Thromboinflammation Model-on-A-Chip by Whole Blood Microfluidics on Fixed Human Endothelium
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Antibodies
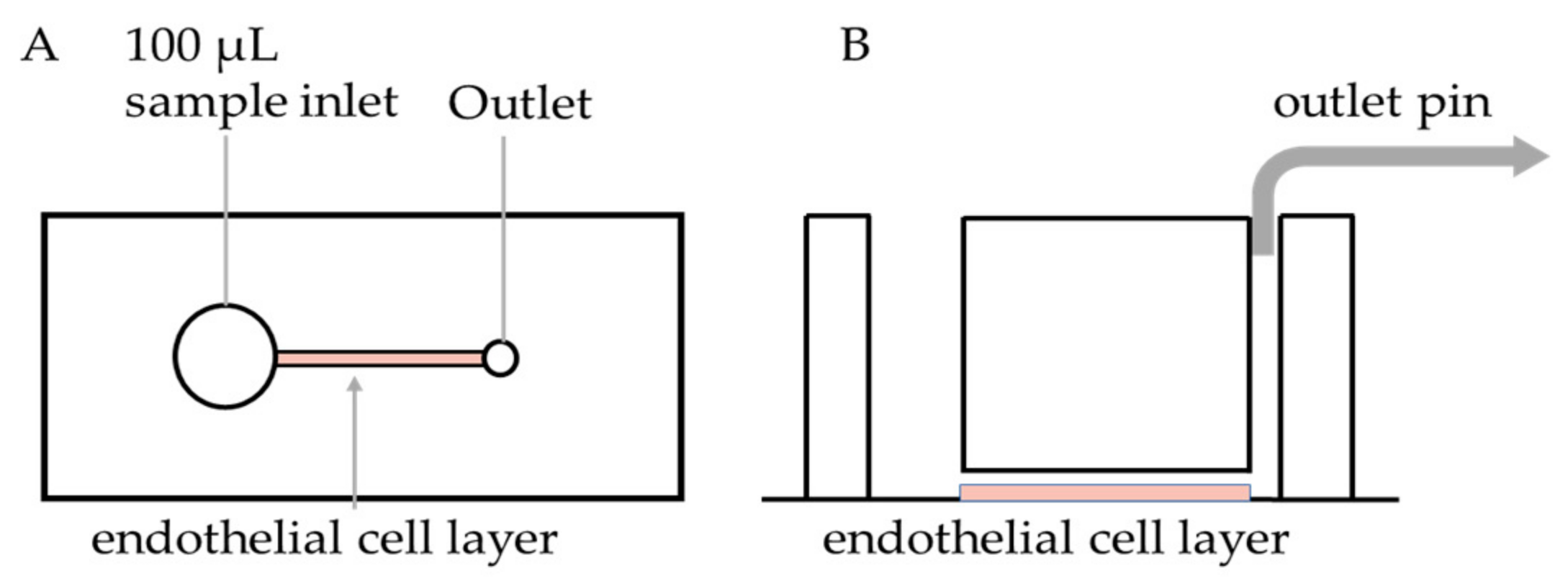
2.2. PDMS Device Fabrication and Endothelialisation
2.3. Microfluidics Assay
2.4. Image Acquisition and Analysis
2.5. Statistical Analysis
3. Results
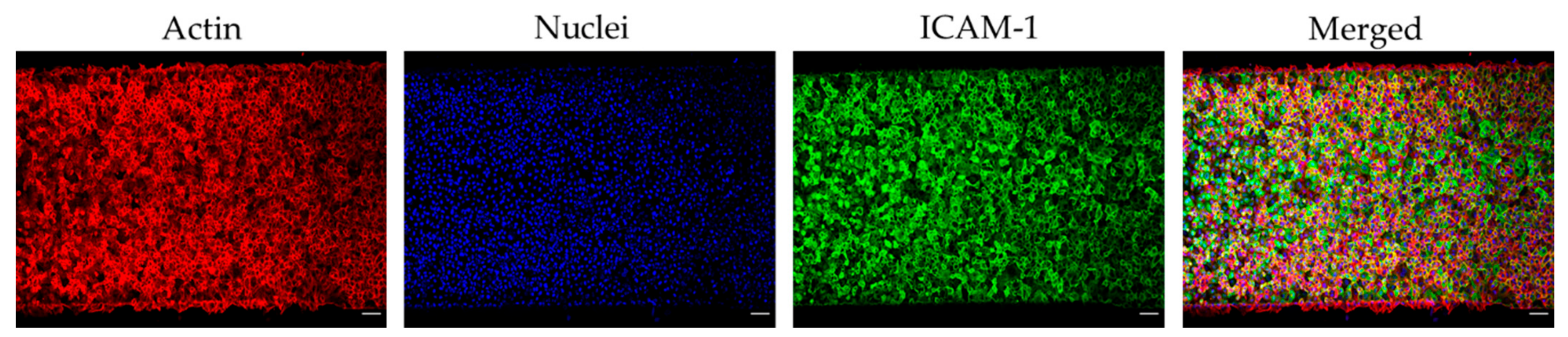
3.1. Microfluidic Device Endothelialisation
3.2. Technical Considerations of Whole Blood Perfusion on Endothelialised Chip
3.2.1. Effect of Anticoagulant on Thromboinflammation Chip
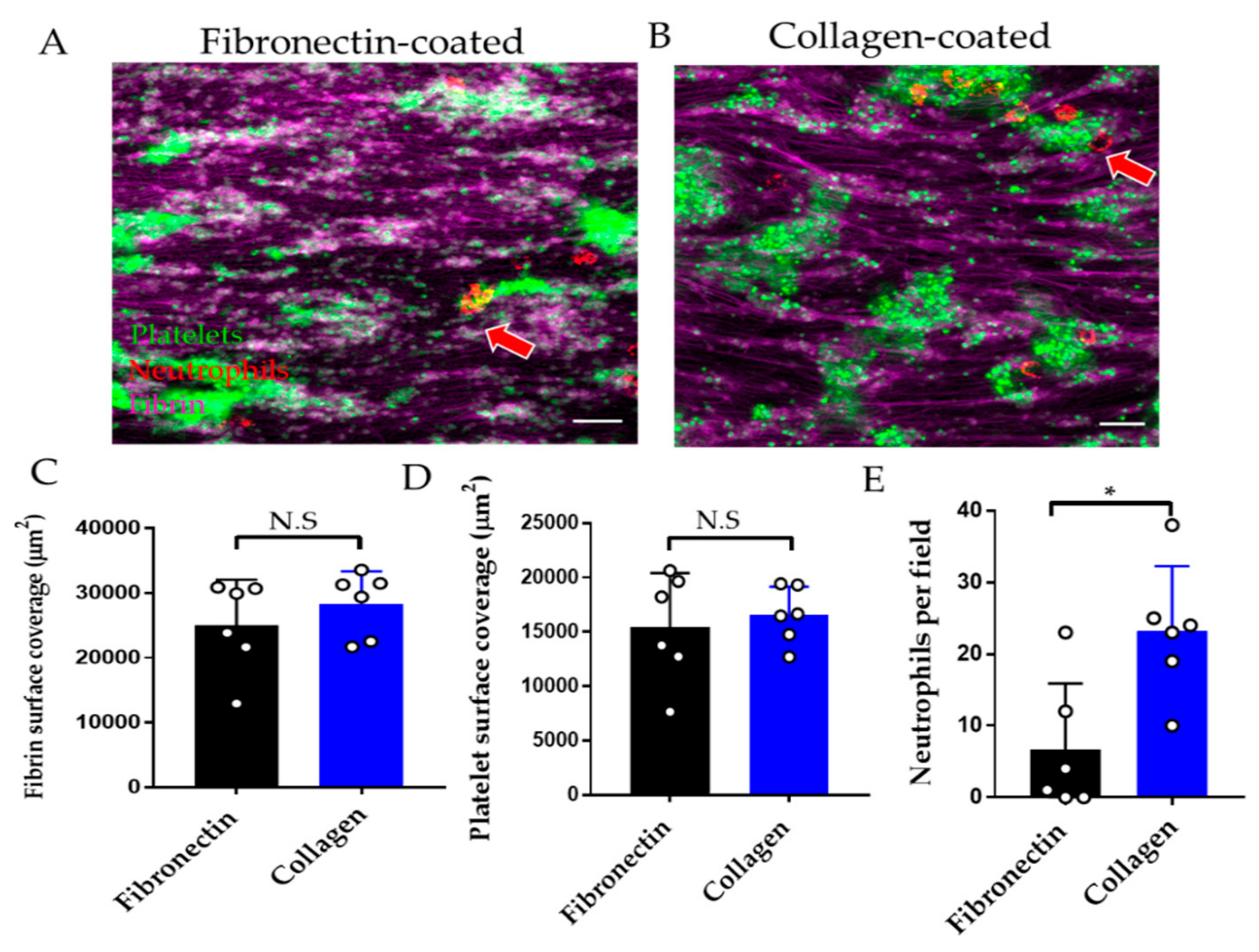
3.2.2. Effect of Endothelial Cell Substrate on Thromboinflammation Chip
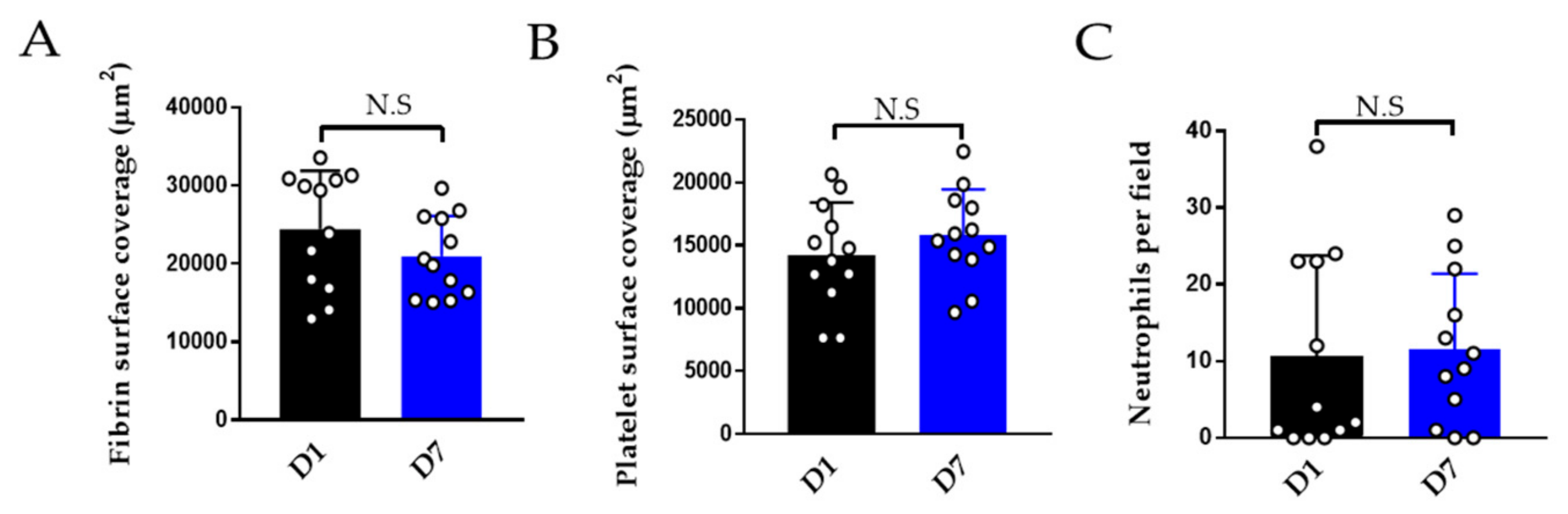
3.2.3. Effect of Storage on Thromboinflammation Chip
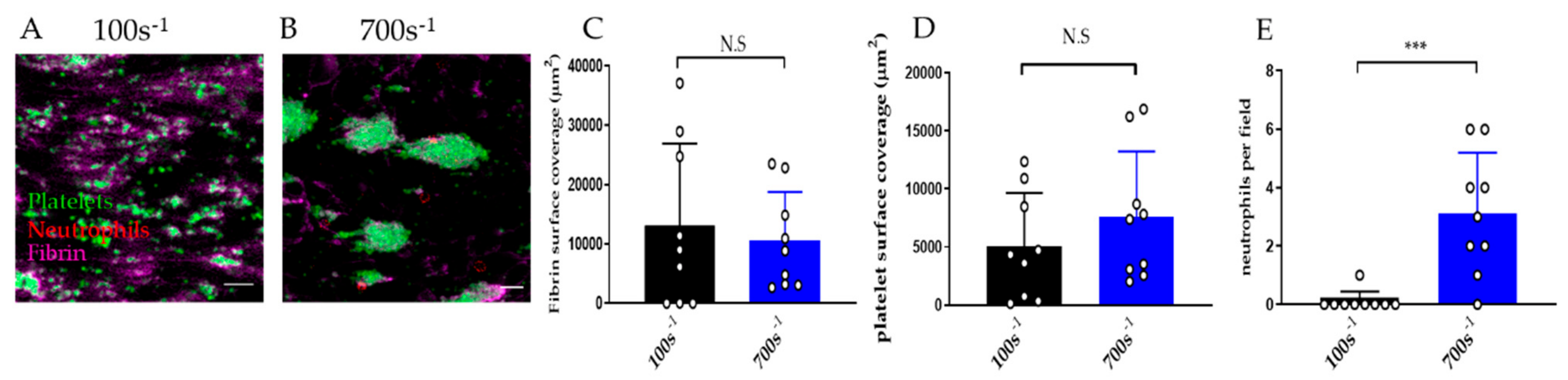
3.2.4. Effect of Shear Rate on Thromboinflammation Chip
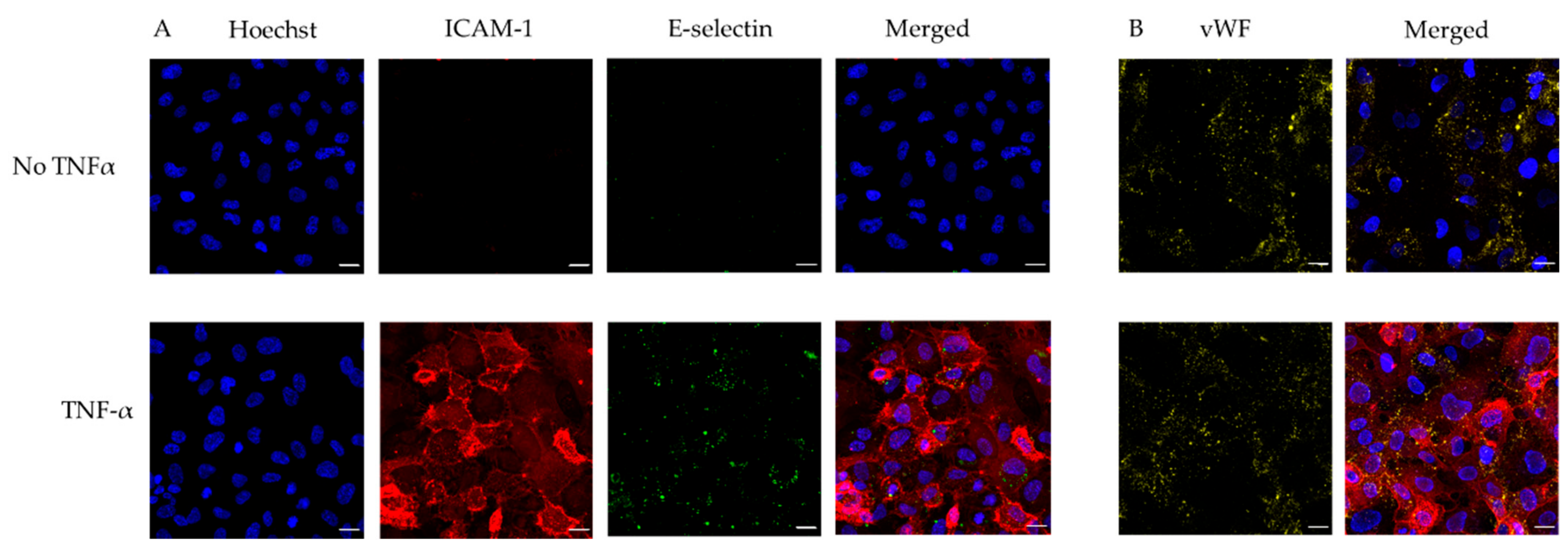
3.3. Use of Endothelialised Biochips to Study Thromboinflammation
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Golia, E.; Limongelli, G.; Natale, F.; Fimiani, F.; Maddaloni, V.; Pariggiano, I.; Bianchi, R.; Crisci, M.; D’Acierno, L.; Giordano, R.; et al. Inflammation and cardiovascular disease: From pathogenesis to therapeutic target. Curr. Atheroscler. Rep. 2014, 16, 435. [Google Scholar] [CrossRef]
- Silvestre-Roig, C.; Braster, Q.; Ortega-Gomez, A.; Soehnlein, O. Neutrophils as regulators of cardiovascular inflammation. Nat. Cardiol. 2020, 17, 327–340. [Google Scholar] [CrossRef] [PubMed]
- Ghasemzadeh, M.; Kaplan, Z.S.; Alwis, I.; Schoenwaelder, S.M.; Ashworth, K.J.; Westein, E.; Hosseini, E.; Salem, H.H.; Slattery, R.; McColl, S.R.; et al. The CXCR1/2 ligand NAP-2 promotes directed intravascular leukocyte migration through platelet thrombi. Blood 2013, 121, 4555–4566. [Google Scholar] [CrossRef] [PubMed]
- Gaul, D.S.; Stein, S.; Matter, C.M. Neutrophils in cardiovascular disease. Eur. Heart J. 2017, 38, 1702–1704. [Google Scholar] [CrossRef] [PubMed]
- Vital, S.A.; Becker, F.; Holloway, P.M.; Russell, J.; Perretti, M.; Granger, N.; Gavins, F. Formyl-Peptide Receptor 2/3/Lipoxin A4 Receptor Regulates Neutrophil-Platelet Aggregation and Attenuates Cerebral Inflammation: Impact for Therapy in Cardiovascular Disease. Circulation 2016, 133, 2169–2179. [Google Scholar] [CrossRef]
- Wolf, D.; Anto-Michel, N.; Blankenbach, H.; Wiedemann, A.; Buscher, K.; Hohmann, J.D.; Lim, B.; Bäuml, M.; Marki, A.; Mauler, M.; et al. A ligand-specific blockade of the integrin Mac-1 selectively targets pathologic inflammation while maintaining protective host-defense. Nat. Commun. 2018, 9, 525. [Google Scholar] [CrossRef]
