Introduction of the Grayscale Median for Ultrasound Tissue Characterization of the Transplanted Kidney
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Population
2.2. Data Collection
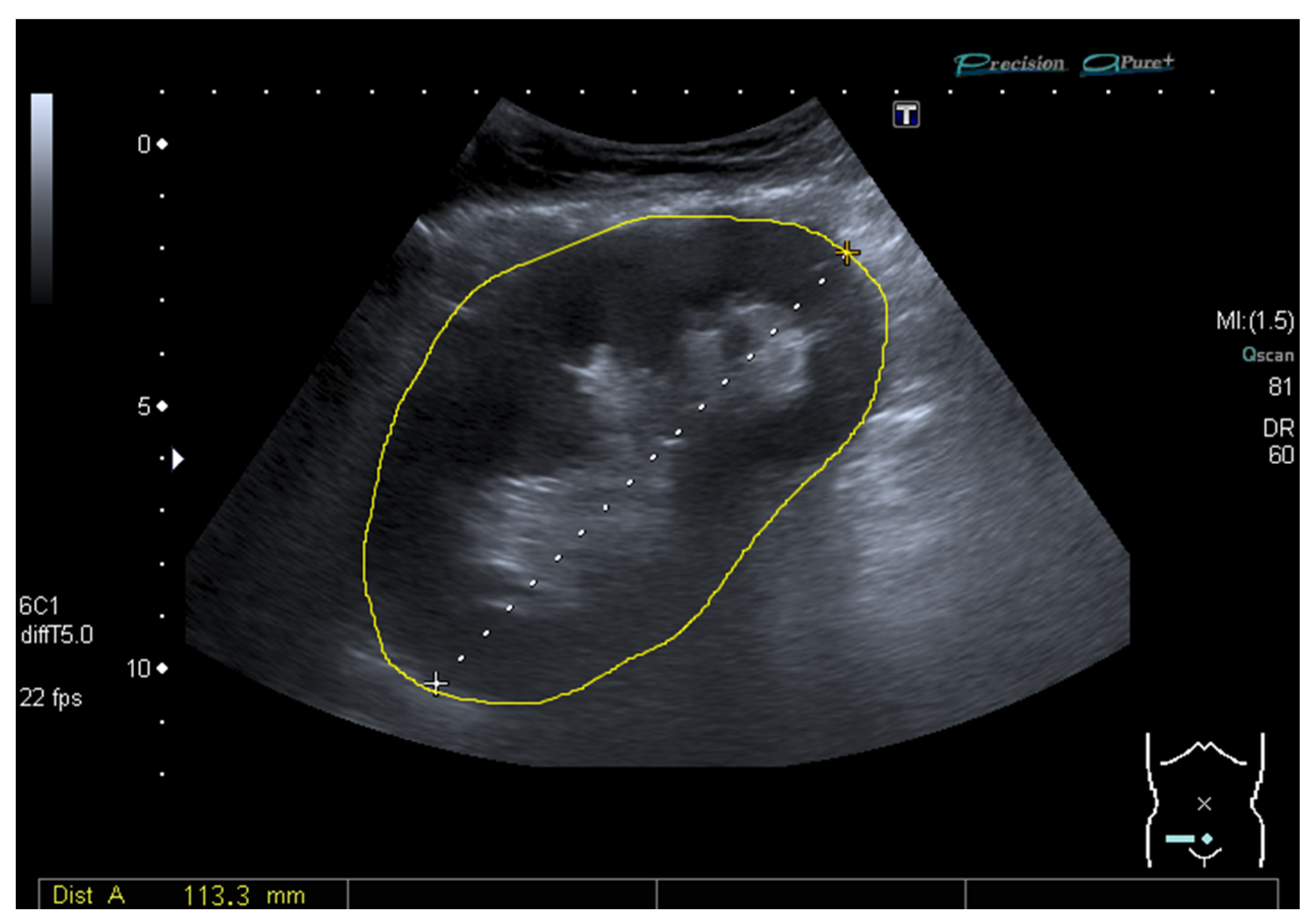
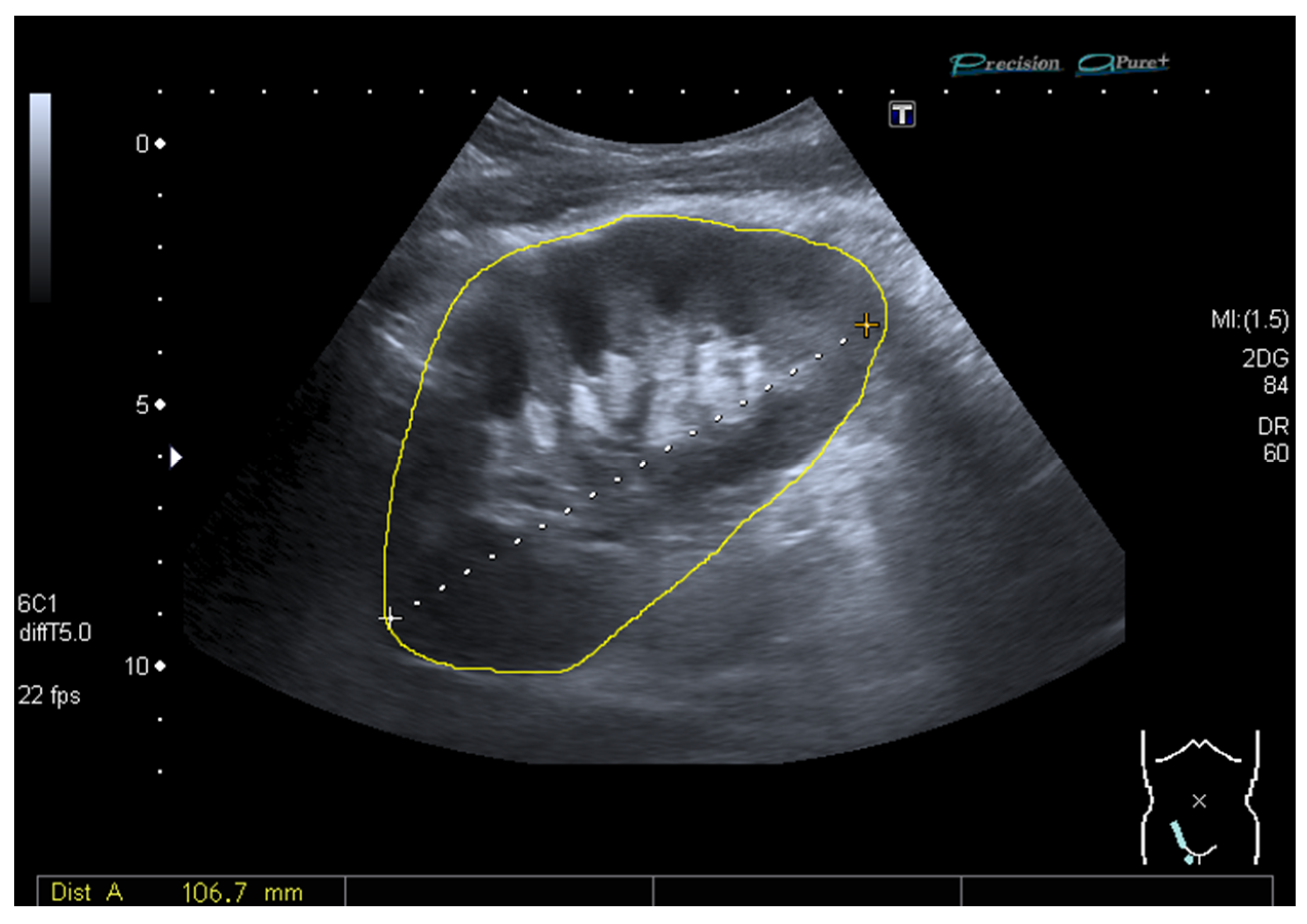
2.3. Ultrasound and Measurement of the Grayscale Median
2.4. Statistical Analyses
3. Results
3.1. Study Population
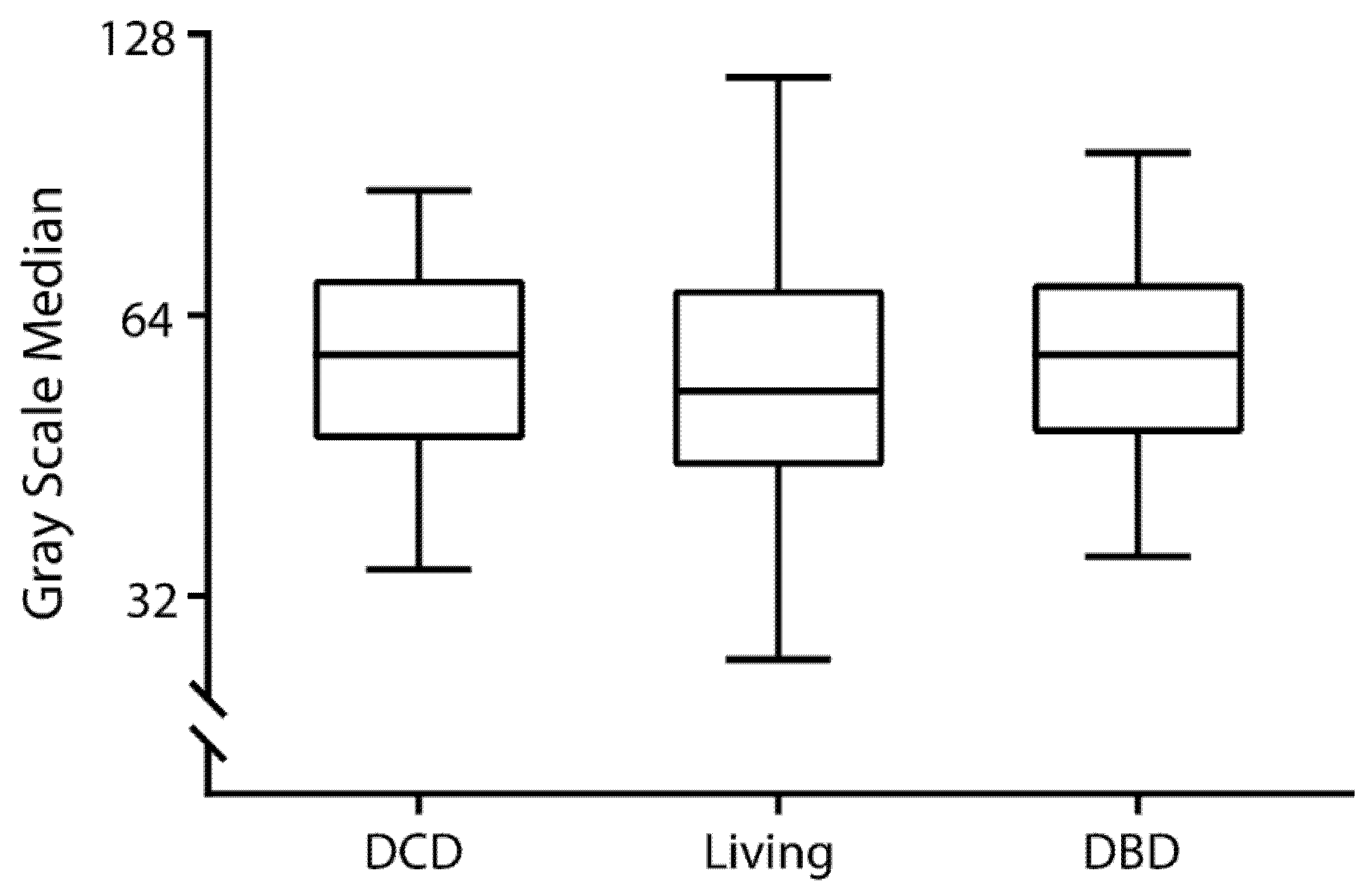
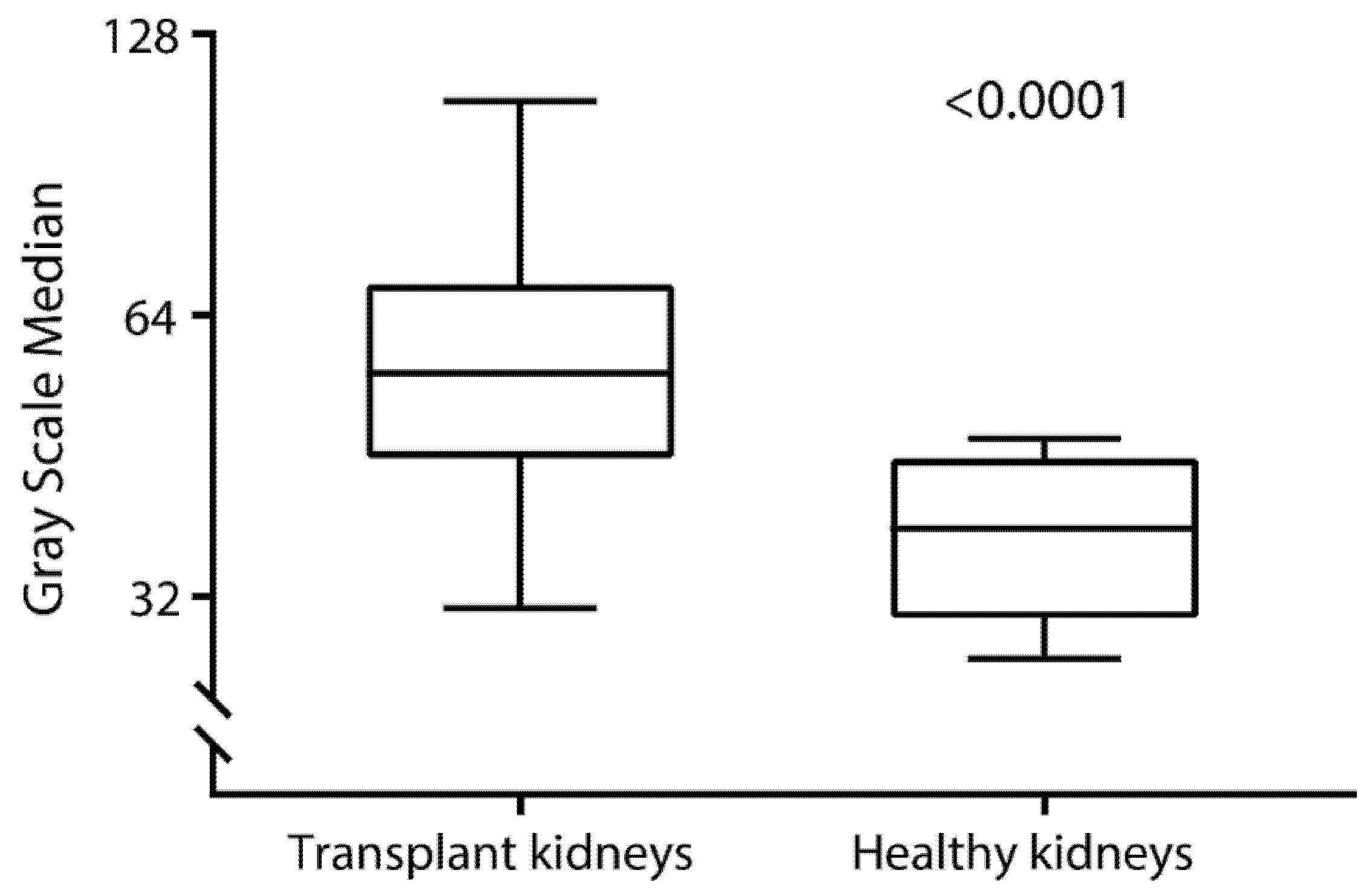
3.2. Grayscale Median in Kidney Transplant Recipients and Nontransplanted Patients
3.3. Cross-Sectional Analyses between Grayscale Median and Transplant Characteristics
3.4. Association between Grayscale Median and Kidney Function Parameters at 1-Year Post-Transplant
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tonelli, M.; Wiebe, N.; Knoll, G.; Bello, A.; Browne, S.; Jadhav, D.; Klarenbach, S.; Gill, J. Systematic review: Kidney transplantation compared with dialysis in clinically relevant outcomes. Am. J. Transplant. 2011, 11, 2093–2109. [Google Scholar] [CrossRef] [PubMed]
- Improving Global Outcomes (KDIGO). Transplant Work Group KDIGO Clinical Practice Guideline for the Care of Kidney Transplant Recipients. Am. J. Transplant. 2009, 9, S1–S157. [Google Scholar] [CrossRef]
- Benjamens, S.; Glaudemans, A.W.J.M.; Berger, S.P.; Slart, R.H.J.A.; Pol, R.A. Have we forgotten imaging prior to and after kidney transplantation? Eur. Radiol. 2018, 28, 3263–3267. [Google Scholar] [CrossRef]
