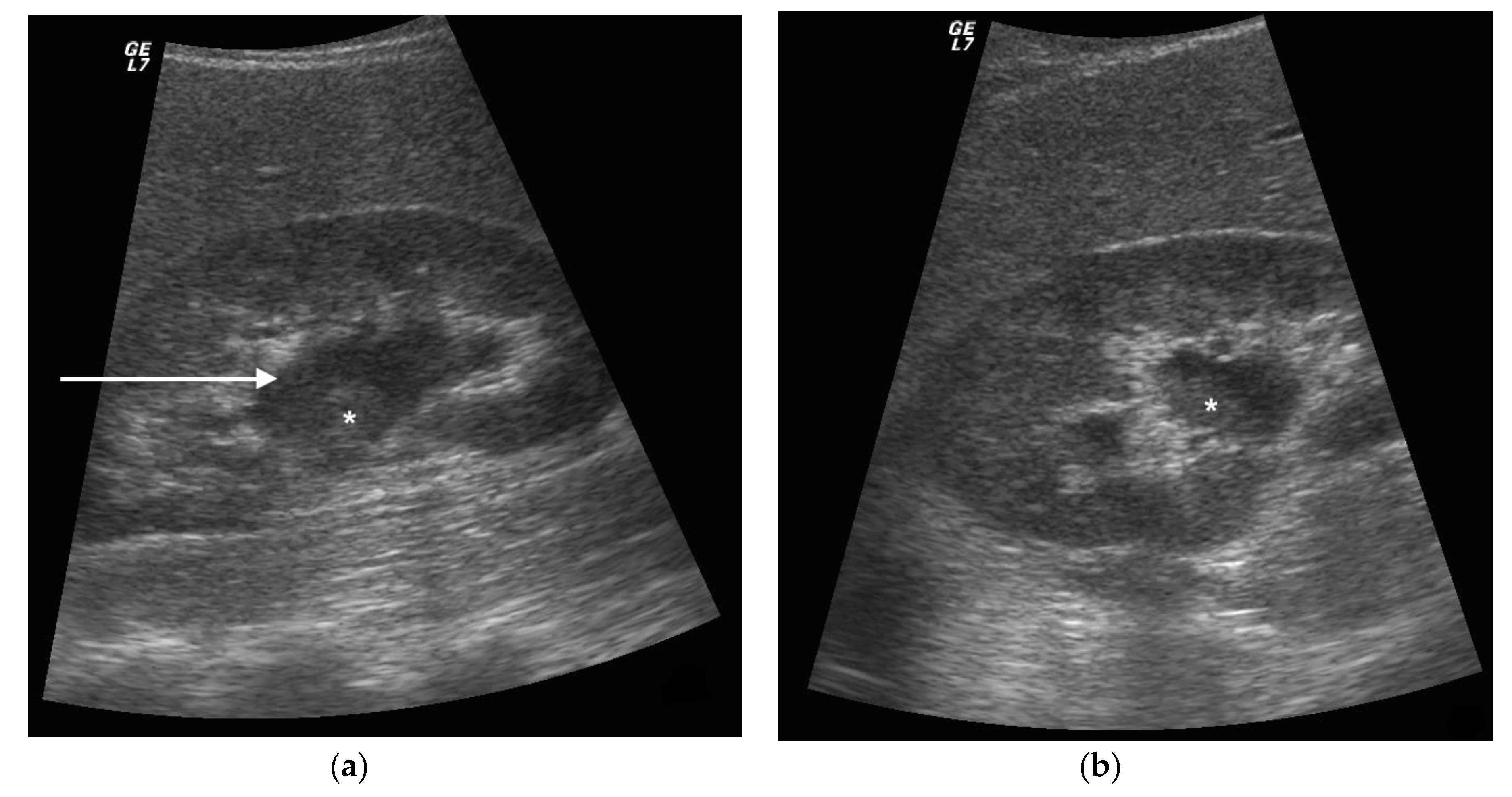
3. Ultrasound
4. CT
5. Discussion
6. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Flores-Mireles, A.L.; Walker, J.N.; Caparon, M.G.; Hultgren, S.J. Urinary tract infections: Epidemiology, mechanisms of infection and treatment options. Nat. Rev. Microbiol. 2015, 13, 269–284. [Google Scholar] [CrossRef] [PubMed]
- Wagenlehner, F.M.E.; Johansen, T.E.B.; Cai, T.; Koves, B.; Kranz, J.; Pilatz, A.; Tandogdu, Z. Epidemiology, definition and treatment of complicated urinary tract infections. Nat. Rev. Urol. 2020, 17, 586–600. [Google Scholar] [CrossRef] [PubMed]
- Wagenlehner, F.M.E.; Pilatz, A.; Weidner, W.; Naber, K.G. Urosepsis: Overview of the Diagnostic and Treatment Challenges. Microbiol. Spectr. 2015, 3. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Tang, K.; Xia, D.; Peng, E.; Li, R.; Liu, H.; Chen, Z. A novel comprehensive predictive model for obstructive pyonephrosis patients with upper urinary tract stones. Int. J. Clin. Exp. Pathol. 2020, 13, 2758–2766. [Google Scholar] [PubMed]
- Patodia, M.; Goel, A.; Singh, V.; Singh, B.P.; Sinha, R.J.; Kumar, M.; Dalela, D.; Sankhwar, S.N. Are there any predictors of pyonephrosis in patients with renal calculus disease? Urolithiasis 2017, 45, 415–420. [Google Scholar] [CrossRef]
- Nicola, R.; Menias, C.O. Urinary Obstruction, Stone Disease, and Infection. In Diseases of the Abdomen and Pelvis 2018–2021: Diagnostic Imaging-IDKD Book; Hodler, J., Kubik-Huch, R.A., von Schulthess, G.K., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 223–228. [Google Scholar]
- Li, H.; Xie, F.; Zhao, C.; Yi, Z.; Chen, J.; Zu, X. Primary mucinous adenocarcinoma of the renal pelvis misdiagnosed as calculous pyonephrosis: A case report and literature review. Transl. Androl. Urol. 2020, 9, 781–788. [Google Scholar] [CrossRef]
- Chen, L.; Li, J.-X.; Huang, X.-B.; Wang, X.-F. Analysis for risk factors of systemic inflammatory response syndrome after one-phase treatment for apyrexic calculous pyonephrosis by percutaneous nephrolithotomy. Beijing Da Xue Xue Bao Yi Xue Ban 2014, 46, 566–569. [Google Scholar]
- Lewis, D.A.; Brown, R.; Williams, J.; White, P.; Jacobson, S.K.; Marchesi, J.R.; Drake, M.J. The human urinary microbiome; bacterial DNA in voided urine of asymptomatic adults. Front. Cell. Infect. Microbiol. 2013, 3, 41. [Google Scholar] [CrossRef]