- Ridker, P.M.; Everett, B.M.; Thuren, T.; MacFadyen, J.G.; Chang, W.H.; Ballantyne, C.; Fonseca, F.; Nicolau, J.; Koenig, W.; Anker, S.D.; et al. Antiinflammatory Therapy with Canakinumab for Atherosclerotic Disease. N. Engl. J. Med. 2017, 377, 1119–1131. [Google Scholar] [CrossRef]
- Lee, H.; Na, W.; Lee, B.; Lim, C.; Shin, S. Recent advances in microfluidic platelet function assays: Moving microfluidics into clinical applications. Clin. Hemorheol. Microcirc. 2019, 71, 249–266. [Google Scholar] [CrossRef]
- Mannino, R.G.; Qiu, Y.; Lam, W.A. Endothelial cell culture in microfluidic devices for investigating microvascular processes. Biomicrofluidics 2018, 12, 042203. [Google Scholar] [CrossRef]
- Smith, C.W. Endothelial adhesion molecules and their role in inflammation. Can. J. Physiol. Pharmacol. 1993, 71, 76–87. [Google Scholar] [CrossRef]
- Bombelli, T.; Schwartz, B.R.; Harlan, J.M. Adhesion of activated platelets to endothelial cells: Evidence for a GPIIbIIIa-dependent bridging mechanism and novel roles for endothelial intercellular adhesion molecule-1 (ICAM-1), αvβ3 integrin, and GP1bα. J. Exp. Med. 1998, 187, 329–339. [Google Scholar] [CrossRef] [PubMed]
- Pircher, J.; Engelmann, B.; Massberg, S.; Schulz, C. Platelet-Neutrophil Crosstalk in Atherothrombosis. Thromb. Haemost. 2019, 119, 1274–1282. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Kim, K.; Jeong, S.Y.; Chiu, J.; Xiong, B.; Petukhov, P.A.; Dai, X.; Li, X.; Andrews, R.K.; Du, X.; et al. Platelet Protein Disulfide Isomerase Promotes Glycoprotein Ibα-Mediated Platelet-Neutrophil Interactions Under Thromboinflammatory Conditions. Circulation 2019, 139, 1300–1319. [Google Scholar] [CrossRef] [PubMed]
- Reinhardt, C.; Brühl, M.L.; Manukyan, D.; Grahl, L.; Lorenz, M.; Altmann, B.; Dlugai, S.; Hess, S.; Konrad, I.; Orschiedt, L.; et al. Protein disulfide isomerase acts as an injury response signal that enhances fibrin generation via tissue factor activation. J. Clin. Investig. 2008, 118, 1110–1122. [Google Scholar] [CrossRef]
- Zwicker, J.I.; Schlechter, B.L.; Stopa, J.D.; Liebman, H.A.; Aggarwal, A.; Puligandla, M.; Caughey, T.; Bauer, K.A.; Kuemmerle, N.; Wong, E.; et al. Targeting protein disulfide isomerase with the flavonoid isoquercetin to improve hypercoagulability in advanced cancer. JCI Insight 2019, 21, e125851. [Google Scholar] [CrossRef] [PubMed]
- Flaumenhaft, R. Protein disulfide isomerase as an antithrombotic target. Trends Cardiovasc. Med. 2013, 23, 264–268. [Google Scholar] [CrossRef]
- Nagy, M.; Heemskerk, J.; Swieringa, F. Use of microfluidics to assess the platelet-based control of coagulation. Platelets 2017, 28, 441–448. [Google Scholar] [CrossRef]
