Abstract
Dopaminergic signaling is believed to be related to autistic traits. We conducted an exploratory 3,4-dihydroxy-6-[18F]-fluoro-L-phenylalanine positron emission tomography/computed tomography ([18F]-FDOPA PET/CT) study, to examine cerebral [18F]-FDOPA influx constant (kicer min−1), reflecting predominantly striatal dopamine synthesis capacity and a mixed monoaminergic innervation in extrastriatal neurons, in 44 adults diagnosed with autism spectrum disorder (ASD) and 22 controls, aged 18 to 30 years. Autistic traits were assessed with the Autism Spectrum Quotient (AQ). Region-of-interest and voxel-based analyses showed no statistically significant differences in kicer between autistic adults and controls. In autistic adults, striatal kicer was significantly, negatively associated with AQ attention to detail subscale scores, although Bayesian analyses did not support this finding. In conclusion, among autistic adults, specific autistic traits can be associated with reduced striatal dopamine synthesis capacity. However, replication of this finding is necessary.
1. Introduction
The atypical functioning of dopaminergic and other monoaminergic systems has long been hypothesized to contribute to autistic traits [1]. For instance, according to the dopamine hypothesis of autism spectrum disorder (ASD; [2,3]), alterations in the midbrain dopaminergic system are associated with clinical and sub-clinical autistic traits, including difficulties in social interaction and communication, and stereotyped behaviors. However, there is a lack of studies assessing in vivo monoamine functioning in autistic adults [4].
Positron emission tomography/computed tomography (PET/CT) studies have used 3,4-dihydroxy-6-[18F]-fluoro-L-phenylalanine ([18F]-FDOPA) to assess the presynaptic dopamine synthesis capacity in ASD. One study reported that [18F]-FDOPA uptake was decreased in the anterior medial prefrontal cortex in (mostly sedated) autistic children (n = 14), relative to typically developing controls (n = 10) [5]. Another study found that [18F]-FDOPA uptake was increased in the frontal and striatal regions in adults with Asperger syndrome (n = 8) relative to controls (n = 5) [6]. However, in both studies, sample sizes were small, and the associations with measures of autistic traits were not examined.
The social defeat hypothesis of schizophrenia posits that a subordinate or outsider position leads to an increased baseline activity or sensitization of the mesolimbic dopamine system and, thereby, to an increased risk of schizophrenia [7]. Since ASD is a risk factor for schizophrenia [8], we recently conducted a large [18F]-FDOPA PET/CT study to test the pre-registered hypothesis of increased striatal dopamine synthesis capacity in non-psychotic individuals with ASD [9]. Contrary to our hypothesis, the results indicated no differences in striatal [18F]-FDOPA uptake between individuals with ASD (n = 44) and controls (n = 22), and no association between this uptake and social defeat.
Here, we extend our previous study with exploratory region of interest (ROI) and voxel-based analyses, in which we compare striatal as well as extrastriatal [18F]-FDOPA uptake between adults with ASD and controls, and examine their associations with self-reported autistic traits.
2. Materials and Methods
2.1. Participants and Procedures
The full procedures are described in our previous publication [9]. We recruited Dutch participants aged 18 to 30 years, who were abstinent from current or recent psychotropic medication use (see Supplementary Methods S1 for details). Those with ASD, had received their diagnosis from a registered mental health clinician, and this diagnosis was confirmed by the first author using the Autism Diagnostic Observation Schedule-2 (ADOS-2) module 4 [10,11]. We included 44 autistic participants and 22 controls (frequency-matched on age, sex, and smoking status). All the participants provided informed consent. The study was approved by the medical ethics committee of the Leiden University Medical Center (reference NL54244.058.15).
2.2. Autism Spectrum Quotient
The Autism Spectrum Quotient (AQ) is a 50 item self-report questionnaire that assesses the presence of autistic traits [12]. Items are scored between 1 (definitely agree) and 4 (definitely disagree). After reverse-scoring, the higher total scores reflect the presence of more autistic traits. Additionally, in line with the original validation of the Dutch AQ [13], we calculated scores on the “social interaction” and “attention to detail” subscales. Higher scores on these subscales indicate greater difficulties in social interactions, and a greater attention to, and interests in, patterns and details, respectively. The AQ was completed by both samples.
