Involvement of Ceramides in Non-Alcoholic Fatty Liver Disease (NAFLD) Atherosclerosis (ATS) Development: Mechanisms and Therapeutic Targets
Abstract
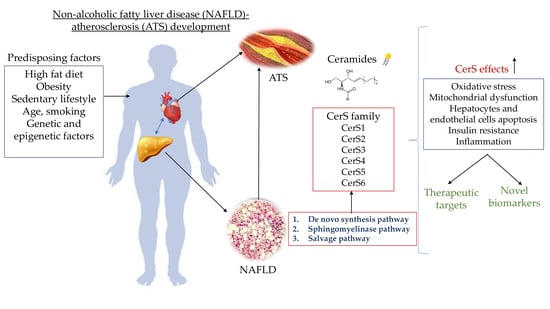
:1. Introduction
2. Ceramides
Ceramides in NAFLD-ATS Development
3. Ceramides as Potential Biomarkers in Atherosclerosis Related Diseases
4. Ceramides as Potential Therapeutic Targets
4.1. Myriocin
4.2. CerS Inhibitors
4.3. Des1 Inhibitors
4.4. MTP Inhibitors
4.5. ASMase Inhibitors
4.6. Lip-C6
4.7. Glucagon-like Peptide-1 (GLP-1)
4.8. Intestinal FXR/SMPD3 Axis
4.9. Other Therapeutic Approaches
| Main Focus | Species | Outcomes | Year | Ref. |
|---|---|---|---|---|
| Fumonisine B1 | Mice | 60% reduction of hepatic SM levels (p < 0.05), increase expression of hepatic SPT (p < 0.01); SPHK 1 maximal at the lowest dose of 0.75 mg/kg (p < 0.05), expression of SPHK2 not affected; | 2006 | [92] |
| Elovl6 | Mice | Reduced: Ceramide(d18:1/18:0)(0.63, p < 0.001); ceramide (d18:2/18:0) (1.68, p < 0.05), oleate (C18:1n-9) and stearate (C18:0); | 2020 | [150] |
| CerS2 | Mice | Reduced lipid accumulation, sphingomyelin levels ~50%, uptake in the liver, reduction in very long chain acyl ceramides, enzymatic activity-decreased; | 2015 | [151] |
| CerS6 | Mice | Reduce C16:0 ceramides, serum insulin concentrations, protects from macrophage infiltration, activation of pro-inflammatory gene expression; improve glucose tolerance and insulin sensitivity; reduced adiposity and increased energy expenditure, (p < 0.05); | 2014 | [152] |
| Diet | Human | Ceramides C22:0, C24:1; C26:0 reduced-29%, (p < 0.05), C24:0 50%, (p < 0.01); at week 8 increase of C16:0 (p < 0.05); | 2017 | [76] |
| P053 | Mice | 5 mg/kg/day reduced C18 ceramide by 31%, (p < 0.01); Reduces whole-body fat mass and the weight of white adipose depots; | 2018 | [17] |
| GW4869 | Mice | Decrease of: the atherosclerotic area, accumulation of macrophages by 68%; atherosclerotic lesions by 69% (p < 0.001), in plasma Cer24:1, Cer22:0, and Cer24:0, (p < 0.05), lipid accumulation by 68% (p < 0.01); | 2018 | [121] |
| CerS1 | Mice | The sphingolipid content in heart, liver, and white adipose tissue—not affect, imprivement of liver glucose metabolism, 95% reduction in C18:0 ceramide; | 2019 | [153] |
| CerS5 | Mice | Improves glucose tolerance, insulin sensitivity, reduces white adipose inflammation, In skeletal muscle without obvious decrease; | 2019 | [153] |
| Bortezomib | Mice | Increase hepatic CerS2 expression, protects from development of NAFLD, decreases weight gain, TG levels lower (p < 0.01); | 2019 | [154] |
| Exendin-4 | Mice | Decrease lobular inflammation (p = 0.18), fibrosis stages (p = 0.24) | 2019 | [155] |
| DEGS1 gene | Mice | Decreased: Cer16:0–0.09, Cer18:0–0.1 (p < 0.001), whole-body insulin sensitivity-restored, selective insulin resistance-reversed in the liver (p < 0.001); | 2019 | [115] |
| Myriocin | Rats | Reduced: serum ceramide content reduced, (p < 0.05), hepatic triglyceride, ALT, AST, hepatic inflammation, amount of inflammatory cell; Bcl-2 expression restored, (p < 0.05); | 2019 | [87] |
| Fenretinide | Mice | Lowered: plaque area 50.8% (p < 0.05), plasma lipid levels by 20.1%, (p < 0.05), and plasma ceramides; | 2020 | [156] |
| Diet | Mice | Flinax reduced lipoperoxidation markers, hepatic fat accumulation restores complex I, III, V (ATP-synthase), lower peroxides levels, (p < 0.05), no difference in complex IV, and higher production of CPT1A and CPT2; | 2021 | [148] |
| Alpha-mangostin | Mice | Inhibits: ceramide content, and, Inhibites aSMase activity, | 2021 | [146] |
| Farnesoid X receptor | Mice | Lower: ceramide content, hepatic cholesterol levels, mRNA levels of Smpd3 elevates hepatic Cyp7a1 mRNA levels Repressed lesion areas in aortas and smaller atherosclerotic lesions; | 2021 | [120] |
| Liraglutide | Mice | Gene expression Sptlc2, cerS4, and cerS6 decreased; C16 and C24 accumulation was limited (p < 0.05); Unchanged: saturated fatty acid, phospholipids with long chains-reduced, phospholipids with very long chains | 2021 | [132] |
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Eslam, M.; Sanyal, A.J.; George, J. International Consensus Panel. MAFLD: A consensus-driven proposed nomenclature for metabolic associated fatty liver disease. Gastroenterology 2020, 158, 1999–2014.e1. [Google Scholar] [CrossRef]
- European Association for the Study of the Liver (EASL); European Association for the Study of Diabetes (EASD); European Association for the Study of Obesity (EASO). EASL-EASD-EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. J. Hepatol. 2016, 64, 1388–1402. [Google Scholar] [CrossRef]
- Yki-Järvinen, H. Ceramides: A cause of insulin resistance in nonalcoholic fatty liver disease in both murine models and humans. Hepatology 2020, 71, 1499–1501. [Google Scholar] [CrossRef] [PubMed]
- Lonardo, A.; Nascimbeni, F.; Mantovani, A.; Targher, G. Hypertension, diabetes, atherosclerosis and NASH: Cause or consequence? J. Hepatol. 2018, 68, 335–352. [Google Scholar] [CrossRef] [PubMed]
- Choi, R.H.; Tatum, S.M.; Symons, J.D.; Summers, S.A.; Holland, W.L. Ceramides and other sphingolipids as drivers of cardiovascular disease [published online ahead of print, 26 March 2021]. Nat. Rev. Cardiol. 2021, 18, 701–711. [Google Scholar] [CrossRef]
- Hajduch, E.; Lachkar, F.; Ferré, P.; Foufelle, F. Roles of Ceramides in non-alcoholic fatty liver disease. J. Clin. Med. 2021, 10, 792. [Google Scholar] [CrossRef]
- Kim, J.W.; You, Y.H.; Jung, S.; Suh-Kim, H.; Lee, I.K.; Cho, J.H.; Yoon, K.H. Regulation of plasma ceramide levels with fatty acid oversupply: Evidence that the liver detects and secretes de novo synthesised ceramide. Diabetologia 2012, 56, 2741–2746. [Google Scholar] [CrossRef] [Green Version]
- Kasumov, T.; Li, L.; Li, M.; Gulshan, K.; Kirwan, J.P.; Liu, X.; Previs, S.; Willard, B.; Smith, J.D.; McCullough, A. Ceramide as a mediator of non-alcoholic fatty liver disease and associated atherosclerosis. PLoS ONE 2015, 10, e0126910. [Google Scholar] [CrossRef]