- Jain, A.; van der Meer, A.D.; Papa, A.L.; Barrile, R.; Lai, A.; Schlechter, B.L.; Otieno, M.A.; Louden, C.S.; Hamilton, G.A.; Michelson, A.D.; et al. Assessment of whole blood thrombosis in a microfluidic device lined by fixed human endothelium. Biomed. Microdevices 2016, 18, 73. [Google Scholar] [CrossRef]
- Barrile, R.; van der Meer, A.D.; Park, H.; Fraser, J.P.; Simic, D.; Teng, F.; Conegliano, D.; Nguyen, J.; Jain, A.; Zhou, M.; et al. Organ-on-Chip Recapitulates Thrombosis Induced by an anti-CD154 Monoclonal Antibody: Translational Potential of Advanced Microengineered Systems. Clin. Pharmacol. Ther. 2018, 104, 1240–1248. [Google Scholar] [CrossRef]
- Branchford, B.R.; Ng, C.J.; Neeves, K.B.; Paola, J.D. Microfluidic technology as an emerging clinical tool to evaluate thrombosis and hemostasis. Thromb. Res. 2015, 136, 13–19. [Google Scholar] [CrossRef]
- Halaidych, O.V.; Hil, F.; Mummery, C.L.; Orlova, V.V. Microfluidic assay for the assessment of leukocyte adhesion to human induced pluripotent stem cell-derived endothelial cells (hiPSC-ECs). J. Vis. Exp. 2018, 141. [Google Scholar] [CrossRef] [PubMed]
- Lamberti, G.; Prabhakarpandian, B.; Garson, C.; Smith, A.; Pant, K.; Wang, B.; Kiani, M.F. Bioinspired microfluidic assay for in vitro modeling of leukocyte-endothelium interactions. Anal. Chem. 2014, 86, 8344–8351. [Google Scholar] [CrossRef] [PubMed]
- Sakurai, Y.; Hardy, E.T.; Ahn, B.; Tran, R.; Fay, M.E.; Ciciliano, J.C.; Mannino, R.G.; Myers, D.R.; Qiu, Y.; Carden, M.A.; et al. A microengineered vascularized bleeding model that integrates the principal components of hemostasis. Nat. Commun. 2018, 9, 509. [Google Scholar] [CrossRef] [PubMed]
- Greineder, C.F.; Johnston, I.H.; Villa, C.H.; Gollomp, K.; Esmon, C.T.; Cines, D.B.; Poncz, M.; Muzykantov, V.R. ICAM-1-targeted thrombomodulin mitigates tissue factor-driven inflammatory thrombosis in a human endothelialised microfluidic model. Blood Adv. 2017, 1, 1452–1465. [Google Scholar] [CrossRef]
- Jenny, L.; Dobó, J.; Gál, P.; Lam, W.A.; Schroeder, V. MASP-1 of the complement system enhances clot formation in a microvascular whole blood flow model. PLoS ONE 2018, 13, e0191292. [Google Scholar] [CrossRef]
- Mathur, T.; Singh, K.A.; Pandian, N.K.; Tsai, S.H.; Hein, T.W.; Gaharwar, A.K.; Flanagan, J.M.; Jain, A. Organ-on-chips made of blood: Endothelial progenitor cells from blood reconstitute vascular thromboinflammation in vessel-chips. Lab Chip 2019, 19, 2500–2511. [Google Scholar] [CrossRef]