2.3. MRI and PET/CT Acquisition and Processing
Details of magnetic resonance imaging (MRI) and PET/CT acquisitions and processing steps have been previously described [9]. In short, a structural T1-weighted MRI scan was obtained on a 3T Ingenia (Philips Healthcare, Best, The Netherlands). A 90 min dynamic PET scan was obtained on a Biograph Horizon with TrueV option (Siemens Healthineers, Erlangen, Germany) or Vereos (Philips Healthcare, Best, The Netherlands), directly after the administration of approximately 150 MBq [18F]-FDOPA. A low dose CT scan (110/120 kVp, 35 mAs) was acquired for attenuation–correction purposes. Participants consumed 150 mg of carbidopa and 400 mg entacapone, 1 hour before starting the PET/CT scan.
We used [18F]-FDOPA uptake in gray matter (GM) cerebellum as a reference to calculate the influx constant (kicer min−1; hereon labeled as kicer) throughout the brain using reference Patlak graphical analysis [14]. The ROIs were automatically identified from the co-registered MRI scan using PVElab (v2.3; Neurobiology Research Unit, Copenhagen, Denmark; [15,16]), using a maximum probability atlas [17]. The ROIs included the GM of the whole striatum and three striatal anatomical sub-regions (putamen, nucleus accumbens, and caudate nucleus), which were selected on the basis of their putative role in ASD [2,3], and their reliability in terms of imaging [18F]-FDOPA uptake [18].
In addition to the ROI analysis, the parametric image of each participant was transformed to standard space to facilitate voxel-based comparisons. To do so, we first normalized the participant’s co-registered MRI scan using SPM12 (Institute of Neurology, London, UK). The resulting transformation matrix was applied to the parametric image, which was then smoothed using an 8 mm full width at half maximum (FWHM) Gaussian filter [18].
2.4. Statistical Analysis
With reference to the ROI method, data were analyzed using JASP version 0.16 [19]. We used multiple linear regression analysis to compare the regional kicer values between ASD and controls and to assess the associations of kicer with the AQ total and subscale scores. We adjusted for four confounders: age, sex, smoker status (yes/no), and scanner type (Vereos/Biograph Horizon). A two-tailed p-value of 0.05 was used to evaluate the statistical significance. In addition, Bayesian analyses were conducted (see Supplementary Methods S2). Voxel-based comparisons were made in SPM12. An independent samples t-test was used to examine group differences in kicer, and multiple linear regression analysis was used to examine associations between kicer and AQ scores. Confounders included age, sex, smoker status, and scanner type. A family-wise error (FWE) rate of α = 0.05 was used to evaluate the statistical significance. We conducted several additional control analyses to assess the robustness of the findings. First, we repeated our analyses restricting ourselves to voxels with kicer values above 0.001 and 0.005, effectively excluding voxels showing little specific [18F]-FDOPA uptake. Second, we repeated our analyses with unsmoothed data and after smoothing with a 4 mm FWHM Gaussian filter (i.e., instead of the 8 mm FWHM Gaussian filter that we used for the main analysis). Third, we conducted the analyses for the two PET/CT scanners separately.
3. Results
3.1. Sample Characteristics
Sample characteristics are reported in Table 1. Participants with ASD had significantly higher AQ total scores (t64 = 8.74, p < 0.001), as well as social interaction (t64 = 8.34, p < 0.001) and attention to detail (t64 = 5.55, p < 0.001) subscale scores. With regard to self-reported lifetime diagnosed mental health conditions, in the control group, participants reported having ever been diagnosed with a depressive disorder (n = 1) and anxiety disorder (n = 1). In the ASD sample, participants reported having ever been diagnosed with a depressive disorder (n = 9), attention deficit/hyperactivity disorder (n = 4), anxiety disorder (n = 2), and post-traumatic stress disorder (n = 2). None of the participants had currently or recently used any medication for these conditions.

Table 1.
Sample characteristics of adults with autism spectrum disorder (ASD) and controls.
3.2. ROI Analyses
Table 2 shows the kicer values in striatal ROIs and their associations with the AQ total and subscale scores. We found no significant differences in the striatal kicer values between ASD and controls. Moreover, within the control sample, and within the combined ASD and control sample, we found no significant associations between the AQ scores and striatal kicer values. In contrast, in the ASD sample, kicer values in the whole striatum, putamen, and nucleus accumbens were significantly negatively associated with AQ attention to detail subscale scores. These associations remained negative when we examined the results without adjusting for confounders or for the two PET/CT scanners separately, although they became statistically non-significant. No other statistically significant associations were observed.