- Raichur, S.; Brunner, B.; Bielohuby, M.; Hansen, G.; Pfenninger, A.; Wang, B.; Bruning, J.C.; Larsen, P.J.; Tennagels, N. The role of C16:0 ceramide in the development of obesity and type 2 diabetes: CerS6 inhibition as a novel therapeutic approach. Mol. Metab. 2019, 21, 36–50. [Google Scholar] [CrossRef]
- Gault, C.R.; Obeid, L.M.; Hannun, Y.A. An overview of sphingolipid metabolism: From synthesis to breakdown. Adv. Exp. Med. Biol. 2010, 688, 1–23. [Google Scholar] [CrossRef] [Green Version]
- Samuel, V.T.; Shulman, G.I. Nonalcoholic fatty liver disease, insulin resistance, and ceramides. N. Engl. J. Med. 2019, 381, 1866–1869. [Google Scholar] [CrossRef]
- McGurk, K.A.; Keavney, B.D.; Nicolaou, A. Circulating ceramides as biomarkers of cardiovascular disease: Evidence from phenotypic and genomic studies. Atherosclerosis 2021, 327, 18–30. [Google Scholar] [CrossRef] [PubMed]
- Younossi, Z.M. Non-alcoholic fatty liver disease—A global public health perspective. J. Hepatol. 2019, 70, 531–544. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Green, C.D.; Maceyka, M.; Cowart, L.A.; Spiegel, S. Sphingolipids in metabolic disease: The good, the bad, and the unknown. Cell Metab. 2021, 33, 1293–1306. [Google Scholar] [CrossRef]
- Carlier, A.; Phan, F.; Szpigel, A.; Hajduch, E.; Salem, J.-E.; Gautheron, J.; Le Goff, W.; Guérin, M.; Lachkar, F.; Ratziu, V.; et al. Dihydroceramides in triglyceride-enriched VLDL are associated with nonalcoholic fatty liver disease severity in type 2 diabetes. Cell Rep. Med. 2020, 1, 100154. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wen, L.; Zhu, F.; Wang, Y.; Xie, Q.; Chen, Z.; Li, Y. Overexpression of ceramide synthase 1 increases C18-ceramide and leads to lethal autophagy in human glioma. Oncotarget 2017, 8, 104022–104036. [Google Scholar] [CrossRef]
- Turner, N.; Lim, X.Y.; Toop, H.D.; Osborne, B.; Brandon, A.E.; Taylor, E.N.; Fiveash, C.E.; Govindaraju, H.; Teo, J.D.; McEwen, H.P.; et al. A selective inhibitor of ceramide synthase 1 reveals a novel role in fat metabolism. Nat. Commun. 2018, 9, 3165. [Google Scholar] [CrossRef]
- Pewzner-Jung, Y.; Park, H.; Laviad, E.L.; Silva, L.; Lahiri, S.; Stiban, J.; Erez-Roman, R.; Brügger, B.; Sachsenheimer, T.; Wieland, F.; et al. A critical role for ceramide synthase 2 in liver homeostasis: I. alterations in lipid metabolic pathways. J. Biol. Chem. 2010, 285, 10902–10910. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nicholson, R.J.; Poss, A.M.; Maschek, J.A.; Cox, J.E.; Hopkins, P.N.; Hunt, S.C.; Playdon, M.C.; Holland, W.L.; Summers, S.A. Characterizing a common CERS2 polymorphism in a mouse model of metabolic disease and in subjects from the Utah CAD study. J. Clin. Endocrinol. Metab. 2021, 106, e3098–e3109. [Google Scholar] [CrossRef]
- Mizutani, Y.; Kihara, A.; Igarashi, Y. LASS3 (longevity assurance homologue 3) is a mainly testis-specific (dihydro) ceramide synthase with relatively broad substrate specificity. Biochem. J. 2006, 398, 531–538. [Google Scholar] [CrossRef] [Green Version]
- Shin, K.O.; Kim, S.; Park, B.D.; Uchida, Y.; Park, K. N-palmitoyl serinol stimulates ceramide production through a CB1-Dependent mechanism in in vitro model of skin inflammation. Int. J. Mol. Sci. 2021, 22, 8302. [Google Scholar] [CrossRef]
- Chen, J.; Li, X.; Ma, D.; Liu, T.; Tian, P.; Wu, C. Ceramide synthase-4 orchestrates the cell proliferation and tumor growth of liver cancer in vitro and in vivo through the nuclear factor-κB signaling pathway corrigendum. Oncol. Lett. 2017, 14, 1477–1483. [Google Scholar] [CrossRef] [Green Version]
- Peters, F.; Tellkamp, F.; Brodesser, S.; Wachsmuth, E.; Tosetti, B.; Karow, U.; Bloch, W.; Utermöhlen, O.; Krönke, M.; Niessen, C.M. Murine epidermal ceramide synthase 4 is a key regulator of skin barrier homeostasis. J. Investig. Dermatol. 2020, 140, 1927–1937.e5. [Google Scholar] [CrossRef]
- Gosejacob, D.; Jäger, P.S.; Vom Dorp, K.; Frejno, M.; Carstensen, A.C.; Köhnke, M.; Degen, J.; Dörmann, P.; Hoch, M. Ceramide synthase 5 is essential to maintain C16:0-ceramide pools and contributes to the development of diet-induced obesity. J. Biol. Chem. 2016, 291, 6989–7003. [Google Scholar] [CrossRef] [Green Version]
- Tosetti, B.; Brodesser, S.; Brunn, A.; Deckert, M.; Blüher, M.; Doehner, W.; Anker, S.D.; Wenzel, D.; Fleischmann, B.; Pongratz, C.; et al. A tissue-specific screen of ceramide expression in aged mice identifies ceramide synthase-1 and ceramide synthase-5 as potential regulators of fiber size and strength in skeletal muscle. Aging Cell 2020, 19, e13049. [Google Scholar] [CrossRef]
- Verlekar, D.; Wei, S.J.; Cho, H. Ceramide synthase-6 confers resistance to chemotherapy by binding to CD95/Fas in T-cell acute lymphoblastic leukemia. Cell Death Dis. 2018, 9, 925. [Google Scholar] [CrossRef]
- Juchnicka, I.; Kuźmicki, M.; Szamatowicz, J. Ceramides and sphingosino-1 phosphate in obesity. Front. Endocrinol. 2021, 12, 635995. [Google Scholar] [CrossRef]
- Tidhar, R.; Futerman, A.H. The complexity of sphingolipid biosynthesis in the endoplasmic reticulum. Biochim. Biophys. Acta 2013, 1833, 2511–2518. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Insausti-Urkia, N.; Solsona-Vilarrasa, E.; Garcia-Ruiz, C.; Fernandez-Checa, J.C. Sphingomyelinases and liver diseases. Biomolecules 2020, 10, 1497. [Google Scholar] [CrossRef] [PubMed]
- Chaurasia, B.; Summers, S.A. Ceramides in metabolism: Key lipotoxic players. Annu. Rev. Physiol. 2021, 83, 303–330. [Google Scholar] [CrossRef] [PubMed]
- Kitatani, K.; Idkowiak-Baldys, J.; Hannun, Y.A. The sphingolipid salvage pathway in ceramide metabolism and signaling. Cell. Signal. 2008, 20, 1010–1018. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sindhu, S.; Leung, Y.H.; Arefanian, H.; Madiraju, S.; Al-Mulla, F.; Ahmad, R.; Prentki, M. Neutral sphingomyelinase-2 and cardiometabolic diseases. Obes. Rev. 2021, 22, e13248. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Larrauri, A.; Presa, N.; Dominguez-Herrera, A.; Ouro, A.; Trueba, M.; Gomez-Muñoz, A. Role of bioactive sphingolipids in physiology and pathology. Essays Biochem. 2020, 64, 579–589. [Google Scholar] [CrossRef]