- Costa, P.F.; Albers, H.J.; Linssen, J.E.A.; Middelkamp, H.H.T.; van der Hout, L.; Passier, R.; van den Berg, A.; Malda, J.; van der Meer, A.D. Mimicking arterial thrombosis in a 3D-printed microfluidic in vitro vascular model based on computed tomography angiography data. Lab Chip 2017, 17, 2785–2792. [Google Scholar] [CrossRef]
- Jenny, L.; Melmer, A.; Laimer, M.; Hardy, E.T.; Lam, W.A.; Schroeder, V. Diabetes affects endothelial cell function and alters fibrin clot formation in a microvascular flow model: A pilot study. Diab. Vasc. Dis. Res. 2020, 17, 1479164120903044. [Google Scholar] [CrossRef]
- Tsou, J.K.; Gower, R.M.; Ting, H.J.; Schaff, U.Y.; Insana, M.F.; Passerini, A.G.; Simon, S.I. Spatial regulation of inflammation by human aortic endothelial cells in a linear gradient of shear stress. Microcirculation 2008, 15, 311–323. [Google Scholar] [CrossRef]
- Tsai, M.; Kita, A.; Leach, J.; Rounsevell, R.; Huang, J.N.; Moake, J.; Ware, R.E.; Fletcher, D.A.; Lam, W.A. In vitro modeling of the microvascular occlusion and thrombosis that occur in hematologic diseases using microfluidic technology. J. Clin. Investig. 2012, 122, 408–418. [Google Scholar] [CrossRef]
- Gollomp, K.; Kim, M.; Johnston, I.; Hayes, V.; Welsh, J.; Arepally, G.M.; Kahn, M.; Lambert, M.P.; Cuker, A.; Cines, D.B.; et al. Neutrophil accumulation and NET release contribute to thrombosis in HIT. JCI Insight 2018, 38, e99445. [Google Scholar] [CrossRef] [PubMed]
- Menon, N.V.; Su, C.; Pang, K.T.; Phua, Z.J.; Tay, H.M.; Dalan, R.; Wang, X.; Li, K.H.H.; Hou, H.W. Recapitulating atherogenic flow disturbances and vascular inflammation in a perfusable 3D stenosis model. Biofabrication 2020, 12, 045009. [Google Scholar] [CrossRef] [PubMed]
- Michels, A.; Swystun, L.L.; Mewburn, J.; Albánez, S.; Lillicrap, D. Investigating von Willebrand Factor Pathophysiology Using a Flow Chamber Model of von Willebrand Factor-platelet String Formation. J. Vis. Exp. 2017, 126, 55917. [Google Scholar] [CrossRef] [PubMed]
- Bernardo, A.; Ball, C.; Nolasco, L.; Choi, H.; Moake, J.L.; Dong, J.F. Platelets adhered to endothelial cellbound ultra-large von Willebrand factor strings support leukocyte tethering and rolling under high shear stress. J. Thromb. Haemost. 2005, 3, 562–570. [Google Scholar] [PubMed]
- Zheng, Y.; Chen, J.; Craven, M.; Choi, N.W.; Totorica, S.; Diaz-Santana, A.; Kermani, P.; Hempstead, B.; Fischbach-Teschl, C.; López, J.A.; et al. In vitro microvessels for the study of angiogenesis and thrombosis. Proc. Natl. Acad. Sci. USA 2012, 109, 9342–9347. [Google Scholar] [CrossRef] [PubMed]
- Adriani, G.; Ma, D.; Pavesi, A.; Kamm, R.D.; Goh, E.L. A 3D neurovascular microfluidic model consisting of neurons, astrocytes and cerebral endothelial cells as a blood-brain barrier. Lab Chip 2017, 17, 448–459. [Google Scholar] [CrossRef] [PubMed]
