Radionuclide Imaging of Invasive Fungal Disease in Immunocompromised Hosts
Abstract
:1. Introduction
2. Host Immunity, Immunodeficiency, and Invasive Fungal Disease
2.1. Host Immunity against Invasive Fungal Disease
2.2. Immunodeficiency States and Invasive Fungal Disease
3. Radionuclide Imaging of Invasive Fungal Disease
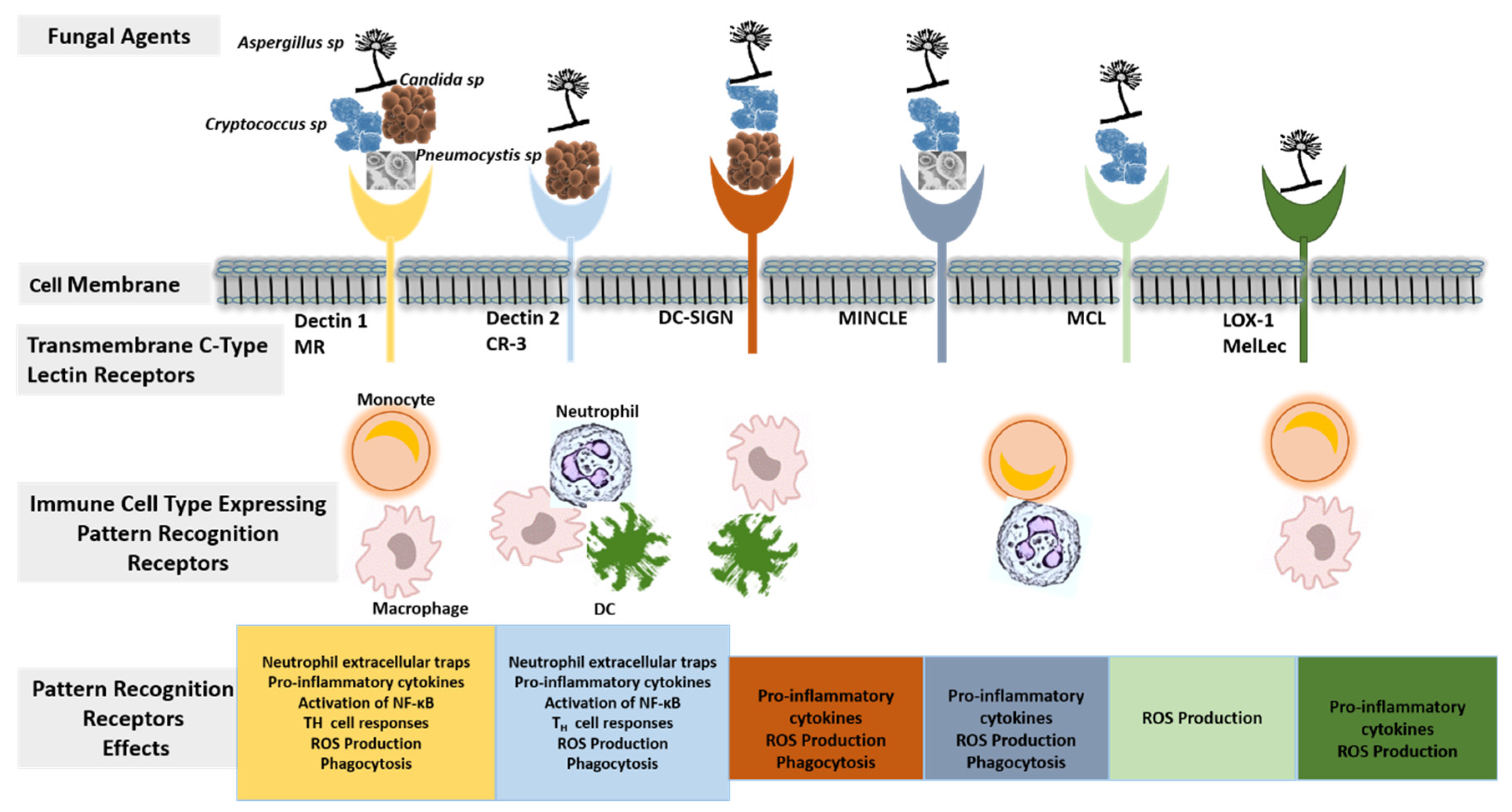
3.1. Targeting Host Immune Response
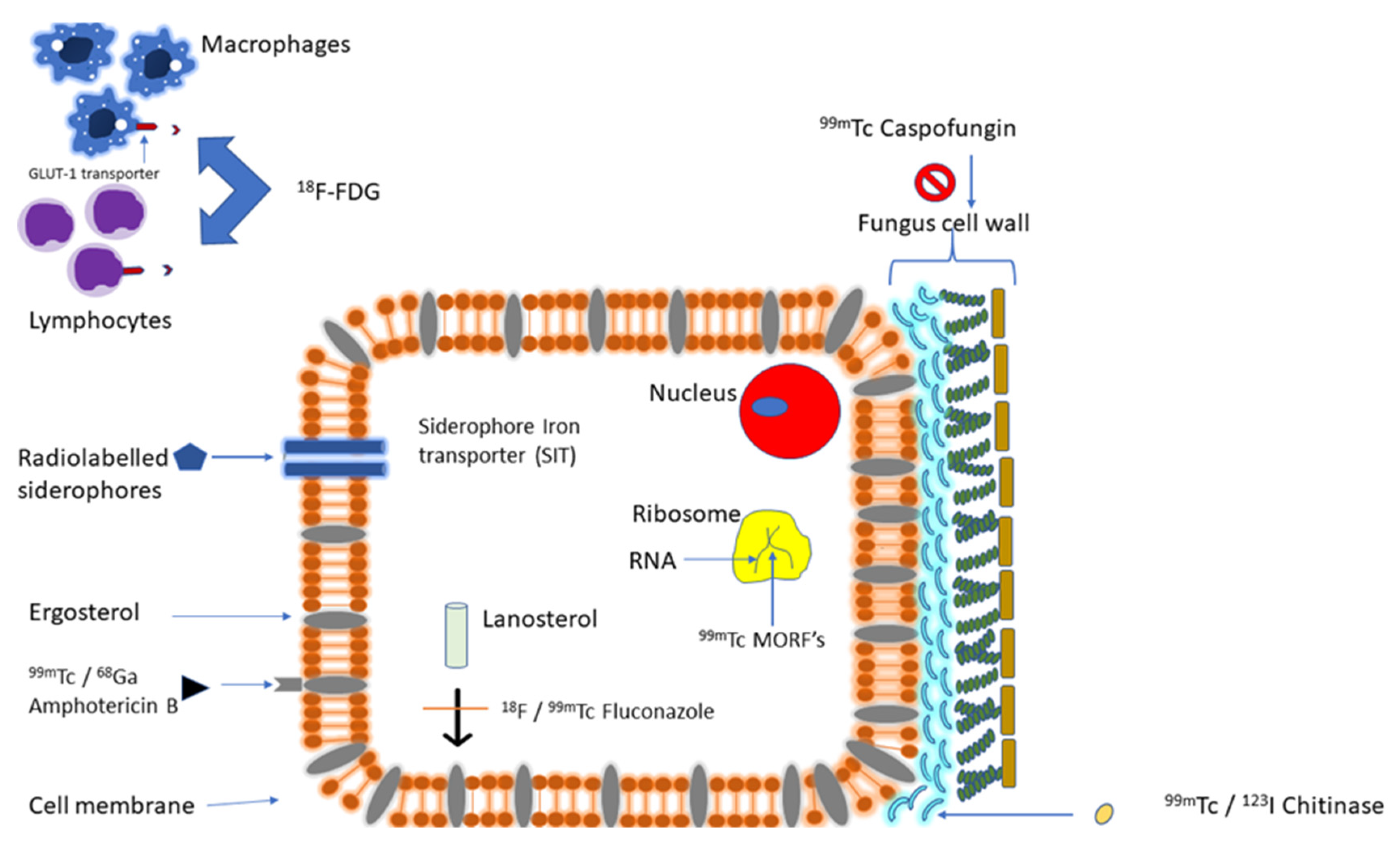
3.2. Targeting Fungal Molecular Structure or Pathway
3.2.1. Targeting Fungal Iron Utilization
3.2.2. Targeting Fungal Cell Membrane/Cell Wall Synthesis
3.2.3. Targeting Fungal-Specific Molecular Structures
3.2.4. Targeting Hyphal-Specific Antigen
3.2.5. Targeting Fungal Cell Wall Chitin
3.2.6. Targeting Fungal Ribosomal RNA
3.3. Non-Specific Antimicrobial Peptides
4. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AIDS: | Acquired immunodeficiency syndrome |
| AMP: | Anti-microbial peptides |
| BTK: | Bruton tyrosine kinase |
| CLRs: | C-type lectin receptors |
| CT: | Computed tomography |
| DAMPs: | Danger-associated molecular patterns |
| DCs: | Dendritic cells |
| [18F]FDG: | Fluorine-18 fluorodeoxyglucose |
| GvHD: | Graft-versus-host disease |
| HCT: | Hematopoietic cell transplantation |
| HIV: | Human immunodeficiency virus |
| IFD: | Invasive fungal disease |
| MORFs: | Morpholino oligomers |
| MRI: | Magnetic resonance imaging |
| NETs: | Neutrophil extracellular traps |
| NK: | Natural killer |
| PAMPs: | Pathogen-associated molecular patterns |
| PET/CT: | Positron emission tomography/computed tomography |
| PJP: | Pneumocystis jirovecii pneumonia |
| PRRs: | Pathogen recognition receptors |
| SIT: | Siderophore–iron transporter |
| SOT: | Solid organ transplant |
| SPECT: | Single-photon emission tomography |
| TAFC: | Triacetylfusarinine C |
| TLG: | Total lesion glycolysis |
| TLRs: | Toll-like receptors |
References
- Lionakis, M.S.; Iliev, I.D.; Hohl, T.M. Immunity against fungi. JCI Insights 2017, 2, e93156. [Google Scholar] [CrossRef]