- Wang, H.H.; Garruti, G.; Liu, M.; Portincasa, P.; Wang, D.Q. Cholesterol and lipoprotein metabolism and atherosclerosis: Recent advances in reverse cholesterol transport. Ann. Hepatol. 2017, 16 (Suppl. 1), S27–S42. [Google Scholar] [CrossRef] [PubMed]
- Paul, A.; Lydic, T.A.; Hogan, R.; Goo, Y.H. Cholesterol acceptors regulate the lipidome of macrophage foam cells. Int. J. Mol. Sci. 2019, 20, 3784. [Google Scholar] [CrossRef] [Green Version]
- Kartsoli, S.; Kostara, C.E.; Tsimihodimos, V.; Bairaktari, E.T.; Christodoulou, D.K. Lipidomics in non-alcoholic fatty liver disease. World J. Hepatol. 2020, 12, 436–450. [Google Scholar] [CrossRef]
- Régnier, M.; Polizzi, A.; Guillou, H.; Loiseau, N. Sphingolipid metabolism in non-alcoholic fatty liver diseases. Biochimie 2019, 159, 9–22. [Google Scholar] [CrossRef]
- Ying, L.H.; Tippetts, T.S.; Chaurasia, B. Ceramide dependent lipotoxicity in metabolic diseases. Nutr. Healthy Aging 2019, 5, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Pan, W.; Dong, H.; Sun, R.; Zhao, L.; Sun, M.; Li, L.; Yu, X.; Liu, J.; Wu, J.; Yang, F.; et al. Plasma ceramides in relation to coronary plaque characterization determined by optical coherence tomography. J. Cardiovasc. Transl. Res. 2021, 14, 140–149. [Google Scholar] [CrossRef] [PubMed]
- Dallio, M.; Sangineto, M.; Romeo, M.; Villani, R.; Romano, A.D.; Loguercio, C.; Serviddio, G.; Federico, A. Immunity as cornerstone of non-alcoholic fatty liver disease: The contribution of oxidative stress in the disease progression. Int. J. Mol. Sci. 2021, 22, 436. [Google Scholar] [CrossRef]
- Moerman, A.M.; Visscher, M.; Slijkhuis, N.; Van Gaalen, K.; Heijs, B.; Klein, T.; Burgers, P.C.; De Rijke, Y.B.; Van Beusekom, H.; Luider, T.M.; et al. Lipid signature of advanced human carotid atherosclerosis assessed by mass spectrometry imaging. J. Lipid Res. 2021, 62, 100020. [Google Scholar] [CrossRef]
- Pei, K.; Gui, T.; Kan, D.; Feng, H.; Jin, Y.; Yang, Y.; Zhang, Q.; Du, Z.; Gai, Z.; Wu, J.; et al. An overview of lipid metabolism and nonalcoholic fatty liver disease. Biomed. Res. Int. 2020, 2020, 4020249. [Google Scholar] [CrossRef] [PubMed]
- Smith, G.I.; Shankaran, M.; Yoshino, M.; Schweitzer, G.G.; Chondronikola, M.; Beals, J.W. Insulin resistance drives hepatic de novo lipogenesis in nonalcoholic fatty liver disease. J. Clin. Investig. 2020, 13, 4165. [Google Scholar] [CrossRef] [PubMed]
- Quinville, B.M.; Deschenes, N.M.; Ryckman, A.E.; Walia, J.S. A comprehensive review: Sphingolipid metabolism and implications of disruption in sphingolipid homeostasis. Int. J. Mol. Sci. 2021, 22, 5793. [Google Scholar] [CrossRef]
- Wali, J.A.; Jarzebska, N.; Raubenheimer, D.; Simpson, S.J.; Rodionov, R.N.; O’Sullivan, J.F. Cardio-metabolic effects of high-fat diets and their underlying mechanisms-a narrative review. Nutrients 2020, 12, 1505. [Google Scholar] [CrossRef]
- Rein-Fischboeck, L.; Haberl, E.M.; Pohl, R.; Feder, S.; Liebisch, G.; Krautbauer, S.; Buechler, C. Variations in hepatic lipid species of age-matched male mice fed a methionine-choline-deficient diet and housed in different animal facilities. Lipids Health Dis. 2019, 18, 172. [Google Scholar] [CrossRef] [Green Version]
- Chatterjee, S.; Balram, A.; Li, W. Convergence: Lactosylceramide-centric signaling pathways induce inflammation, oxidative stress, and other phenotypic outcomes. Int. J. Mol. Sci. 2021, 22, 1816. [Google Scholar] [CrossRef]
- Marra, F.; Svegliati-Baroni, G. Lipotoxicity and the gut-liver axis in NASH pathogenesis. J. Hepatol. 2017, 68, 280–295. [Google Scholar] [CrossRef]
- Simões, I.; Amorim, R.; Teixeira, J.; Karkucinska-Wieckowska, A.; Carvalho, A.; Pereira, S.P.; Simões, R.F.; Szymanska, S.; Dąbrowski, M.; Janikiewicz, J.; et al. The alterations of mitochondrial function during NAFLD progression-an independent effect of mitochondrial ros production. Int. J. Mol. Sci. 2021, 22, 6848. [Google Scholar] [CrossRef]
- Quehenberger, O.; Armando, A.M.; Brown, A.H.; Milne, S.B.; Myers, D.S.; Merrill, A.H.; Bandyopadhyay, S.; Jones, K.N.; Kelly, S.; Shaner, R.L.; et al. Lipidomics reveals a remarkable diversity of lipids in human plasma. J. Lipid Res. 2010, 51, 3299–3305. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bellanti, F.; Villani, R.; Tamborra, R.; Blonda, M.; Iannelli, G.; di Bello, G.; Facciorusso, A.; Poli, G.; Iuliano, L.; Avolio, C.; et al. Synergistic interaction of fatty acids and oxysterols impairs mitochondrial function and limits liver adaptation during nafld progression. Redox Biol. 2018, 15, 86–96. [Google Scholar] [CrossRef] [PubMed]
- Bays, H.E.; Taub, P.R.; Epstein, E.; Michos, E.D.; Ferraro, R.A.; Bailey, A.L.; Kelli, H.M.; Ferdinand, K.C.; Echols, M.R.; Weintraub, H.; et al. Ten things to know about ten cardiovascular disease risk factors. Am. J. Prev. Cardiol. 2021, 5, 100149. [Google Scholar] [CrossRef]
- Petersen, M.C.; Shulman, G.I. Roles of diacylglycerols and ceramides in hepatic insulin resistance. Trends Pharmacol. Sci. 2017, 38, 649–665. [Google Scholar] [CrossRef] [PubMed]
- Gehrke, N.; Schattenberg, J.M. Metabolic inflammation—A role for hepatic inflammatory pathways as drivers of comorbidities in nonalcoholic fatty liver disease? Gastroenterology 2020, 158, 1929–1947.e6. [Google Scholar] [CrossRef]
- Wolsk, E.; Claggett, B.; Pfeffer, M.A.; Diaz, R.; Dickstein, K.; Gerstein, H.C.; Lawson, F.C.; Lewis, E.F.; Maggioni, A.P.; McMurray, J.J.V.; et al. Role of B-type natriuretic peptide and N-terminal prohormone BNP as predictors of cardiovascular morbidity and mortality in patients with a recent coronary event and type 2 diabetes mellitus. J. Am. Heart Assoc. 2017, 6, e004743. [Google Scholar] [CrossRef]
- Sipos, B.; Jirak, P.; Paar, V.; Rezar, R.; Mirna, M.; Kopp, K.; Hoppe, U.C.; Berezin, A.E.; Lichtenauer, M. Promising novel biomarkers in cardiovascular diseases. Appl. Sci. 2021, 11, 3654. [Google Scholar] [CrossRef]