- Hui, K.Y.; Haber, E.; Matsueda, G.R. Monoclonal antibodies to a synthetic fibrin-like peptide bind to human fibrin but not fibrinogen. Science 1983, 222, 1129–1132. [Google Scholar] [CrossRef]
- Setiadi, H.; Sedgewick, G.; Erlandsen, S.L.; McEver, R.P. Interactions of the cytoplasmic domain of P-selectin with clathrin-coated pits enhance leukocyte adhesion under flow. J. Cell. Biol. 1998, 142, 859–871. [Google Scholar] [CrossRef]
- Dupuy, A.; Ju, A.; Passam, F. Straight channel microfluidic chips for the study of platelet adhesion under flow. Bioprotocol 2019, 9, e3195. [Google Scholar]
- Coleman, P.R.; Lay, A.J.; Ting, K.K.; Zhao, Y.; Li, J.; Jarrah, S.; Vadas, M.A.; Gamble, J.R. YAP and the RhoC regulator ARHGAP18, are required to mediate flow-dependent endothelial cell alignment. Cell Commun. Signal. 2020, 18, 18. [Google Scholar] [CrossRef]
- Woollard, K.J.; Suhartoyo, A.; Harris, E.E.; Eisenhardt, S.U.; Jackson, S.P.; Peter, K.; Dart, A.M.; Hickey, M.J.; Chin-Dusting, J.P. Pathophysiological levels of soluble P-selectin mediate adhesion of leukocytes to the endothelium through Mac-1 activation. Circ. Res. 2008, 103, 1128–1138. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Gopal, S.; Sharda, A.; Passam, F.; Bowley, S.R.; Stopa, J.; Xue, G.; Yuan, C.; Furie, B.C.; Flaumenhaft, R.; et al. Quercetin-3-rutinoside inhibits protein disulfide isomerase by binding to its b’x domain. J. Biol. Chem. 2015, 290, 23543–23552. [Google Scholar] [CrossRef] [PubMed]
- Sakariassen, K.S.; Orning, L.; Turitto, V.T. The impact of blood shear rate on arterial thrombus formation. Future Sci. OA 2015, 1. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Kucik, D.F.; Szalai, A.J.; Edberg, J.C. Human neutrophil flow chamber adhesion assay. J. Vis. Exp. 2014, 89, 51410. [Google Scholar] [CrossRef]
- Lehmann, M.; Wallbank, A.M.; Dennis, K.A.; Wufsus, A.R.; Davis, K.M.; Rana, K.; Neeves, K.B. On-chip recalcification of citrated whole blood using a microfluidic herringbone mixer. Biomicrofluidics 2015, 9, 064106. [Google Scholar] [CrossRef]
- Muthard, R.W.; Diamond, S. Rapid on-chip recalcification and drug dosing of citrated whole blood using microfluidic buffer sheath flow. Biorheology 2014, 51, 227–237. [Google Scholar] [CrossRef]
- Wang, Y.; Gao, H.; Shi, C.; Erhardt, P.W.; Pavlovsky, A.A.; Soloviev, D.; Bledzka, K.; Ustinov, V.; Zhu, L.; Qin, J.; et al. Leukocyte integrin Mac-1 regulates thrombosis via interaction with platelet GPIbα. Nat. Commun. 2017, 8, 15559. [Google Scholar] [CrossRef]
- Cho, J.; Furie, B.C.; Coughlin, S.R.; Furie, B. A critical role for extracellular protein disulfide isomerase during thrombus formation in mice. J. Clin. Investig. 2008, 118, 1123–1131. [Google Scholar] [CrossRef]