- Siddiqui, H.; Lagesen, K.; Nederbragt, A.J.; Jeansson, S.L.; Jakobsen, K.S. Alterations of microbiota in urine from women with interstitial cystitis. BMC Microbiol. 2012, 12, 205. [Google Scholar] [CrossRef]
- Nelson, D.E.; Van Der Pol, B.; Dong, Q.; Revanna, K.V.; Fan, B.; Easwaran, S.; Sodergren, E.; Weinstock, G.M.; Diao, L.; Fortenberry, J.D. Characteristic Male Urine Microbiomes Associate with Asymptomatic Sexually Transmitted Infection. PLoS ONE 2010, 5, e14116. [Google Scholar] [CrossRef]
- Dong, Q.; Nelson, D.E.; Toh, E.; Diao, L.; Gao, X.; Fortenberry, J.D.; Van Der Pol, B. The Microbial Communities in Male First Catch Urine Are Highly Similar to Those in Paired Urethral Swab Specimens. PLoS ONE 2011, 6, e19709. [Google Scholar] [CrossRef]
- Alverdy, J.C.; Krezalek, M.A. Collapse of the Microbiome, Emergence of the Pathobiome, and the Immunopathology of Sepsis. Crit. Care Med. 2017, 45, 337–347. [Google Scholar] [CrossRef] [PubMed]
- Fay, K.T.; Ford, M.L.; Coopersmith, C.M. The intestinal microenvironment in sepsis. Biochim. Biophys. Acta Mol. Basis Dis. 2017, 1863 Pt B, 2574–2583. [Google Scholar] [CrossRef]
- Klingensmith, N.J.; Coopersmith, C.M. The Gut as the Motor of Multiple Organ Dysfunction in Critical Illness. Crit. Care Clin. 2016, 32, 203–212. [Google Scholar] [CrossRef] [PubMed]
- Florido, C.; Herren, J.L.; Pandhi, M.B.; Niemeyer, M.M. Emergent Percutaneous Nephrostomy for Pyonephrosis: A Primer for the On-Call Interventional Radiologist. Semin. Interv. Radiol. 2020, 37, 74–84. [Google Scholar] [CrossRef]
- Thornton, R.H.; Covey, A.M. Urinary Drainage Procedures in Interventional Radiology. Tech. Vasc. Interv. Radiol. 2016, 19, 170–181. [Google Scholar] [CrossRef] [PubMed]
- Flukes, S.; Hayne, D.; Kuan, M.; Wallace, M.; McMillan, K.; Rukin, N.J. Retrograde ureteric stent insertion in the management of infected obstructed kidneys. BJU Int. 2015, 115 (Suppl. 5), 31–34. [Google Scholar] [CrossRef]
- Chang, C.W.; Huang, C.N. Pyonephrosis drained by double-J catheter. Clin. Case Rep. 2020, 8, 3586–3587. [Google Scholar] [CrossRef]
- Shifti, D.M.; Bekele, K. Generalized peritonitis after spontaneous rupture of pyonephrosis: A case report. Int. Med. Case Rep. J. 2018, 11, 113–116. [Google Scholar] [CrossRef]