Table 2.
Striatal [18F]-FDOPA uptake (kicer min−1) in ASD adults and controls, and its association with self-reported autistic traits.
Bayesian analyses supported the observed null findings over the alternative hypotheses (Supplementary Method S2 and Supplementary Table S1). Notably, these analyses also did not provide support for a relationship between AQ attention to detail subscale scores and kicer values.
3.3. Voxel-Based Comparisons
Figure 1 (panels A and B) shows the average kicer for ASD and control participants in voxels throughout the brain. In striatal as well as extrastriatal regions, we found no statistically significant differences in the kicer values between ASD and controls, and in neither sample did we observe significant associations between the kicer values and the AQ total or social interaction subscale scores. These results were similar, regardless of whether we adjusted for confounders, applied varying kicer thresholds, used unsmoothed data or data smoothed with a 4 FWHM Gaussian filter, or examined the results for the two PET/CT scanners separately. We did observe, in accordance with the ROI analysis, that kicer values in a small cluster of voxels in the left nucleus accumbens, significantly negatively correlated with the AQ attention to detail subscale in the ASD sample (and not in controls) (Figure 1C). At more lenient p-value thresholds, this association extended to larger parts of the striatum bilaterally.
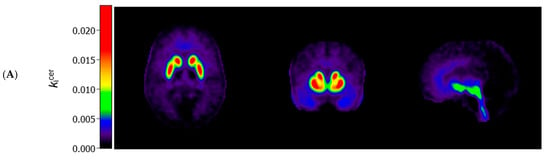
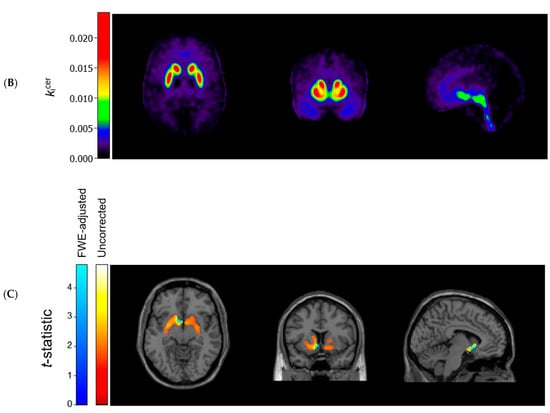
Figure 1.
Axial (left), coronal (middle), and sagittal (right) view of the mean cerebral [18F]-FDOPA uptake (kicer min−1; unadjusted and unsmoothed) in (A) adults with autism spectrum disorder (n = 44) and (B) controls (n = 22). Panel (C) shows statistically significant negative associations between scores on the autism spectrum quotient attention to detail subscale and kicer values in autistic adults, overlaid on a single subject T1-weighted MRI scan, when family-wise error (FWE) rate-adjusted or unadjusted p-values of 0.05 are used (8 mm smoothing, threshold of kicer ≥ 0.005).
4. Discussion
In this exploratory study, we found no significant differences in the striatal and extrastriatal [18F]-FDOPA uptake between unmedicated autistic adults and controls. In the ASD sample, but not in the control or combined samples, the AQ attention to detail subscale scores were significantly and negatively correlated with dopamine synthesis capacity in the whole striatum, the putamen, and particularly the nucleus accumbens, although these findings were not supported by Bayesian analyses.
The results of our exploratory analyses confirm our previous ROI analysis, in which we found no differences in [18F]-FDOPA uptake in the striatum and its functional sub-regions (i.e., associative, limbic, and sensorimotor striatum) between ASD and controls [9]. We extend these findings by showing that [18F]-FDOPA uptake does not differ in anatomical sub-regions of the striatum (i.e., putamen, nucleus accumbens, and caudate nucleus) nor in extrastriatal brain regions. In the striatum, [18F]-FDOPA is decarboxylated to fluorodopamine through amino acid decarboxylase (AADC) and stored in vesicles within presynaptic terminals [20]. Although it is well-established that striatal [18F]-FDOPA uptake represents dopamine synthesis capacity, the radiotracer is taken up and stored by all AADC-containing, monoaminergic neurons [21]. On the one hand, this can be considered a limitation of the method, since to some extent it remains unknown what [18F]-FDOPA uptake in extrastriatal regions reflects. On the other hand, since we observed no significant difference in uptake in the whole brain, this can indicate that, for instance, also serotonergic functioning in the raphe nuclei is unaltered in ASD [22].