- Hahm, E.; Li, J.; Kim, K.; Huh, S.; Rogelj, S.; Cho, J. Extracellular protein disulfide isomerase regulates ligand-binding activity of αMβ2 integrin and neutrophil recruitment during vascular inflammation. Blood 2013, 121, 3789–3800. [Google Scholar] [CrossRef]
- Pendu, R.; Terraube, V.; Christophe, O.D. P-selectin glycoprotein ligand 1 and beta2-integrins cooperate in the adhesion of leukocytes to von Willebrand factor. Blood 2006, 108, 3746–3752. [Google Scholar]
- Hirasashi, J.; Hishikawa, K.; Kaname, S.; Tsuboi, N.; Wang, Y.; Simon, D.I.; Stavrakis, G.; Shimosawa, T.; Xiao, L.; Nagahama, Y.; et al. Mac-1 (CD11b/CD18) is a critical molecular link between inflammation and thrombosis following glomerular injury. Circulation 2009, 120, 1255–1265. [Google Scholar] [CrossRef] [PubMed]
- Dove, A. CD18 trials disappoint again. Nat. Biotechnol. 2000, 18, 817–818. [Google Scholar] [CrossRef] [PubMed]
- Hertog, M.G.; Feskens, E.J.; Hollman, P.C.; Katan, M.B.; Kromhout, D. Dietary antioxidant flavonoids and risk of coronary heart disease: The Zutphen Elderly Study. Lancet 1993, 342, 1007–1011. [Google Scholar] [CrossRef] [PubMed]
- Jayachandran, M.; Wu, Z.; Ganesan, K.; Khalid, S.; Chung, S.M.; Xu, B. Isoquercetin upregulates antioxidant genes, suppresses inflammatory cytokines and regulates AMPK pathway in streptozotocin-induced diabetic rats. Chem. Biol. Interact. 2019, 303, 62–69. [Google Scholar] [CrossRef] [PubMed]
- Jasuja, R.; Passam, F.H.; Kennedy, D.R.; Kim, S.H.; van Hessem, L.; Lin, L.; Bowley, S.R.; Joshi, S.S.; Dilks, J.R.; Furie, B.; et al. Protein disulfide isomerase inhibitors constitute a new class of antithrombotic agents. J. Clin. Investig. 2012, 122, 2104–2113. [Google Scholar] [CrossRef]
- Yu, X.; Diamond, S.L. Fibrin modulates shear-induced NETosis in sterile occlusive thrombi formed under hemodynamic flow. Thromb. Haemost. 2019, 119, 586–593. [Google Scholar] [CrossRef]
- Perdomo, J.; Leung, H.H.L.; Ahmadi, Z.; Yan, F.; Chong, J.J.H.; Passam, F.H.; Chong, B.H. Neutrophil activation and NETosis are major drivers of thrombosis in heparin-induced thrombocytopenia. Nat. Commun. 2019, 10, 1322. [Google Scholar] [CrossRef]
- Neeves, K.B.; McCarty, O.J.; Reininger, A.J.; Sugimoto, M.; King, M.R. Biorheology Subcommittee of the SSC of the ISTH. Flow-dependent thrombin and fibrin generation in vitro: Opportunities for standardization: Communication from SSC of the ISTH. J. Thromb. Haemost. 2014, 12, 418–420. [Google Scholar] [CrossRef]


| Microfluidic Device Description | Endothelial Treatment | Perfusion Sample | Function Measured | Clinical/Diagnostics Potential | Reference |