- Cardellini, M.; Rizza, S.; Casagrande, V.; Cardolini, I.; Ballanti, M.; Davato, F.; Porzio, O.; Canale, M.P.; Legramante, J.M.; Mavilio, M.; et al. Soluble ST2 is a biomarker for cardiovascular mortality related to abnormal glucose metabolism in high-risk subjects. Acta Diabetol. 2019, 56, 273–280. [Google Scholar] [CrossRef] [PubMed]
- Rezar, R.; Jirak, P.; Gschwandtner, M.; Derler, R.; Felder, T.K.; Haslinger, M.; Kopp, K.; Seelmaier, C.; Granitz, C.; Hoppe, U.C.; et al. Heart-type fatty acid-binding protein (H-FABP) and its role as a biomarker in heart failure: What do we know so far? J. Clin. Med. 2020, 9, 164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, J.; Cui, Y.; Huang, A.; Li, Q.; Jia, W.; Liu, K.; Qi, X. Additional diagnostic value of growth differentiation factor-15 (GDF-15) to N-terminal B-type natriuretic peptide (NT-proBNP) in patients with different stages of heart failure. Med. Sci. Monit. 2018, 24, 4992–4999. [Google Scholar] [CrossRef]
- Heraclides, A.; Jensen, T.M.; Rasmussen, S.S.; Eugen-Olsen, J.; Haugaard, S.B.; Borch-Johnsen, K.; Sandbæk, A.; Lauritzen, T.; Witte, D.R. The pro-inflammatory biomarker soluble urokinase plasminogen activator receptor (suPAR) is associated with incident type 2 diabetes among overweight but not obese individuals with impaired glucose regulation: Effect modification by smoking and body weight status. Diabetologia 2013, 56, 1542–1546. [Google Scholar] [CrossRef] [Green Version]
- Zeier, M.; Reiser, J. suPAR and chronic kidney disease—A podocyte story. Pflugers Arch. Eur. J. Physiol. 2017, 469, 1017–1020. [Google Scholar] [CrossRef] [PubMed]
- Holland, W.L.; Summers, S.A. Strong heart, low ceramides. Diabetes 2018, 67, 1457–1460. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kurz, J.; Parnham, M.J.; Geisslinger, G.; Schiffmann, S. Ceramides as novel disease biomarkers. Trends Mol. Med. 2019, 25, 20–32. [Google Scholar] [CrossRef]
- Hilvo, M.; Meikle, P.J.; Pedersen, E.R.; Tell, G.S.; Dhar, I.; Brenner, H.; Schöttker, B.; Lääperi, M.; Kauhanen, D.; Koistinen, K.M.; et al. Development and validation of a ceramide- And phospholipid-based cardiovascular risk estimation score for coronary artery disease patients. Eur. Heart J. 2020, 41, 371–380. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sokolowska, E.; Blachnio-Zabielska, A. The role of ceramides in insulin resistance. Front. Endocrinol. 2019, 10, 577. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tu, C.; Xie, L.; Wang, Z.; Zhang, L.; Wu, H.; Ni, W.; Li, C.; Li, L.; Zeng, Y. Association between ceramides and coronary artery stenosis in patients with coronary artery disease. Lipids Health Dis. 2020, 19, 151. [Google Scholar] [CrossRef]
- Li, Y.; Talbot, C.L.; Chaurasia, B. Ceramides in adipose tissue. Front. Endocrinol. 2020, 11, 407. [Google Scholar] [CrossRef] [PubMed]
- McGrath, E.R.; Himali, J.J.; Xanthakis, V.; Duncan, M.S.; Schaffer, J.E.; Ory, D.S.; Peterson, L.R.; DeCarli, C.; Pase, M.P.; Satizabal, C.L.; et al. Circulating ceramide ratios and risk of vascular brain aging and dementia. Ann. Clin. Transl. Neurol. 2020, 7, 160–168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dinoff, A.; Herrmann, N.; Lanctôt, K.L. Ceramides and depression: A systematic review. J. Affect. Disord. 2017, 15, 35–43. [Google Scholar] [CrossRef]
- Field, B.C.; Gordillo, R.; Scherer, P.E. The role of ceramides in diabetes and cardiovascular disease regulation of ceramides by adipokines. Front. Endocrinol. 2020, 11, 569250. [Google Scholar] [CrossRef]
- Öörni, K.; Jauhiainen, M.; Kovanen, P.T. Why and how increased plasma ceramides predict future cardiovascular events? Atherosclerosis 2020, 314, 71–73. [Google Scholar] [CrossRef]
- Meeusen, J.W.; Donato, L.J.; Bryant, S.C.; Baudhuin, L.M.; Berger, P.B.; Jaffe, A.S. Plasma ceramides. Arterioscler. Thromb. Vasc. Biol. 2018, 38, 1933–1939. [Google Scholar] [CrossRef] [Green Version]
- Bodini, A.; Michelucci, E.; Di Giorgi, N.; Caselli, C.; Signore, G.; Neglia, D.; Smit, J.M.; Scholte, A.; Mincarone, P.; Leo, C.G.; et al. Predictive added value of selected plasma lipids to a re-estimated minimal risk tool. Front. Cardiovasc. Med. 2021, 8, 682785. [Google Scholar] [CrossRef] [PubMed]
- Laaksonen, R.; Ekroos, K.; Sysi-Aho, M.; Hilvo, M.; Vihervaara, T.; Kauhanen, D.; Suoniemi, M.; Hurme, R.; März, W.; Scharnagl, H.; et al. Plasma ceramides predict cardiovascular death in patients with stable coronary artery disease and acute coronary syndromes beyond LDL-cholesterol. Eur. Heart J. 2016, 37, 1967–1976. [Google Scholar] [CrossRef]
- Havulinna, A.S.; Sysi-Aho, M.; Hilvo, M.; Kauhanen, D.; Hurme, R.; Ekroos, K.; Salomaa, V.; Laaksonen, R. Circulating ceramides predict cardiovascular outcomes in the population-based FINRISK 2002 cohort. Arterioscler. Thromb. Vasc. Biol. 2016, 36, 2424–2430. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, D.D.; Toledo, E.; Hruby, A.; Rosner, B.A.; Willett, W.C.; Sun, Q.; Razquin, C.; Zheng, Y.; Ruiz-Canela, M.; Guasch-Ferré, M.; et al. Plasma ceramides, mediterranean diet, and incident cardiovascular disease in the PREDIMED trial (Prevención con Dieta Mediterránea). Circulation 2017, 135, 2028–2040. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vasile, V.C.; Meeusen, J.W.; Medina Inojosa, J.R.; Donato, L.J.; Scott, C.G.; Hyun, M.S.; Vinciguerra, M.; Rodeheffer, R.R.; Lopez-Jimenez, F.; Jaffe, A.S. Ceramide scores predict cardiovascular risk in the community. Arterioscler. Thromb. Vasc. Biol. 2021, 41, 1558–1569. [Google Scholar] [CrossRef]
- Poss, A.M.; Maschek, J.A.; Cox, J.E.; Hauner, B.J.; Hopkins, P.N.; Hunt, S.C.; Holland, W.L.; Summers, S.A.; Playdon, M.C. Machine learning reveals serum sphingolipids as cholesterol-independent biomarkers of coronary artery disease. J. Clin. Investig. 2020, 130, 1363–1376. [Google Scholar] [CrossRef] [Green Version]
- Echouffo-Tcheugui, J.B.; Jain, M.; Cheng, S. Breaking through the surface: More to learn about lipids and cardiovascular disease. J. Clin. Investig. 2020, 130, 1084–1086. [Google Scholar] [CrossRef] [PubMed]