- Pathakumari, B.; Liang, G.; Liu, W. Immune defence to invasive fungal infections: A comprehensive review. Biomed. Pharm. 2020, 130, 110550. [Google Scholar] [CrossRef]
- Brown, G.D.; Denning, D.W.; Gow, N.A.R.; Levitz, S.M.; Netea, M.G.; White, T.C. Hidden killers: Human fungal infections. Sci. Transl. Med. 2012, 4, 165rv13. [Google Scholar] [CrossRef] [Green Version]
- Denning, D.W.; Bromley, M.J. Infectious Disease. How to bolster the antifungal pipeline. Science 2015, 347, 1414–1416. [Google Scholar] [CrossRef] [Green Version]
- De Pauw, B.; Walsh, T.J.; Donnelly, J.P.; Stevens, D.A.; Edwards, J.E.; Calandra, T.; Pappas, P.G.; Maertens, J.; Lortholary, O.; Kauffman, C.A.; et al. Revised Definitions of Invasive Fungal Disease from the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group. Clin. Infect. Dis 2008, 46, 1813–1821. [Google Scholar]
- Formanek, P.E.; Dilling, F.D. Advances in the diagnosis and management of invasive fungal disease. Chest 2019, 156, 834–842. [Google Scholar] [CrossRef] [Green Version]
- Bays, D.J.; Thompson, G.R., III. Fungal infections of the stem cell transplant recipient and hematologic malignancy patients. Infect. Dis. Clin. N. Am. 2019, 33, 545–566. [Google Scholar] [CrossRef]
- Pappas, P.G.; Alexander, B.D.; Andes, D.R.; Hadley, S.; Kauffman, C.A.; Freifeld, A.; Anaissie, E.J.; Brumble, L.M.; Herwaldt, L.; Ito, J.; et al. Invasive fungal infections among organ transplant recipients: Results of the Transplant-Associated Infection Surveillance Network (TRANSNET). Clin. Infect. Dis. 2010, 50, 1101–1111. [Google Scholar] [CrossRef]
- Lee, P.P.; Lau, Y.L. Cellular and molecular defects underlying invasive fungal infections—Revelations from endemic mycoses. Front. Immunol. 2017, 8, 735. [Google Scholar] [CrossRef] [Green Version]