|---|---|---|---|---|---|
| Hemostasis | |||||
| HUVEC-lined straight channel | Mechanical injury by pneumatic valve | Re-calcified citrated whole blood | Hemostasis | Detection of hemostatic defects (hemophilia) and screening of anticoagulants | [23] |
| Thrombosis | |||||
| HUVEC-lined straight channel | TNF-α and fixation | Re-calcified citrated whole blood | Platelet adhesion and fibrin generation | Long-term storage of chips for study of thrombosis | [18] |
| HUVEC-lined flow chambers | TNF-α | Re-calcified citrated whole blood | Fibrin formation, platelet and neutrophil adhesion | Testing anti-thrombotic potential of single-chain antibody fragment | [24] |
| HUVEC-lined straight channel | - | Re-calcified citrated whole blood | Fibrin formation | Study of complement-driven thrombosis | [25] |
| BOEC-lined straight channel | TNF-α | Re-calcified citrated whole blood | Platelet adhesion and fibrin generation | Non-invasive assessment of endothelial function and thrombus formation in diabetes | [26] |
| HUVEC-lined 3D channel with stenoses | - | Re-calcified citrated whole blood | Platelet adhesion and thrombus formation | Employing medical CTA imaging data to produce patient-specific microfluidic chips | [27] |
| HMVEC-lined straight channel | - | Re-calcified citrated whole blood | Fibrin formation | Study of endothelial function and thrombus formation in diabetes | [28] |
| Thromboinflammation | |||||
| HAECS-lined curved channel with gradient shear | - | Isolated neutrophils, and monocytes | Neutrophil and monocyte adhesion | Study of the effect of shear stress on vascular inflammation | [29] |
| HUVEC, HLMVEC-lined 3D branching microchannels | TNF-α, STX2 | Heparin, EDTA whole blood | Platelet, neutrophil and monocyte adhesion | Study of microvascular occlusion in sickle cell disease and HUS and effect of medications | [30] |
| HUVEC-lined 3D microvascular network | TNF-α | Isolated neutrophils | Neutrophil rolling, adhesion, migration | Study of vascular inflammation | [22] |
| HUVEC-lined straight channel | TNF-α | Re-calcified whole blood | NETosis | Study of NETosis and thrombosis in patient groups (HIT) | [31] |
| HUVEC-lined 3D channel with stenosis | TNF-α | Citrated whole blood | Platelet and neutrophil adhesion | Study of antithrombotic potential of aspirin and metformin | [32] |
| BOEC-lined straight channel | PMA, histone | Isolated platelets | Platelet adhesion to vWF strings | Study of thromboinflammation | [33] |
| HUVEC-lined parallel flow chamber | histamine | Isolated platelets, reconsitituted blood | Platelet and neutrophil adhesion to vWF | Study of neutrophil involvement in thrombosis under high shear | [34] |