- Sugi, M.D.; Joshi, G.; Maddu, K.K.; Dahiya, N.; Menias, C.O. Imaging of renal transplant complications throughout the life of the allograft: Comprehensive multimodality review. Radiographics 2019, 39, 1327–1355. [Google Scholar] [CrossRef] [PubMed]
- Kolofousi, C.; Stefanidis, K.; Cokkinos, D.D.; Karakitsos, D.; Antypa, E.; Piperopoulos, P. Ultrasonographic features of kidney transplants and their complications: An imaging review. ISRN Radiol. 2013, 2013. [Google Scholar] [CrossRef]
- Naesens, M.; Heylen, L.; Lerut, E.; Claes, K.; De Wever, L.; Claus, F.; Oyen, R.; Kuypers, D.; Evenepoel, P.; Bammens, B.; et al. Intrarenal resistive index after renal transplantation. N. Engl. J. Med. 2013, 369, 1797–1806. [Google Scholar] [CrossRef]
- Van de Kuit, A.; Benjamens, S.; Sotomayor, C.G.; Rijkse, E.; Berger, S.P.; Moers, C.; Bakker, S.J.L.; Minnee, R.C.; Yakar, D.; Pol, R.A.M. Postoperative ultrasound in kidney transplant recipients: Association between intrarenal resistance index and cardiovascular events. Transplant. Direct 2020, 6, e581. [Google Scholar] [CrossRef]
- Kolonko, A.; Chudek, J.; Wiecek, A. Initial kidney graft resistance index and the long-term cardiovascular mortality in transplanted patients: A paired grafts analysis. Nephrol. Dial. Transplant. 2015, 30, 1218–1224. [Google Scholar] [CrossRef]
- Radermacher, J.; Mengel, M.; Ellis, S.; Stuht, S.; Hiss, M.; Schwarz, A.; Eisenberger, U.; Burg, M.; Luft, F.C.; Gwinner, W.; et al. The renal arterial resistance index and renal allograft survival. N. Engl. J. Med. 2003, 349, 115–124. [Google Scholar] [CrossRef]