- Govender, N.P.; Todd, J.; Nel, J.; Mer, M.; Karstaedt, A.; Cohen, C.; GERMS-SA1. HIV Infection as Risk Factor for Death among Hospitalized Persons with Candidemia, South Africa, 2012–2017. Emerg. Infect. Dis. 2021, 27, 1607–1615. [Google Scholar] [CrossRef]
- Lao, M.; Li, C.; Li, J.; Chen, D.; Ding, M.; Gong, Y. Opportunistic invasive fungal disease in patients with type 2 diabetes mellitus from Southern China: Clinical features and associated factors. J. Diabetes Investig. 2020, 1, 731–744. [Google Scholar] [CrossRef] [Green Version]
- Bassetti, M.; Bouza, E. Invasive mold infections in the ICU setting: Complexities and solutions. J. Antimicrob. Chemother. 2017, 72, i39–i47. [Google Scholar] [CrossRef] [Green Version]
- Mishra, Y.; Prashar, M.; Sharma, D.; Akash; Kumar, V.P.; Tilak, T.V.S.V.G.K. Diabetes, COVID 19 and mucormycosis: Clinical spectrum and outcome in a tertiary care medical center in Western India. Diabetes Metab. Syndr. 2021, 15, 102196. [Google Scholar] [CrossRef]
- Pal, R.; Singh, B.; Bhadada, S.K.; Banerjee, M.; Bhogal, R.S.; Hage, N.; Kumar, A. COVID-19-associated mucormycosis: An updated systematic review of literature. Mycoses 2021. Epub ahead of print. [Google Scholar] [CrossRef]
- Donnelly, J.P.; Chen, S.C.; Kauffman, C.A.; Steinbach, W.J.; Baddley, J.W.; Verweij, P.E.; Clancy, C.J.; Wingard, J.R.; Lockhart, S.R.; Groll, A.H.; et al. Revision and Update of the Consensus Definitions of Invasive Fungal Disease from the European Organization for Research and Treatment of Cancer and the Mycoses Study Group Education and Research Consortium. Clin. Infect. Dis. 2020, 71, 1367–1376. [Google Scholar] [CrossRef] [Green Version]
- Weiss, Z.F.; Leon, A.; Koo, S. The evolving landscape of fungal diagnostics, current and emerging microbiological approaches. J. Fungi 2021, 7, 127. [Google Scholar] [CrossRef]
- Duarte, R.F.; Sánchez-Ortega, I.; Cuesta, I.; Arnan, M.; Patiño, B.; Fernández de Sevilla, A.; Gudiol, C.; Ayats, J.; Cuenca-Estrella, M. Serum galactomannan-based early detection of invasive aspergillosis in hematology patients receiving effective antimold prophylaxis. Clin. Infect. Dis. 2014, 59, 1696–1702. [Google Scholar] [CrossRef] [Green Version]