| Endothelial function | |||||
| HUVEC, HUASMC, HBVPC-seeded multi-channel network | PMA, Media containing angiogenic growth factors | Citrated whole blood | Angiogenesis, vessel permeability, thrombosis | Study of angiogenesis and microvascular thrombosis in CV medicine | [35] |
| Neuron, astrocytes and HUVEC-lined 3D multi-channel | - | Fluorescent dextran | Blood–brain barrier | Screening of drugs for blood–brain barrier permeability | [36] |
| Target Antigen | Clone | Supplier | Fluorescent Label |
|---|---|---|---|
| CD42a | ALMA.16 | Becton Dickinson, Franklin Lakes, NJ, USA | PE |
| CD18 | H52 | Developmental Studies Hybridoma Bank, university of Iowa, USA | AlexaFluor 488, labelled using protein labelling kit A10235 (Invitrogen) |
| CD66b | 80H3 | Beckman Coulter, Brea, CA, USA | APC |
| Fibrin | 59D8 | - | AlexaFluor 594, labelled using protein labelling kit A10239 (Invitrogen) |
| CD41a | P2 | Beckman Coulter | FITC |
| CD11b | M1/70 | Invitrogen | - |
| ICAM-1 | HA58 | Invitrogen | APC |
| VE-Cadherin | D87F2 | Cell Signalling Technologies, Danvers, MA, USA | - |
| CD62P | G1 | - | - |
| CD62E | UZ4 | Thermofisher | - |
| vWF | rabbit polyclonal | Dako | AlexaFluor 594, labelled using protein labelling kit A10239 (Invitrogen) |
| Rabbit IgG | N/A | Invitrogen | AlexaFluor 488 |
| Rat Ig | N/A | Becton Dickinson | FITC |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dupuy, A.; Hagimola, L.; Mgaieth, N.S.A.; Houlahan, C.B.; Preketes-Tardiani, R.E.; Coleman, P.R.; Passam, F.H. Thromboinflammation Model-on-A-Chip by Whole Blood Microfluidics on Fixed Human Endothelium. Diagnostics 2021, 11, 203. https://doi.org/10.3390/diagnostics11020203
Dupuy A, Hagimola L, Mgaieth NSA, Houlahan CB, Preketes-Tardiani RE, Coleman PR, Passam FH. Thromboinflammation Model-on-A-Chip by Whole Blood Microfluidics on Fixed Human Endothelium. Diagnostics. 2021; 11(2):203. https://doi.org/10.3390/diagnostics11020203
Chicago/Turabian StyleDupuy, Alexander, Lejla Hagimola, Neil S. A. Mgaieth, Callum B. Houlahan, Renee E. Preketes-Tardiani, Paul R. Coleman, and Freda H. Passam. 2021. "Thromboinflammation Model-on-A-Chip by Whole Blood Microfluidics on Fixed Human Endothelium" Diagnostics 11, no. 2: 203. https://doi.org/10.3390/diagnostics11020203
APA StyleDupuy, A., Hagimola, L., Mgaieth, N. S. A., Houlahan, C. B., Preketes-Tardiani, R. E., Coleman, P. R., & Passam, F. H. (2021). Thromboinflammation Model-on-A-Chip by Whole Blood Microfluidics on Fixed Human Endothelium. Diagnostics, 11(2), 203. https://doi.org/10.3390/diagnostics11020203