- Hilvo, M.; Jylhä, A.; Lääperi, M.; Jousilahti, P.; Laaksonen, R. Absolute and relative risk prediction in cardiovascular primary prevention with a modified SCORE chart incorporating ceramide-phospholipid risk score and diabetes mellitus. Eur. Heart J. Open 2021, oeab010. [Google Scholar] [CrossRef]
- Vasile, V.C.; Jaffe, A.S. An enhanced ceramide-based approach for primary prevention of atherosclerotic events. Eur. Heart J. Open 2021, oeab016. [Google Scholar] [CrossRef]
- Wang, D.; Xu, P.; Mesaros, C. Analytical considerations for reducing the matrix effect for the sphingolipidome quantification in whole blood. Bioanalysis 2021, 13, 1037–1049. [Google Scholar] [CrossRef] [PubMed]
- Luukkonen, P.K.; Sädevirta, S.; Zhou, Y.; Kayser, B.; Ali, A.; Ahonen, L.; Lallukka, S.; Pelloux, V.; Gaggini, M.; Jian, C.; et al. Saturated fat is more metabolically harmful for the human liver than unsaturated fat or simple sugars. Diabetes Care 2018, 41, 1732–1739. [Google Scholar] [CrossRef] [Green Version]
- Rosqvist, F.; Kullberg, J.; Ståhlman, M.; Cedernaes, J.; Heurling, K.; Johansson, H.E.; Iggman, D.; Wilking, H.; Larsson, A.; Eriksson, O.; et al. Overeating Saturated Fat Promotes Fatty Liver and Ceramides Compared With Polyunsaturated Fat: A Randomized Trial. J. Clin. Endocrinol. Metab. 2019, 104, 6207–6219. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raichur, S. Ceramide synthases are attractive drug targets for treating metabolic diseases. Front. Endocrinol. 2020, 11, 483. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Peng, Q.; Li, S.; Hao, H.; Deng, J.; Meng, L.; Shen, Z.; Yu, W.; Nan, D.; Bai, Y.; et al. Myriocin and D-PDMP ameliorate atherosclerosis in ApoE−/− mice via reducing lipid uptake and vascular inflammation. Clin. Sci. 2020, 134, 439–458. [Google Scholar] [CrossRef] [Green Version]
- Ruuth, M.; Nguyen, S.D.; Vihervaara, T.; Hilvo, M.; Laajala, T.D.; Kondadi, P.K.; Gisterå, A.; Lähteenmäki, H.; Kittilä, T.; Huusko, J.; et al. Susceptibility of low-density lipoprotein particles to aggregate depends on particle lipidome, is modifiable, and associates with future cardiovascular deaths. Eur. Heart J. 2018, 39, 2562–2573. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, R.X.; Pan, Q.; Liu, X.L.; Zhou, D.; Xin, F.Z.; Zhao, Z.H.; Zhang, R.N.; Zeng, J.; Qiao, L.; Hu, C.X.; et al. Therapeutic effect and autophagy regulation of myriocin in nonalcoholic steatohepatitis. Lipids Health Dis. 2019, 18, 179. [Google Scholar] [CrossRef] [Green Version]
- Jiang, M.; Li, C.; Liu, Q.; Wang, A.; Lei, M. Inhibiting ceramide synthesis attenuates hepatic steatosis and fibrosis in rats with non-alcoholic fatty liver disease. Front. Endocrinol. 2019, 10, 665. [Google Scholar] [CrossRef]
- Hilvo, M.; Simolin, H.; Metso, J.; Ruuth, M.; Öörni, K.; Jauhiainen, M.; Laaksonen, R.; Baruch, A. PCSK9 inhibition alters the lipidome of plasma and lipoprotein fractions. Atherosclerosis 2018, 269, 159–165. [Google Scholar] [CrossRef] [Green Version]
- Laviad, E.L.; Albee, L.; Pankova-Kholmyansky, I.; Epstein, S.; Park, H.; Merrill, A.H.; Futerman, A.H., Jr. Characterization of ceramide synthase 2: Tissue distribution, substrate specificity, and inhibition by sphingosine 1-phosphate. J. Biol. Chem. 2008, 283, 5677–5684. [Google Scholar] [CrossRef] [Green Version]
- He, Q.; Suzuki, H.; Sharma, N.; Sharma, R.P. Ceramide synthase inhibition by fumonisin B1 treatment activates sphingolipid-metabolizing systems in mouse liver. Toxicol. Sci. 2006, 94, 388–397. [Google Scholar] [CrossRef] [Green Version]
- Gelineau-van Waes, J.; Rainey, M.A.; Maddox, J.R.; Voss, K.A.; Sachs, A.J.; Gardner, N.M.; Wilberding, J.D.; Riley, R.T. Increased sphingoid base-1-phosphates and failure of neural tube closure after exposure to fumonisin or FTY720. Birth Defects Res. A Clin. Mol. Teratol. 2012, 94, 790–803. [Google Scholar] [CrossRef]
- Dellafiora, L.; Galaverna, G.; Dall’Asta, C. Mechanisms of fumonisin B1 toxicity: A computational perspective beyond the ceramide synthases inhibition. Chem. Res. Toxicol. 2018, 31, 1203–1212. [Google Scholar] [CrossRef] [PubMed]
- Riley, R.T.; Merrill, A.H., Jr. Ceramide synthase inhibition by fumonisins: A perfect storm of perturbed sphingolipid metabolism, signaling, and disease. J. Lipid Res. 2019, 60, 1183–1189. [Google Scholar] [CrossRef] [Green Version]
- Hammad, S.M.; Hardin, J.R.; Wilson, D.A.; Twal, W.O.; Nietert, P.J.; Oates, J.C. Race disparity in blood sphingolipidomics associated with lupus cardiovascular comorbidity. PLoS ONE 2019, 14, e0224496. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harden, O.C.; Hammad, S.M. Sphingolipids and diagnosis, prognosis, and organ damage in systemic lupus erythematosus. Front. Immunol. 2020, 11, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Nowling, T.K.; Mather, A.R.; Thiyagarajan, T.; Hernández-Corbacho, M.J.; Powers, T.W.; Jones, E.E.; Snider, A.J.; Oates, J.C.; Drake, R.R.; Siskind, L.J. Renal glycosphingolipid metabolism is dysfunctional in lupus nephritis. J. Am. Soc. Nephrol. 2015, 26, 1402–1413. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, W.J.; Song, J.H.; Kim, G.T.; Park, T.S. Ceramide and sphingosine 1-phosphate in liver diseases. Mol. Cells. 2020, 43, 419–430. [Google Scholar] [CrossRef] [PubMed]
- Nikolova-Karakashian, M. Sphingolipids at the crossroads of NAFLD and senescence. Adv. Cancer Res. 2018, 140, 155–190. [Google Scholar] [CrossRef]
- Rohrbach, T.D.; Asgharpour, A.; Maczis, M.A.; Montefusco, D.; Cowart, L.A.; Bedossa, P.; Sanyal, A.J.; Spiegel, S. FTY720/fingolimod decreases hepatic steatosis and expression of fatty acid synthase in diet-induced nonalcoholic fatty liver disease in mice. J. Lipid Res. 2019, 60, 1311–1322. [Google Scholar] [CrossRef]
- Keul, P.; Tölle, M.; Lucke, S.; von Wnuck Lipinski, K.; Heusch, G.; Schuchardt, M.; van der Giet, M.; Levkau, B. The sphingosine-1-phosphate analogue FTY720 reduces atherosclerosis in apolipoprotein E-deficient mice. Arterioscler. Thromb. Vasc. Biol. 2007, 27, 607–613. [Google Scholar] [CrossRef] [Green Version]