Our findings partially differ from the study by Ernst et al. [5], who reported a decreased [18F]-FDOPA uptake in the anterior medial prefrontal cortex in autistic children (n = 14), and from the study by Nieminen von Wendt et al. [6], who found an increased [18F]-FDOPA uptake in the striatal and frontal regions in adults with Asperger syndrome (n = 8). Future studies can assess whether the differences between samples in factors, such as age, ASD diagnosis, and symptom severity, can have contributed to these partially discrepant findings.
Although we found no group differences in [18F]-FDOPA uptake, we did find significant negative associations between AQ attention to detail subscale scores and dopamine synthesis capacity in striatal ROIs among ASD adults. These findings should be interpreted with caution since multiple tests were performed, and Bayesian analyses were inconsistent with these observations. Nevertheless, it is of interest that a recent study also showed that in individuals with ASD (n = 18), but not in controls (n = 20), striatal dopamine D1 receptor binding was negatively associated with the same AQ attention to detail subscale [23]. This finding can be accounted for by either increased endogenous dopamine or by the expression of fewer D1 receptors. This latter explanation seems more plausible, since the authors note that the assessment of D1 receptor binding is unlikely to be strongly influenced by the availability of endogenous dopamine. Our findings of no increased striatal dopamine synthesis capacity in ASD, and a recent report of a decreased striatal dopamine release in response to monetary reward in adults with ASD (n = 10) compared to controls (n = 12) [24], support this interpretation, as these suggest that endogenous synaptic dopamine is not higher in ASD. Together, these findings can then be interpreted as indicating that a reduction in striatal dopamine signaling is associated with attentional processes relevant to ASD, which accords with previous theoretical and empirical work on the role of striatal dopamine in ASD [1,2,3,25].
If striatal dopamine is indeed related to attentional processing in ASD, and we emphasize that this finding requires replication in an independent cohort, then it can do so in different ways. For example, striatal dopamine can be involved in the direction of attention to salient information [25,26,27], which would fit with our finding that associations were strongest in the nucleus accumbens, a region known to play a role in these cognitive processes [28]. It is also possible that our findings reflect alterations secondary to the perturbations in other neurotransmitter systems. Future studies, combining molecular imaging methods with objective assessments of cognitive functioning would be useful in this respect. Of note, there has been a relative scarcity of molecular imaging studies in ASD [4], and future (preferably longitudinal) assessments of different aspects of the dopamine and other neurotransmitter systems would help elucidate the role of these systems in ASD. Such assessments can also increase our knowledge on the reasons why certain medications might (not) work in autistic individuals. Given their high prescription rates [29], this seems useful and necessary.
The strengths of the present study are its large sample size and the completion of additional analyses to ensure the robustness of our findings. Note that, as reported previously [9], the mean cerebellar standardized uptake values were comparable for ASD and controls and, therefore, possible differences in non-specific uptake of [18F]-FDOPA can be excluded. A first limitation of the study is the exclusion of participants who used medication or had been diagnosed with a low IQ or a psychotic disorder, as we do not know how our findings generalize to those populations. Second, autistic traits were assessed by self-report only. Third, since the study was exploratory, we did not conduct a priori sample size calculations for the present study purposes. Fourth, data were collected on two PET/CT systems. However, reconstruction parameters for the two scanners were harmonized using published guidelines [30], and scanner type was added as a covariate to the analyses. Fifth, we chose to use [18F]-FDOPA analyses methods based on previous literature (e.g., [18]) and conducted additional sensitivity analyses (e.g., with varying kicer thresholds); however, future studies should explore the added value of more sophisticated analysis methods. For example, the role of partial volume correction should be further investigated, as some studies have indicated GM/WM differences between autistic individuals and healthy controls [31,32].
In conclusion, our exploratory findings indicate that [18F]-FDOPA uptake in the brain does not significantly differ between autistic adults and controls. The striatal dopamine synthesis capacity can be negatively associated with scores on the AQ attention to detail subscale in autistic adults, but replication of this finding is necessary.
Supplementary Materials
The following are available online at www.mdpi.com/article/10.3390/diagnostics11122404/s1. Table S1: Bayes factors (BF10) for the analyses examining striatal [18F]-FDOPA uptake (kicer min−1) in autistic adults and controls, and its association with self-reported autistic traits.