- Biasi, G.M.; Froio, A.; Diethrich, E.B.; Deleo, G.; Galimberti, S.; Mingazzini, P.; Nicolaides, A.N.; Griffin, M.; Raithel, D.; Reid, D.B.; et al. Carotid plaque echolucency increases the risk of stroke in carotid stenting: The imaging in carotid angioplasty and risk of stroke (ICAROS) study. Circulation 2004, 110, 756–762. [Google Scholar] [CrossRef]
- Pavela, J.; Ahanchi, S.; Steerman, S.N.; Higgins, J.A.; Panneton, J.M. Grayscale median analysis of primary stenosis and restenosis after carotid endarterectomy. J. Vasc. Surg. 2014, 59, 978–982. [Google Scholar] [CrossRef]
- Nicolaides, A.N.; Kakkos, S.K.; Kyriacou, E.; Griffin, M.; Sabetai, M.; Thomas, D.J.; Tegos, T.; Geroulakos, G.; Labropoulos, N.; Doré, C.J.; et al. Asymptomatic internal carotid artery stenosis and cerebrovascular risk stratification. J. Vasc. Surg. 2010, 52, 1486–1496. [Google Scholar] [CrossRef]
- Valiente Engelhorn, A.L.D.; Engelhorn, C.A.; Salles-Cunha, S.X.; Ehlert, R.; Akiyoshi, F.K.; Assad, K.W. Ultrasound tissue characterization of the normal kidney. Ultrasound Q. 2012, 28, 275–280. [Google Scholar] [CrossRef]
- Valiente Engelhorn, A.L.D.; Engelhorn, C.A.; Salles-Cunha, S.X.; Andruska, G.; Batisti, K. Ultrasonographic tissue characterization of kidneys in patients with unilateral renal artery stenosis. J. Vasc. Ultrasound 2016, 40, 70–75. [Google Scholar] [CrossRef]
- Valiente Engelhorn, A.L.D.; Engelhorn, C.A.; Salles-Cunha, S.X. Initial evaluation of virtual histology ultrasonographic techniques applied to a case of renal transplant. J. Vasc. Ultrasound 2015, 39, 142–144. [Google Scholar] [CrossRef]