- Colemen, B.G.; Arger, P.H.; Mulhern, C.B., Jr.; Pollack, H.M.; Banner, M.P. Pyonephrosis: Sonography in the diagnosis and management. AJR Am. J. Roentgenol. 1981, 137, 939–943. [Google Scholar] [CrossRef] [PubMed]
- Manno, E.; Navarra, M.; Faccio, L.; Motevallian, M.; Bertolaccini, L.; Mfochive, A.; Pesce, M.; Evangelista, A. Deep impact of ultrasound in the intensive care unit: The “ICU-sound” protocol. Anesthesiology 2012, 117, 801–809. [Google Scholar] [CrossRef] [PubMed]
- Narasimhan, M.; Koenig, S.J.; Mayo, P.H. A Whole-Body Approach to Point of Care Ultrasound. Chest 2016, 150, 772–776. [Google Scholar] [CrossRef]
- Kamboj, M.; Loy, J.L.; Koratala, A. Renal ultrasonography: A reliable diagnostic tool for pyonephrosis. Clin. Case Rep. 2018, 6, 1176–1178. [Google Scholar] [CrossRef]
- Subramanyam, B.R.; Raghavendra, B.N.; Bosniak, M.A.; Lefleur, R.S.; Rosen, R.J.; Horii, S.C. Sonography of pyonephrosis: A prospective study. AJR Am. J. Roentgenol. 1983, 140, 991–993. [Google Scholar] [CrossRef]
- Gupta, R.; Gupta, S.; Choudhary, A.; Basu, S. Giant Pyonephrosis Due to Ureteropelvic Junction Obstruction: A Case Report. J. Clin. Diagn. Res. 2017, 11, PD17–PD18. [Google Scholar] [CrossRef] [PubMed]
- Demertzis, J.; Menias, C.O. State of the art: Imaging of renal infections. Emerg. Radiol. 2007, 14, 13–22. [Google Scholar] [CrossRef]
- Browne, L.P.; Mason, E.O.; Kaplan, S.L.; Cassady, C.I.; Krishnamurthy, R.; Guillerman, R.P. Optimal imaging strategy for community-acquired Staphylococcus aureus musculoskeletal infections in children. Pediatr. Radiol. 2008, 38, 841–847. [Google Scholar] [CrossRef] [PubMed]
- Basmaci, I.; Sefik, E. A novel use of attenuation value (Hounsfield unit) in non-contrast CT: Diagnosis of pyonephrosis in obstructed systems. Int. Urol. Nephrol. 2020, 52, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Boeri, L.; Fulgheri, I.; Palmisano, F.; Lievore, E.; Lorusso, V.; Ripa, F.; D’Amico, M.; Spinelli, M.G.; Salonia, A.; Carrafiello, G.; et al. Hounsfield unit attenuation value can differentiate pyonephrosis from hydronephrosis and predict septic complications in patients with obstructive uropathy. Sci. Rep. 2020, 10, 18546. [Google Scholar] [CrossRef] [PubMed]