- Nádró, B.; Juhász, L.; Szentpéteri, A.; Páll, D.; Paragh, G.; Harangi, M. The role of apolipoprotein M and sphingosine 1-phosphate axis in the prevention of atherosclerosis. Orv. Hetil. 2018, 159, 168–175. [Google Scholar] [CrossRef]
- Ruiz, M.; Frej, C.; Holmér, A.; Guo, L.J.; Tran, S.; Dahlbäck, B. High-density lipoprotein-associated apolipoprotein m limits endothelial inflammation by delivering sphingosine-1-phosphate to the sphingosine-1-phosphate receptor 1. Arterioscler. Thromb. Vasc. Biol. 2017, 37, 118–129. [Google Scholar] [CrossRef] [Green Version]
- He, Q.; Ding, G.; Zhang, M.; Nie, P.; Yang, J.; Liang, D.; Bo, J.; Zhang, Y.; Liu, Y. Trends in the use of sphingosine 1 phosphate in age-related diseases: A scientometric research study (1992–2020). J. Diabetes Res. 2021, 2021, 4932974. [Google Scholar] [CrossRef]
- Ishimaru, K.; Yoshioka, K.; Kano, K.; Kurano, M.; Saigusa, D.; Aoki, J.; Yatomi, Y.; Takuwa, N.; Okamoto, Y.; Proia, R.L.; et al. Sphingosine kinase-2 prevents macrophage cholesterol accumulation and atherosclerosis by stimulating autophagic lipid degradation. Sci. Rep. 2019, 9, 18329. [Google Scholar] [CrossRef] [PubMed]
- Blitzer, J.T.; Wang, L.; Summers, S.A. DES1: A key driver of lipotoxicity in metabolic disease. DNA Cell Biol. 2020, 39, 733–737. [Google Scholar] [CrossRef] [PubMed]
- Chaurasia, B.; Tippetts, T.S.; Mayoral Monibas, R.; Liu, J.; Li, Y.; Wang, L.; Wilkerson, J.L.; Sweeney, C.R.; Pereira, R.F.; Sumida, D.H.; et al. Targeting a ceramide double bond improves insulin resistance and hepatic steatosis. Science 2019, 365, 386–392. [Google Scholar] [CrossRef]
- Kusminski, C.M.; Scherer, P.E. Lowering ceramides to overcome diabetes. Science 2019, 365, 319–320. [Google Scholar] [PubMed]
- Casasampere, M.; Ordoñez, Y.F.; Pou, A.; Casas, J. Inhibitors of dihydroceramide desaturase 1: Therapeutic agents and pharmacological tools to decipher the role of dihydroceramides in cell biology. Chem. Phys. Lipids 2016, 197, 33–44. [Google Scholar] [CrossRef]
- Iqbal, J.; Jahangir, Z.; Al-Qarni, A.A. Microsomal triglyceride transfer protein: From lipid metabolism to metabolic diseases. Adv. Exp. Med. Biol. 2020, 1276, 37–52. [Google Scholar] [CrossRef]
- Iqbal, J.; Walsh, M.T.; Hammad, S.M.; Hussain, M.M. Sphingolipids and lipoproteins in health and metabolic disorders. Trends Endocrinol. Metab. 2017, 28, 506–518. [Google Scholar] [CrossRef]
- Abulizi, A.; Vatner, D.F.; Ye, Z.; Wang, Y.; Camporez, J.P.; Zhang, D.; Kahn, M.; Lyu, K.; Sirwi, A.; Cline, G.W.; et al. Membrane-bound sn-1,2-diacylglycerols explain the dissociation of hepatic insulin resistance from hepatic steatosis in MTTP knockout mice. J. Lipid Res. 2020, 61, 1565–1576. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, J.; Walsh, M.T.; Hammad, S.M.; Cuchel, M.; Tarugi, P.; Hegele, R.A.; Davidson, N.O.; Rader, D.J.; Klein, R.L.; Hussain, M.M. Microsomal triglyceride transfer protein transfers and determines plasma concentrations of ceramide and sphingomyelin but not glycosylceramide. J. Biol. Chem. 2015, 290, 25863–25875. [Google Scholar] [CrossRef] [Green Version]
- Khatun, I.; Zeissig, S.; Iqbal, J.; Wang, M.; Curiel, D.; Shelness, G.S.; Blumberg, R.S.; Hussain, M.M. Phospholipid transfer activity of microsomal triglyceride transfer protein produces apolipoprotein B and reduces hepatosteatosis while maintaining low plasma lipids in mice. Hepatology 2012, 55, 1356–1368. [Google Scholar] [CrossRef] [Green Version]
- Hewing, B.; Parathath, S.; Mai, C.K.; Fiel, M.I.; Guo, L.; Fisher, E.A. Rapid regression of atherosclerosis with MTP inhibitor treatment. Atherosclerosis 2013, 227, 125–129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, X.; Lu, Z.; Perry, D.M.; Li, Y.; Russo, S.B.; Cowart, L.A.; Hannun, Y.A.; Huang, Y. Acid sphingomyelinase plays a key role in palmitic acid-amplified inflammatory signaling triggered by lipopolysaccharide at low concentrations in macrophages. Am. J. Physiol. Endocrinol. Metab. 2013, 305, 853–867. [Google Scholar] [CrossRef]
- Lu, Z.; Li, Y.; Brinson, C.W.; Lopes-Virella, M.F.; Huang, Y. Cooperative stimulation of atherogenesis by lipopolysaccharide and palmitic acid-rich high fat diet in low-density lipoprotein receptor-deficient mice. Atherosclerosis 2017, 265, 231–241. [Google Scholar] [CrossRef]
- Cai, B.B.; Lu, Y.N.; Xu, M. Acid sphingomyelinase downregulation alleviates vascular endothelial leptin resistance in rats. Acta Pharmacol. Sin. 2020, 41, 650–660. [Google Scholar] [CrossRef] [PubMed]
- Edsfeldt, A.; Dunér, P.; Ståhlman, M.; Mollet, I.G.; Asciutto, G.; Grufman, H.; Nitulescu, M.; Persson, A.F.; Fisher, R.M.; Melander, O.; et al. Sphingolipids Contribute to Human Atherosclerotic Plaque Inflammation. Arterioscler. Thromb. Vasc. Biol. 2016, 36, 1132–1140. [Google Scholar] [CrossRef] [Green Version]
- Lallemand, T.; Rouahi, M.; Swiader, A.; Grazide, M.H.; Geoffre, N.; Alayrac, P.; Recazens, E.; Coste, A.; Salvayre, R.; Nègre-Salvayre, A.; et al. nSMase2 (Type 2-Neutral Sphingomyelinase) deficiency or inhibition by GW4869 reduces inflammation and atherosclerosis in Apoe−/− Mice. Arterioscler. Thromb. Vasc. Biol. 2018, 38, 1479–1492. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, Z.; Li, Y.; Syn, W.K.; Wang, Z.; Lopes-Virella, M.F.; Lyons, T.J.; Huang, Y. Amitriptyline inhibits nonalcoholic steatohepatitis and atherosclerosis induced by high-fat diet and LPS through modulation of sphingolipid metabolism. Am. J. Physiol. Endocrinol. Metab. 2020, 318, E131–E144. [Google Scholar] [CrossRef]
- Al-Rashed, F.; Ahmad, Z.; Thomas, R.; Melhem, M.; Snider, A.J.; Obeid, L.M.; Al-Mulla, F.; Hannun, Y.A.; Ahmad, R. Neutral sphingomyelinase 2 regulates inflam-matory responses in monocytes/macrophages induced by TNF-α. Sci. Rep. 2020, 10, 1–12. [Google Scholar] [CrossRef]
- Song, J.H.; Kim, G.T.; Park, K.H.; Park, W.J.; Park, T.S. Bioactive sphingolipids as major regulators of coronary artery disease. Biomol. Ther. 2021, 29, 373–383. [Google Scholar] [CrossRef]