- Kruskal, W.H.; Wallis, W.A. Use of Ranks in One-Criterion Variance Analysis. J. Am. Stat. Assoc. 1952, 47, 583–621. [Google Scholar] [CrossRef]
- Matsumoto, M.; Nishimura, T. Mersenne twister: A 623-dimensionally equidistributed uniform pseudo-random number generator. ACM Trans. Model. Comput. Simulat. 1998, 8, 3–30. [Google Scholar] [CrossRef]
- Badulescu, M.R.; Socaciu, M.A.; Moisoiu, T.; Andries, A.; Iacob, G.; Badea, R. Current status of imaging diagnosis in the transplanted kidney. A review of the literature with a special focus on contrast-enhanced ultrasonography. Med. Pharm. Rep. 2020, 93, 133. [Google Scholar] [CrossRef]
- Thölking, G.; Schuette-Nuetgen, K.; Kentrup, D.; Pawelski, H.; Reuter, S. Imaging-based diagnosis of acute renal allograft rejection. World J. Transpl. 2016, 6, 174–182. [Google Scholar] [CrossRef]
- Mayor, I.; Momjian, S.; Lalive, P.; Sztajzel, R. Carotid plaque: Comparison between visual and grey-scale median analysis. Ultrasound Med. Biol. 2003, 29, 961–966. [Google Scholar] [CrossRef]
- Kramann, R.; Frank, D.; Brandenburg, V.M.; Heussen, N.; Takahama, J.; Krüger, T.; Riehl, J.; Floege, J. Prognostic impact of renal arterial resistance index upon renal allograft survival: The time point matters. Nephrol. Dial. Transplant. 2012, 27, 3958–3963. [Google Scholar] [CrossRef]
- Baxter, G.M. Imaging in renal transplantation. Ultrasound Q. 2003, 19, 123–138. [Google Scholar] [CrossRef]
- Philosophe, B.; Malat, G.E.; Soundararajan, S.; Barth, R.N.; Manitpisikul, W.; Wilson, N.S.; Ranganna, K.; Drachenberg, C.B.; Papadimitriou, J.C.; Neuman, B.P.; et al. Validation of the Maryland Aggregate Pathology Index (MAPI), a pre-implantation scoring system that predicts graft outcome. Clin. Transplant. 2014, 28, 897–905. [Google Scholar] [CrossRef] [PubMed]