- Yuruk, E.; Tuken, M.; Sulejman, S.; Colakerol, A.; Serefoglu, E.C.; Sarica, K.; Muslumanoglu, A.Y. Computerized tomography attenuation values can be used to differentiate hydronephrosis from pyonephrosis. World J. Urol. 2017, 35, 437–442. [Google Scholar] [CrossRef]
- Kawashima, A.; Leroy, A.J. Radiologic evaluation of patients with renal infections. Infect. Dis. Clin. N. Am. 2003, 17, 433–456. [Google Scholar] [CrossRef]
- Zulfiqar, M.; Ubilla, C.V.; Nicola, R.; Menias, C.O. Imaging of Renal Infections and Inflammatory Disease. Radiol. Clin. N. Am. 2020, 58, 909–923. [Google Scholar] [CrossRef] [PubMed]
- Vourganti, S.; Agarwal, P.K.; Bodner, D.R.; Dogra, V.S. Ultrasonographic Evaluation of Renal Infections. Radiol. Clin. N. Am. 2006, 44, 763–775. [Google Scholar] [CrossRef]
- Hansen, K.L.; Nielsen, M.B.; Ewertsen, C. Ultrasonography of the Kidney: A Pictorial Review. Diagnostics 2015, 6, 2. [Google Scholar] [CrossRef]
- Joseph, R.C.; Amendola, M.A.; Artze, M.E.; Casillas, J.; Jafri, S.Z.; Dickson, P.R.; Morillo, G. Genitourinary tract gas: Imaging evaluation. Radiographics 1996, 16, 295–308. [Google Scholar] [CrossRef]
- Chung, S.-D.; Lai, M.-K.; Chueh, S.-C.; Wang, S.-M.; Yu, H.-J. An unusual cause of pyonephrosis and intra-peritoneal abscess: Ureteral urothelial carcinoma. Int. J. Infect. Dis. 2009, 13, e39–e40. [Google Scholar] [CrossRef]
- Jalbani, I.K.; Khurrum, M.; Aziz, W. Spontaneous rupture of pyonephrosis leading to pyoperitoneum. Urol. Case Rep. 2019, 26, 100928. [Google Scholar] [CrossRef] [PubMed]
- Quaresima, S.; Manzelli, A.; Ricciardi, E.; Petrou, A.; Brennan, N.; Mauriello, A.; Rossi, P. Spontaneous intraperitoneal rupture of pyonephrosis in a patient with unknown kidney carcinosarcoma: A case report. World J. Surg. Oncol. 2011, 9, 39. [Google Scholar] [CrossRef]
- Spinelli, M.G.; Palmisano, F.; Zanetti, S.P.; Boeri, L.; Gadda, F.; Talso, M.; Dell’Orto, P.G.; Montanari, E. Spontaneous upper urinary tract rupture caused by ureteric stones: A prospective high-volume single centre observational study and proposed management. Arch. Esp. Urol. 2019, 72, 590–595. [Google Scholar]
- Kim, C.J.; Kato, K.; Yoshiki, T.; Okada, Y.; Tani, T. Intractable duodenocutaneous fistula after nephrectomy for stone pyonephrosis: Report of a case. Hinyokika Kiyo 2003, 49, 547–550. [Google Scholar]
- Kondapi, D.; Tambe, V.; Hegazy, H. Pyeloduodenal fistula as a result of pyonephrosis. Urol. Case Rep. 2018, 21, 36–37. [Google Scholar] [CrossRef] [PubMed]