- Dechecchi, M.C.; Nicolis, E.; Mazzi, P.; Cioffi, F.; Bezzerri, V.; Lampronti, I.; Huang, S.; Wiszniewski, L.; Gambari, R.; Scupoli, M.T.; et al. Modulators of sphingolipid metabolism reduce lung inflammation. Am. J. Respir. Cell Mol. Biol. 2011, 45, 825–833. [Google Scholar] [CrossRef] [PubMed]
- Liangpunsakul, S.; Rahmini, Y.; Ross, R.A.; Zhao, Z.; Xu, Y.; Crabb, D.W. Imipramine blocks ethanol-induced ASMase activation, ceramide generation, and PP2A activation, and ameliorates hepatic steatosis in ethanol-fed mice. Am. J. Physiol. Gastrointest. Liver Physiol. 2012, 302, G515–G523. [Google Scholar] [CrossRef] [Green Version]
- Meakin, P.J.; Chowdhry, S.; Sharma, R.S.; Ashford, F.B.; Walsh, S.V.; McCrimmon, R.J.; Dinkova-Kostova, A.T.; Dillon, J.F.; Hayes, J.D.; Ashford, M.L. Susceptibility of Nrf2-null mice to steatohepatitis and cirrhosis upon consumption of a high-fat diet is associated with oxidative stress, perturbation of the unfolded protein response, and disturbance in the expression of metabolic enzymes but not with insulin resistance. Mol. Cell. Biol. 2014, 34, 3305–3320. [Google Scholar] [CrossRef] [Green Version]
- Kester, M.; Bassler, J.; Fox, T.E.; Carter, C.J.; Davidson, J.A.; Parette, M.R. Preclinical development of a C6-ceramide NanoLiposome, a novel sphingolipid therapeutic. Biol. Chem. 2015, 396, 737–747. [Google Scholar] [CrossRef]
- Zanieri, F.; Levi, A.; Montefusco, D.; Longato, L.; De Chiara, F.; Frenguelli, L.; Omenetti, S.; Andreola, F.; Luong, T.V.; Massey, V.; et al. Exogenous liposomal ceramide-C6 ameliorates lipidomic profile, energy homeostasis, and anti-oxidant systems in NASH. Cells 2020, 9, 1237. [Google Scholar] [CrossRef] [PubMed]
- Sofogianni, A.; Filippidis, A.; Chrysavgis, L.; Tziomalos, K.; Cholongitas, E. Glucagon-like peptide-1 receptor agonists in non-alcoholic fatty liver disease: An update. World J. Hepatol. 2020, 12, 493–505. [Google Scholar] [CrossRef] [PubMed]
- Drucker, D.J. Mechanisms of action and therapeutic application of glucagon-like peptide-1. Cell Metab. 2018, 27, 740–756. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Somm, E.; Montandon, S.A.; Loizides-Mangold, U.; Gaïa, N.; Lazarevic, V.; De Vito, C.; Perroud, E.; Bochaton-Piallat, M.L.; Dibner, C.; Schrenzel, J.; et al. The GLP-1R agonist liraglutide limits hepatic lipotoxicity and inflammatory response in mice fed a methionine-choline deficient diet. Transl. Res. 2021, 227, 75–88. [Google Scholar] [CrossRef]
- Zobel, E.H.; Wretlind, A.; Ripa, R.S.; Rotbain Curovic, V.; Von Scholten, B.J.; Suvitaival, T.; Hansen, T.W.; Kjær, A.; Legido-Quigley, C.; Rossing, P. Ceramides and phospholipids are downregulated with liraglutide treatment: Results from the LiraFlame randomized controlled trial. BMJ Open Diabetes Res. Care 2021, 9, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Jia, H.; Jiang, Y.; Wang, L.; Zhang, Y.; Mu, Y.; Liu, Y. Anti-atherosclerotic effects of the glucagon-like peptide-1 (GLP-1) based therapies in patients with type 2 diabetes mellitus: A meta-analysis. Sci. Rep. 2015, 5, 10202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marquis-Gravel, G.; Tardif, J.C. Glucagon-like peptide 1 receptor agonists, carotid atherosclerosis, and cardiovascular outcomes. Diabetes Care 2021, 44, 1252–1253. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, F.J.; Jiang, C.; Patterson, A.D. An intestinal microbiota–farnesoid x receptor axis modulates metabolic disease. Gastroenterology 2016, 151, 845–859. [Google Scholar] [CrossRef] [Green Version]
- Wu, Q.; Sun, L.; Hu, X.; Wang, X.; Xu, F.; Chen, B.; Liang, X.; Xia, J.; Wang, P.; Aibara, D.; et al. Suppressing the intestinal farnesoid X receptor/sphingomyelin phosphodiesterase 3 axis decreases atherosclerosis. J. Clin. Investig. 2021, 131, e142865. [Google Scholar] [CrossRef]
- Liu, H.; Pathak, P.; Boehme, S.; Chiang JY, L. Cholesterol 7α-hydroxylase protects the liver from inflammation and fibrosis by maintaining cholesterol homeostasis. J. Lipid Res. 2016, 57, 1831–1844. [Google Scholar] [CrossRef] [Green Version]
- Velázquez, A.M.; Roglans, N.; Bentanachs, R.; Gené, M.; Sala-Vila, A.; Lázaro, I.; Rodríguez-Morató, J.; Sánchez, R.M.; Laguna, J.C.; Alegret, M. Effects of a low dose of caffeine alone or as part of a green coffee extract, in a rat dietary model of lean non-alcoholic fatty liver disease without inflammation. Nutrients 2020, 12, 3240. [Google Scholar] [CrossRef]
- Ebadi, M.; Ip, S.; Bhanji, R.A.; Montano-Loza, A.J. Effect of coffee consumption on non-alcoholic fatty liver disease incidence, prevalence and risk of significant liver fibrosis: Systematic review with meta-analysis of observational studies. Nutrients 2021, 13, 3042. [Google Scholar] [CrossRef]
- Hayat, U.; Siddiqui, A.A.; Okut, H.; Afroz, S.; Tasleem, S.; Haris, A. The effect of coffee consumption on the non-alcoholic fatty liver disease and liver fibrosis: A meta-analysis of 11 epidemiological studies. Ann. Hepatol. 2021, 20, 100254. [Google Scholar] [CrossRef] [PubMed]
- Raichur, S.; Wang, S.T.; Chan, P.W.; Li, Y.; Ching, J.; Chaurasia, B.; Dogra, S.; Öhman, M.K.; Takeda, K.; Sugii, S.; et al. CerS2 haploinsufficiency inhibits β-oxidation and confers susceptibility to diet-induced steatohepatitis and insulin resistance. Cell Metab. 2014, 20, 687–695. [Google Scholar] [CrossRef] [Green Version]
- Mathews, A.T.; Famodu, O.A.; Olfert, M.D.; Murray, P.J.; Cuff, C.F.; Downes, M.T.; Haughey, N.J.; Colby, S.E.; Chantler, P.D.; Olfert, I.M.; et al. W Efficacy of nutritional interventions to lower circulating ceramides in young adults: FRUVEDomic pilot study. Physiol. Rep. 2017, 5, e13329. [Google Scholar] [CrossRef]
- Tuttolomondo, A.; Simonetta, I.; Daidone, M.; Mogavero, A.; Ortello, A.; Pinto, A. Metabolic and vascular effect of the mediterranean diet. Int. J. Mol. Sci. 2019, 20, 4716. [Google Scholar] [CrossRef] [Green Version]
- Mekseepralard, C.; Areebambud, C.; Suksamrarn, S.; Jariyapongskul, A. Effects of long-term α-mangostin supplementation on hyperglycemia and insulin resistance in type 2 diabetic rats induced by high fat diet and low dose streptozotocin. J. Med. Assoc. Thai. 2015, 98 (Suppl. 10), S23–S30. [Google Scholar]