| Baseline Characteristics | Overall | Tertiles of GSM | p | ||
|---|---|---|---|---|---|
| (n = 282) | Tertile 1 (n = 94) | Tertile 2 (n = 94) | Tertile 3 (n = 94) | ||
| GSM | 55 (45–69) | 42 (38–45) | 56 (51–60) | 73 (69–81) | - |
| Recipients characteristics | |||||
| Age, years, mean ± SD | 54 ± 15 | 54 ± 15 | 55 ± 14 | 54 ± 16 | 0.84 |
| Sex, male, n (%) | 170 (60) | 49 (52) | 60 (64) | 61 (65) | 0.14 |
| BMI, kg/m2, mean ± SD | 26.2 ± 4.3 | 26.5 ± 4.9 | 25.9 ± 4.0 | 26.0 ± 4.1 | 0.60 |
| Systolic blood pressure, mmHg, mean ± SD | 143 ± 21 | 138 ± 20 | 142 ± 19 | 148 ± 23 | 0.005 |
| Diastolic blood pressure, mmHg, mean ± SD | 80 ± 13 | 79 ± 12 | 80 ± 13 | 82 ± 13 | 0.23 |
| Use of antihypertensives, n (%) | 211 (75) | 70 (75) | 75 (80) | 66 (70) | 0.32 |
| Pre-emptive transplantation, n (%) | 83 (28) | 33 (35) | 22 (23) | 28 (30) | 0.21 |
| Dialysis vintage, months, median (IQR) | 22 (13–37) | 23 (16–37) | 20 (12–34) | 23 (13–41) | 0.76 |
| Smoking history, n (%) | 59 (21) | 21 (22) | 15 (16) | 23 (25) | 0.33 |
| History of DM, n (%) | 57 (20) | 17 (18) | 17 (18) | 23 (25) | 0.28 |
| History of CVD, n (%) | 65 (23) | 30 (32) | 19 (20) | 16 (17) | 0.02 |
| Donors characteristics | |||||
| Age, years, mean ± SD | 54 ± 13 | 52 ± 16 | 56 ± 11 | 53 ± 13 | 0.15 |
| Sex, male, n (%) | 152 (54) | 52 (55) | 52 (55) | 48 (51) | 0.56 |
| BMI, kg/m2, mean ± SD | 26.0 ± 3.8 | 25.7 ± 4.4 | 26.2 ± 3.4 | 26.0 ± 3.4 | 0.70 |