Author Contributions
Conceptualization, R.S., L.-F.d.G.-O., J.-P.S., T.v.A., J.B. and F.H.P.v.V.; Methodology, R.S., L.-F.d.G.-O., J.-P.S., M.Y., A.S., T.v.A., J.B. and F.H.P.v.V.; Software, R.S., F.H.P.v.V., A.S. and M.Y.; Validation, R.S., F.H.P.v.V., A.S. and M.Y.; Formal Analysis, R.S. and F.H.P.v.V.; Investigation, R.S., L.-F.d.G.-O. and F.H.P.v.V.; Resources, L.-F.d.G.-O., J.-P.S., M.Y., A.S., T.v.A., J.B. and F.H.P.v.V.; Data Curation, R.S., L.-F.d.G.-O., J.-P.S. and F.H.P.v.V.; Writing—Original Draft Preparation, R.S.; Writing—Review and Editing, L.-F.d.G.-O., J.-P.S., M.Y., A.S., T.v.A., J.B. and F.H.P.v.V.; Visualization, R.S., F.H.P.v.V., A.S. and M.Y.; Supervision, L.-F.d.G.-O., J.-P.S., J.B. and F.H.P.v.V.; Project Administration, R.S., L.-F.d.G.-O., J.-P.S. and F.H.P.v.V.; Funding Acquisition, L.-F.d.G.-O., J.-P.S. and F.H.P.v.V. All authors have read and agreed to the published version of the manuscript.
Funding
The study was funded in part by Stichting J.M.C. Kapteinfonds.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Medical Ethical Committee of Leiden University Medical Center (reference NL54244.058.15; date of approval: 5 July 2016).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Acknowledgments
We would like to thank Jacqueline Aanholt-Bijlemeer, Ina Boot, Neanke Bouwman, Robert Bovenkerk, Johan van Brecht, Michael Bruijns, Paul de Bruin, Mark van Buchem, Petra Dibbets-Schneider, Demi Jansen, Jordi Vonk-van Oosten, and Patrick van der Zwet for facilitating the MRI and PET/CT scans. Furthermore, we would like to express our gratitude to Daniëlle Bos, Carlijn Clemens, Truda Driesen, Debora Op ‘t Eijnde, Erik Giltay, Jori Henke, and Jessie Kosterman for their assistance with conducting this study. We would also like to thank Ronald Boellaard, Patricia Cambraia Lopes, Elsmarieke van de Giessen, Sandeep Golla, Claus Svarer, and Charlotte van der Vos for their support with PET/CT data processing, and Fabian Termorshuizen for his support with the statistical analyses. Finally, we would like to thank JADOS (in particular Elles van Woerkum and Paul Stoffer), Anna Souverijn, Marcel Melchers, Els van der Ven, Villa Abel, PAS Nederland, Leviaan, aspergersyndroom.nl, autsider.net, RIAN Autismenetwerk, and the Dutch Association for Autism (Nederlandse Vereniging voor Autisme) for their help with recruiting study participants.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Baron-Cohen, S.; Wheelwright, S.; Skinner, R.; Martin, J.; Clubley, E. The autism-spectrum quotient (AQ): Evidence from asperger syndrome/high-functioning autism, males and females, scientists and mathematicians. J. Autism Dev. Disord. 2001, 31, 5–17. [Google Scholar] [CrossRef] [PubMed]
- Brown, W.D.; Taylor, M.; Roberts, A.D.; Oakes, T.R.; Schueller, M.; Holden, J.E.; Malischke, L.; DeJesus, O.T.; Nickles, R.J. FluoroDOPA PET shows the nondopaminergic as well as dopaminergic destinations of levodopa. Neurology 1999, 53, 1212–1218. [Google Scholar] [CrossRef] [PubMed]
- Cauda, F.; Geda, E.; Sacco, K.; D’Agata, F.; Duca, S.; Geminiani, G.; Keller, R. Grey matter abnormality in autism spectrum disorder: An activation likelihood estimation meta-analysis study. J. Neurol. Neurosurg. Psychiatry 2011, 82, 1304–1313. [Google Scholar] [CrossRef] [PubMed]
- Cools, R.; D’Esposito, M. Inverted-U–shaped dopamine actions on human working memory and cognitive control. Biol. Psychiatry 2011, 69, e113–e125. [Google Scholar] [CrossRef] [PubMed]