- Jiang, M.; Huang, S.; Duan, W.; Liu, Q.; Lei, M. α-mangostin improves endothelial dysfunction in db/db mice through inhibition of aSMase/ceramide pathway. J. Cell. Mol. Med. 2021, 25, 3601–3609. [Google Scholar] [CrossRef]
- Pickett-Blakely, O.; Young, K.; Carr, R.M. Micronutrients in nonalcoholic fatty liver disease pathogenesis. CMGH 2018, 6, 451–462. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sangineto, M.; Bukke, V.N.; Bellanti, F.; Tamborra, R.; Moola, A.; Duda, L.; Villani, R.; Romano, A.D.; Serviddio, G. A novel nutraceuticals mixture improves liver steatosis by preventing oxidative stress and mitochondrial dysfunction in a NAFLD model. Nutrients 2021, 13, 652. [Google Scholar] [CrossRef]
- Sangineto, M.; Villani, R.; Cavallone, F.; Romano, A.; Loizzi, D.; Serviddio, G. Lipid metabolism in development and progression of hepatocellular carcinoma. Cancers 2020, 12, 1419. [Google Scholar] [CrossRef]
- Matsuzaka, T.; Kuba, M.; Koyasu, S.; Yamamoto, Y.; Motomura, K.; Arulmozhiraja, S.; Ohno, H.; Sharma, R.; Shimura, T.; Okajima, Y.; et al. Hepatocyte ELOVL Fatty Acid Elongase 6 Determines Ceramide Acyl-Chain Length and Hepatic Insulin Sensitivity in Mice. Hepatology 2020, 71, 1609–1625. [Google Scholar] [CrossRef]
- Spassieva, S.D.; Mullen, T.D.; Townsend, D.M.; Obeid, L.M. Disruption of ceramide synthesis by CerS2 down-regulation leads to autophagy and the unfolded protein response. Biochem. J. 2009, 424, 273–283. [Google Scholar] [CrossRef] [Green Version]
- Turpin, S.M.; Nicholls, H.T.; Willmes, D.M.; Mourier, A.; Brodesser, S.; Wunderlich, C.M.; Mauer, J.; Xu, E.; Hammerschmidt, P.; Brönneke, H.S.; et al. Obesity-induced CerS6-dependent C16:0 ceramide production promotes weight gain and glucose intolerance. Cell Metab. 2014, 20, 678–686. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Turpin-Nolan, S.M.; Hammerschmidt, P.; Chen, W.; Jais, A.; Timper, K.; Awazawa, M.; Brodesser, S.; Brüning, J.C. CerS1-derived C18:0 ceramide in skeletal muscle promotes obesity-induced insulin resistance. Cell Rep. 2019, 26, 1–10.e7. [Google Scholar] [CrossRef] [Green Version]
- Kim, Y.R.; Lee, E.J.; Shin, K.O.; Kim, M.H.; Pewzner-Jung, Y.; Lee, Y.M.; Park, J.W.; Futerman, A.H.; Park, W.J. Hepatic triglyceride accumulation via endoplasmic reticulum stress-induced SREBP-1 activation is regulated by ceramide synthases. Exp. Mol. Med. 2019, 51, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Kalavalapalli, S.; Bril, F.; Guingab, J.; Vergara, A.; Garrett, T.J.; Sunny, N.E.; Cusi, K. Impact of exenatide on mitochondrial lipid metabolism in mice with nonalcoholic steatohepatitis. J. Endocrinol. 2019, 241, 293–305. [Google Scholar] [CrossRef]
- Busnelli, M.; Manzini, S.; Bonacina, F.; Soldati, S.; Barbieri, S.S.; Amadio, P.; Sandrini, L.; Arnaboldi, F.; Donetti, E.; Laaksonen, R.; et al. Fenretinide treatment accelerates atherosclerosis development in apoE-deficient mice in spite of beneficial metabolic effects. Br. J. Pharmacol. 2020, 177, 328–345. [Google Scholar] [CrossRef] [Green Version]

| Ceramide Synthases (CerS) | Tissue Distribution | Acyl-CoA Specificity | The Biophysical Properties | Ref. |
|---|---|---|---|---|
| CerS 1 | Brain Skeletal muscle | C18, C18:1 | Play important roles in signaling and sphingolipid development; Promotes insulin resistance in humans; | [16,17] |
| CerS 2 | Liver Kidney | C20, C22,C24, C24:1, C26 | Play vital roles in postnatal liver development and physiology; Have major molecular roles in the maintenance of normal liver homeostasis; | [18,19] |
| CerS 3 | Intestine Skin Testes | C18, C18:1,C20, C22, C24, C24:1 | Maintain the water permeability barrier function; Involved in sperm formation and androgen production; | [20,21] |
| CerS 4 | Heart Liver | C18, C20 | Highly expressed in liver cancer tissues and can facilitate HCC (Hepatocellular Carcinoma) formation; Controls homeostatic epidermal barrier maintenance; | [22,23] |
| CerS 5 | Lung Prostate Thymus Spleen Skeletal muscle | C14, C16, C18, C18:1 | Contributes to the development of diet-induced obesity; Play roles in sphingosine salvage pathway signaling and in the response to cellular stress; | [24,25] |
| CerS 6 | Lymph node Intestine | C14, C16, C18 | May serve as biomarkers in determining the effectiveness of anticancer agents; C16:0 is a significant factor in the development of obesity and its related complications. | [26,27] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tanase, D.M.; Gosav, E.M.; Petrov, D.; Jucan, A.E.; Lacatusu, C.M.; Floria, M.; Tarniceriu, C.C.; Costea, C.F.; Ciocoiu, M.; Rezus, C. Involvement of Ceramides in Non-Alcoholic Fatty Liver Disease (NAFLD) Atherosclerosis (ATS) Development: Mechanisms and Therapeutic Targets. Diagnostics 2021, 11, 2053. https://doi.org/10.3390/diagnostics11112053
Tanase DM, Gosav EM, Petrov D, Jucan AE, Lacatusu CM, Floria M, Tarniceriu CC, Costea CF, Ciocoiu M, Rezus C. Involvement of Ceramides in Non-Alcoholic Fatty Liver Disease (NAFLD) Atherosclerosis (ATS) Development: Mechanisms and Therapeutic Targets. Diagnostics. 2021; 11(11):2053. https://doi.org/10.3390/diagnostics11112053
Chicago/Turabian StyleTanase, Daniela Maria, Evelina Maria Gosav, Daniela Petrov, Alina Ecaterina Jucan, Cristina Mihaela Lacatusu, Mariana Floria, Claudia Cristina Tarniceriu, Claudia Florida Costea, Manuela Ciocoiu, and Ciprian Rezus. 2021. "Involvement of Ceramides in Non-Alcoholic Fatty Liver Disease (NAFLD) Atherosclerosis (ATS) Development: Mechanisms and Therapeutic Targets" Diagnostics 11, no. 11: 2053. https://doi.org/10.3390/diagnostics11112053
APA StyleTanase, D. M., Gosav, E. M., Petrov, D., Jucan, A. E., Lacatusu, C. M., Floria, M., Tarniceriu, C. C., Costea, C. F., Ciocoiu, M., & Rezus, C. (2021). Involvement of Ceramides in Non-Alcoholic Fatty Liver Disease (NAFLD) Atherosclerosis (ATS) Development: Mechanisms and Therapeutic Targets. Diagnostics, 11(11), 2053. https://doi.org/10.3390/diagnostics11112053