- Arvind, N.K.; Singh, O.; Gupta, S.S.; Sahay, S.; Ali, K.; Dharaskar, A. Laparoscopic nephrectomy for pyonephrosis during pregnancy: Case report and review of the literature. Rev. Urol. 2011, 13, 98–103. [Google Scholar]
- Stojadinovic, M.; Micic, S.; Milovanovic, D. Ultrasonographic and computed tomography findings in renal suppurations: Performance indicators and risks for diagnostic failure. Urol. Int. 2008, 80, 389–397. [Google Scholar] [CrossRef] [PubMed]
- Allen, B.C.; Barnhart, H.; Bashir, M.; Nieman, C.; Breault, S.; Jaffe, T.A. Diagnostic Accuracy of Intra-abdominal Fluid Collection Characterization in the Era of Multidetector Computed Tomography. Am. Surg. 2012, 78, 185–189. [Google Scholar] [CrossRef] [PubMed]
- Cullu, N.; Kalemci, S.; Karakas, O.; Eser, I.; Yalcin, F.; Boyaci, F.N.; Karakas, E. Efficacy of CT in diagnosis of transudates and exudates in patients with pleural effusion. Diagn. Interv. Radiol. 2014, 20, 116–120. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tamburrini, S.; Lugarà, M.; Iannuzzi, M.; Cesaro, E.; De Simone, F.; Del Biondo, D.; Toto, R.; Iulia, D.; Marrone, V.; Faella, P.; et al. Pyonephrosis Ultrasound and Computed Tomography Features: A Pictorial Review. Diagnostics 2021, 11, 331. https://doi.org/10.3390/diagnostics11020331
Tamburrini S, Lugarà M, Iannuzzi M, Cesaro E, De Simone F, Del Biondo D, Toto R, Iulia D, Marrone V, Faella P, et al. Pyonephrosis Ultrasound and Computed Tomography Features: A Pictorial Review. Diagnostics. 2021; 11(2):331. https://doi.org/10.3390/diagnostics11020331
Chicago/Turabian StyleTamburrini, Stefania, Marina Lugarà, Michele Iannuzzi, Edoardo Cesaro, Fiore De Simone, Dario Del Biondo, Roberta Toto, Dora Iulia, Valeria Marrone, Pierluigi Faella, and et al. 2021. "Pyonephrosis Ultrasound and Computed Tomography Features: A Pictorial Review" Diagnostics 11, no. 2: 331. https://doi.org/10.3390/diagnostics11020331
APA StyleTamburrini, S., Lugarà, M., Iannuzzi, M., Cesaro, E., De Simone, F., Del Biondo, D., Toto, R., Iulia, D., Marrone, V., Faella, P., Liguori, C., & Marano, I. (2021). Pyonephrosis Ultrasound and Computed Tomography Features: A Pictorial Review. Diagnostics, 11(2), 331. https://doi.org/10.3390/diagnostics11020331