- Damasio, A.R.; Maurer, R.G. A neurological model for childhood autism. Arch. Neurol. 1978, 35, 777–786. [Google Scholar] [CrossRef] [PubMed]
- Egerton, A.; Demjaha, A.; McGuire, P.; Mehta, M.A.; Howes, O.D. The test–retest reliability of 18F-DOPA PET in assessing striatal and extrastriatal presynaptic dopaminergic function. Neuroimage 2010, 50, 524–531. [Google Scholar] [CrossRef]
- Ernst, M.; Zametkin, A.; Matochik, J.; Pascualvaca, D.; Cohen, R. Low medial prefrontal dopaminergic activity in autistic children. Lancet 1997, 350, 638. [Google Scholar] [CrossRef]
- Fuccillo, M.V. Striatal circuits as a common node for autism pathophysiology. Front. Neurosci. 2016, 10, 27. [Google Scholar] [CrossRef]
- Hammers, A.; Allom, R.; Koepp, M.J.; Free, S.L.; Myers, R.; Lemieux, L.; Mitchell, T.N.; Brooks, D.J.; Duncan, J.S. Three-dimensional maximum probability atlas of the human brain, with particular reference to the temporal lobe. Hum. Brain Mapp. 2003, 19, 224–247. [Google Scholar] [CrossRef]
- Hoekstra, R.A.; Bartels, M.; Cath, D.C.; Boomsma, D.I. Factor structure, reliability and criterion validity of the Autism-Spectrum Quotient (AQ): A study in Dutch population and patient groups. J. Autism Dev. Disord. 2008, 38, 1555–1566. [Google Scholar] [CrossRef]
- Hus, V.; Lord, C. The autism diagnostic observation schedule, module 4: Revised algorithm and standardized severity scores. J. Autism Dev. Disord. 2014, 44, 1996–2012. [Google Scholar] [CrossRef] [PubMed]
- JASP Team. JASP (Version 0.16); University of Amsterdam: Amsterdam, The Netherlands, 2021. [Google Scholar]
- Kapur, S.; Mizrahi, R.; Li, M. From dopamine to salience to psychosis—Linking biology, pharmacology and phenomenology of psychosis. Schizophr. Res. 2005, 79, 59–68. [Google Scholar] [CrossRef]
- Kubota, M.; Fujino, J.; Tei, S.; Takahata, K.; Matsuoka, K.; Tagai, K.; Sano, Y.; Yamamoto, Y.; Shimada, H.; Takado, Y. Binding of Dopamine D1 receptor and noradrenaline transporter in individuals with autism spectrum disorder: A PET study. Cereb. Cortex 2020, 30, 6458–6468. [Google Scholar] [CrossRef] [PubMed]
- Lai, M.-C.; Kassee, C.; Besney, R.; Bonato, S.; Hull, L.; Mandy, W.; Szatmari, P.; Ameis, S.H. Prevalence of co-occurring mental health diagnoses in the autism population: A systematic review and meta-analysis. Lancet Psychiatry 2019, 6, 819–829. [Google Scholar] [CrossRef]
- Lord, C.; Petkova, E.; Hus, V.; Gan, W.; Lu, F.; Martin, D.M.; Ousley, O.; Guy, L.; Bernier, R.; Gerdts, J. A multisite study of the clinical diagnosis of different autism spectrum disorders. Arch. Gen. Psychiatry 2012, 69, 306–313. [Google Scholar] [CrossRef]
- Moore, R.Y.; Whone, A.L.; McGowan, S.; Brooks, D.J. Monoamine neuron innervation of the normal human brain: An 18F-DOPA PET study. Brain Res. 2003, 982, 137–145. [Google Scholar] [CrossRef]
- Nieminen-von Wendt, T.S.; Metsähonkala, L.; Kulomäki, T.A.; Aalto, S.; Autti, T.H.; Vanhala, R.; Eskola, O.; Bergman, J.; Hietala, J.A.; von Wendt, L.O. Increased presynaptic dopamine function in Asperger syndrome. Neuroreport 2004, 15, 757–760. [Google Scholar] [CrossRef]
- Patlak, C.S.; Blasberg, R.G. Graphical evaluation of blood-to-brain transfer constants from multiple-time uptake data. Generalizations. J. Cereb. Blood Flow Metab. 1985, 5, 584–590. [Google Scholar] [CrossRef] [PubMed]