| Systolic blood pressure, mmHg, mean ± SD | 127 ± 18 | 127 ± 17 | 126 ± 19 | 128 ± 19 | 0.71 |
| Diastolic blood pressure, mmHg, mean ± SD | 73 ± 11 | 73 ± 11 | 71 ± 11 | 74 ± 11 | 0.05 |
| Use of antihypertensives, n (%) | 26 (9) | 10 (11) | 10 (11) | 6 (6) | 0.50 |
| Type of donation | |||||
| Living donor, n (%) | 166 (59) | 62 (66) | 51 (54) | 53 (56) | 0.22 |
| DCD, n (%) | 66 (22) | 19 (20) | 23 (25) | 24 (26) | 0.66 |
| DBD, n (%) | 50 (18) | 13 (14) | 20 (21) | 17 (18) | 0.41 |
| Transplant characteristics | |||||
| Side donor kidney, right, n (%) | 85 (30) | 32 (34) | 32 (34) | 21 (22) | 0.10 |
| Cold ischemia time, minutes, median (IQR) | 30 (16–41) | 29 (17–40) | 33 (16–43) | 31 (15–44) | 0.64 |
| Cold ischemia time, >12 h, n (%) | 56 (20) | 16 (17) | 21 (22) | 19 (20) | 0.66 |
| Ultrasound | |||||
| Time between PO and US, minutes, median (IQR) | 53 (29–86) | 62 (35–114) | 50 (28–85) | 49 (29–76) | 0.08 |
| Position of the kidney, right iliac fossa, n (%) | 228 (81) | 77 (82) | 74 (79) | 77 (82) | 0.76 |
| Central resistive index, mean ± SD | 0.68 ± 0.09 | 0.68 ± 0.08 | 0.69 ± 0.08 | 0.67 ± 0.10 | 0.60 |
| Peripheral resistive index, mean ± SD | 0.64 ± 0.08 | 0.64 ± 0.08 | 0.65 ± 0.08 | 0.64 ± 0.09 | 0.70 |
| Post-operative outcomes | |||||
| DGF, n (%) | 49 (17) | 12 (13) | 16 (17) | 21 (22) | 0.22 |
| DGF, days, median (IQR) | 9 (6–12) | 8 (6–9) | 6 (4–12) | 10 (7–13) | 0.13 |
| CCI, median (IQR) | 9 (0–23) | 9 (0–21) | 9 (0–23) | 9 (0–23) | 0.45 |
| Length of hospital stay, days, median (IQR) | 8 (7–11) | 8 (7–10) | 8 (7–11) | 8 (6–13) | 0.77 |