- Marr, K.A.; Laverdiere, M.; Gugel, A.; Leisenring, W. Antifungal therapy decreases sensitivity of the Aspergillus galactomannan enzyme immunoassay. Clin. Infect. Dis. 2005, 40, 1762–1769. [Google Scholar] [CrossRef]
- Park, S.Y.; Kim, S.H.; Choi, S.H.; Sung, H.; Kim, M.N.; Woo, J.H.; Kim, Y.S.; Park, S.K.; Lee, J.H.; Lee, K.H.; et al. Clinical and radiological features of invasive pulmonary aspergillosis in transplant recipients and neutropenic patients. Transpl. Infect. Dis. 2010, 12, 309–315. [Google Scholar] [CrossRef]
- Nam, B.D.; Kim, T.J.; Lee, K.S.; Kim, T.S.; Han, J.; Chung, M.J. Pulmonary mucormycosis: Serial morphologic changes on computed tomography correlate with clinical and pathologic findings. Eur. Radiol. 2018, 28, 788–795. [Google Scholar] [CrossRef]
- Lim, C.; Seo, J.B.; Park, S.Y.; Hwang, H.J.; Lee, H.J.; Lee, S.O.; Chae, E.J.; Do, K.H.; Song, J.W.; Kim, M.Y.; et al. Analysis of initial and follow-up CT findings in patients with invasive pulmonary aspergillosis after solid organ transplantation. Clin. Radiol. 2012, 67, 1179–1186. [Google Scholar] [CrossRef] [PubMed]
- Lawal, I.; Zeevaart, J.; Ebenhan, T.; Ankrah, A.; Vorster, M.; Kruger, H.G.; Govender, T.; Sathekge, M. Metabolic Imaging of Infection. J. Nucl. Med. 2017, 58, 1727–1732. [Google Scholar] [CrossRef]
- Sathekge, M.M.; Ankrah, A.O.; Lawal, I.; Vorster, M. Monitoring Response to Therapy. Semin. Nucl. Med. 2018, 48, 166–181. [Google Scholar] [CrossRef] [Green Version]
- Ankrah, A.O.; Sathekge, M.M.; Dierckx, R.A.; Glaudemans, A.W. Imaging fungal infections in children. Clin. Transl. Imaging 2016, 4, 57–72. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ankrah, A.O.; Klein, H.C.; Span, L.F.R.; de Vries, E.F.J.; Dierckx, R.A.J.O.; Sathekge, M.M.; Glaudemans, A.W.J.M. The Role of PET in Monitoring Therapy in Fungal Infections. Curr. Pharm. Des. 2018, 24, 795–805. [Google Scholar] [CrossRef] [Green Version]