- Pavăl, D. A dopamine hypothesis of autism spectrum disorder. Dev. Neurosci. 2017, 39, 355–360. [Google Scholar] [CrossRef]
- Pavăl, D.; Micluția, I.V. The dopamine hypothesis of autism spectrum disorder revisited: Current status and future prospects. Dev. Neurosci. 2021, 43, 73–83. [Google Scholar] [CrossRef]
- Pavese, N.; Simpson, B.; Metta, V.; Ramlackhansingh, A.; Chaudhuri, K.R.; Brooks, D.J. [18F]FDOPA uptake in the raphe nuclei complex reflects serotonin transporter availability. A combined [18F]FDOPA and [11C]DASB PET study in Parkinson’s disease. Neuroimage 2012, 59, 1080–1084. [Google Scholar] [CrossRef]
- Quarantelli, M.; Berkouk, K.; Prinster, A.; Landeau, B.; Svarer, C.; Balkay, L.; Alfano, B.; Brunetti, A.; Baron, J.-C.; Salvatore, M. Integrated software for the analysis of brain PET/SPECT studies with partial-volume-effect correction. J. Nucl. Med. 2004, 45, 192–201. [Google Scholar]
- Radua, J.; Via, E.; Catani, M.; Mataix-Cols, D. Voxel-based meta-analysis of regional white-matter volume differences in autism spectrum disorder versus healthy controls. Psychol. Med. 2011, 41, 1539–1550. [Google Scholar] [CrossRef] [PubMed]
- Salgado, S.; Kaplitt, M.G. The nucleus accumbens: A comprehensive review. Stereotact. Funct. Neurosurg. 2015, 93, 75–93. [Google Scholar] [CrossRef]
- Schalbroeck, R.; van Velden, F.H.P.; de Geus-Oei, L.-F.; Yaqub, M.; van Amelsvoort, T.; Booij, J.; Selten, J.-P. Striatal dopamine synthesis capacity in autism spectrum disorder and its relation with social defeat: An [18F]-FDOPA PET/CT study. Transl. Psychiatry 2021, 11, 47. [Google Scholar] [CrossRef]
- Selten, J.-P.; Booij, J.; Buwalda, B.; Meyer-Lindenberg, A. Biological mechanisms whereby social exclusion may contribute to the etiology of psychosis: A narrative review. Schizophr. Bull. 2017, 43, 287–292. [Google Scholar] [CrossRef][Green Version]
- Svarer, C.; Madsen, K.; Hasselbalch, S.G.; Pinborg, L.H.; Haugbøl, S.; Frøkjær, V.G.; Holm, S.; Paulson, O.B.; Knudsen, G.M. MR-based automatic delineation of volumes of interest in human brain PET images using probability maps. Neuroimage 2005, 24, 969–979. [Google Scholar] [CrossRef] [PubMed]
- Verwer, E.E.; Golla, S.; Kaalep, A.; Lubberink, M.; van Velden, F.; Bettinardi, V.; Yaqub, M.; Sera, T.; Rijnsdorp, S.; Lammertsma, A.A.; et al. Harmonisation of PET/CT contrast recovery performance for brain studies. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 2856–2870. [Google Scholar] [CrossRef]
- Wong, A.Y.; Hsia, Y.; Chan, E.W.; Murphy, D.G.; Simonoff, E.; Buitelaar, J.K.; Wong, I.C. The variation of psychopharmacological prescription rates for people with autism spectrum disorder (ASD) in 30 countries. Autism Res. 2014, 7, 543–554. [Google Scholar] [CrossRef] [PubMed]
- Zürcher, N.R.; Bhanot, A.; McDougle, C.J.; Hooker, J.M. A systematic review of molecular imaging (PET and SPECT) in autism spectrum disorder: Current state and future research opportunities. Neurosci. Biobehav. Rev. 2015, 52, 56–73. [Google Scholar] [CrossRef]
- Zürcher, N.R.; Walsh, E.C.; Phillips, R.D.; Cernasov, P.M.; Tseng, C.-E.J.; Dharanikota, A.; Smith, E.; Li, Z.; Kinard, J.L.; Bizzell, J.C. A simultaneous [11C]raclopride positron emission tomography and functional magnetic resonance imaging investigation of striatal dopamine binding in autism. Transl. Psychiatry 2021, 11, 33. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).