| Characteristics | Unadjusted Linear Regression | Stepwise Backwards Linear Regression | ||
|---|---|---|---|---|
| Std. β | p | Std. β | p | |
| Recipients characteristics | ||||
| Age, years | 0.04 | 0.56 | ||
| Sex, male, n | −0.08 | 0.26 | ||
| BMI, kg/m2 | −0.06 | 0.34 | ||
| Systolic blood pressure, mmHg | 0.15 | 0.03 | - | |
| Diastolic blood pressure, mmHg | 0.02 | 0.78 | ||
| Use of antihypertensives, n | −0.04 | 0.55 | ||
| Pre-emptive transplantation, n | −0.001 | 0.99 | ||
| Dialysis vintage, months | 0.03 | 0.72 | ||
| Smoking history, n | 0.07 | 0.29 | ||
| History of DM, n | 0.06 | 0.40 | ||
| History of CVD, n | −0.20 | 0.003 | ||
| Donors characteristics | ||||
| Age, years | 0.13 | 0.05 | 0.39 | 0.02 |
| Sex, male, n | 0.03 | 0.39 | ||
| BMI, kg/m2 | 0.05 | 0.48 | ||
| Systolic blood pressure, mmHg | 0.06 | 0.37 | ||
| Diastolic blood pressure, mmHg | 0.02 | 0.76 | ||
| Use of antihypertensives, n | 0.01 | 0.90 | ||
| Type of donation | ||||
| Living donor, n | −0.01 | 0.87 | ||
| DCD, n | 0.01 | 0.92 | ||
| DBD, n | 0.01 | 0.92 | ||
| Transplant characteristics | ||||
| Side donor kidney, right, n | −0.04 | 0.58 | ||
| Cold ischemia time, minutes | 0.03 | 0.65 | ||
| Cold ischemia time, >12 h, n | 0.04 | 0.56 | ||
| Ultrasound | ||||
| Time between PO and US, minutes | −0.11 | 0.10 | - | |
| Position of the kidney, right iliac fossa, n | −0.03 | 0.67 | ||
| Central resistive index | 0.01 | 0.84 | ||
| Peripheral resistive inde | −0.06 | 0.41 | ||
| Follow-up | ||||
| DGF, n | 0.11 | 0.11 | - | |
| DGF, days | 0.23 | 0.17 | - | |
| CCI | 0.05 | 0.53 | ||
| Length of hospital stay, days | 0.09 | 0.16 | - | |
| Kidney Function Parameters | Linear Regression Analyses | |
|---|---|---|
| Std. β | p | |
| Glomerular filtration rate | −0.07 | 0.35 |
| Serum creatinine | 0.003 | 0.97 |
| Creatinine clearance | 0.02 | 0.82 |
| Estimated glomerular filtration rate | −0.02 | 0.76 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sotomayor, C.G.; Benjamens, S.; Dijkstra, H.; Yakar, D.; Moers, C.; Bakker, S.J.L.; Pol, R.A. Introduction of the Grayscale Median for Ultrasound Tissue Characterization of the Transplanted Kidney. Diagnostics 2021, 11, 390. https://doi.org/10.3390/diagnostics11030390
Sotomayor CG, Benjamens S, Dijkstra H, Yakar D, Moers C, Bakker SJL, Pol RA. Introduction of the Grayscale Median for Ultrasound Tissue Characterization of the Transplanted Kidney. Diagnostics. 2021; 11(3):390. https://doi.org/10.3390/diagnostics11030390
Chicago/Turabian StyleSotomayor, Camilo G., Stan Benjamens, Hildebrand Dijkstra, Derya Yakar, Cyril Moers, Stephan J. L. Bakker, and Robert A. Pol. 2021. "Introduction of the Grayscale Median for Ultrasound Tissue Characterization of the Transplanted Kidney" Diagnostics 11, no. 3: 390. https://doi.org/10.3390/diagnostics11030390
APA StyleSotomayor, C. G., Benjamens, S., Dijkstra, H., Yakar, D., Moers, C., Bakker, S. J. L., & Pol, R. A. (2021). Introduction of the Grayscale Median for Ultrasound Tissue Characterization of the Transplanted Kidney. Diagnostics, 11(3), 390. https://doi.org/10.3390/diagnostics11030390