- Ankrah, A.O.; Sathekge, M.M.; Dierckx, R.A.J.O.; Glaudemans, A.W.J.M. Radionuclide Imaging of Fungal Infections and Correlation with the Host Defense Response. J. Fungi 2021, 7, 407. [Google Scholar] [CrossRef] [PubMed]
- Cassone, A.; Cauda, R. Candida and candidiasis in HIV-infected patients: Where commensalism, opportunistic behavior and frank pathogenicity lose their borders. AIDS 2012, 26, 1457–1472. [Google Scholar] [CrossRef]
- Del Poeta, M.; Casadevall, A. Ten challenges on Cryptococcus and cryptococcosis. Mycopathologia 2012, 173, 303–310. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brunke, S.; Mogavero, S.; Kasper, L.; Hube, B. Virulence factors in fungal pathogens of man. Curr. Opin. Microbiol. 2016, 32, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, K.E.; Carter, D.A. Cellular plasticity of pathogenic fungi during infection. PLoS Pathog. 2020, 16, e1008571. [Google Scholar] [CrossRef] [PubMed]
- Moyes, D.L.; Wilson, D.; Richardson, J.P.; Mogavero, S.; Tang, S.X.; Wernecke, J.; Höfs, S.; Gratacap, R.L.; Robbins, J.; Runglall, M.; et al. Candidalysin is a fungal peptide toxin critical for mucosal infection. Nature 2016, 532, 64–68. [Google Scholar] [CrossRef] [Green Version]
- Guinea, J.; Torres-Narbona, M.; Gijón, P.; Muñoz, P.; Pozo, F.; Peláez, T.; de Miguel, J.; Bouza, E. Pulmonary aspergillosis in patients with chronic obstructive pulmonary disease: Incidence, risk factors, and outcome. Clin. Microbiol. Infect. 2010, 16, 870–877. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiao, C.; Puddicombe, S.M.; Field, S.; Haywood, J.; Broughton-Head, V.; Puxeddu, I.; Haitchi, H.M.; Vernon-Wilson, E.; Sammut, D.; Bedke, N.; et al. Defective epithelial barrier function in asthma. J. Allergy Clin. Immunol. 2011, 128, 549–556. [Google Scholar] [CrossRef]
- Yang, D.; Han, Z.; Oppenheim, J.J. Alarmins and immunity. Immunol. Rev. 2017, 280, 41–56. [Google Scholar] [CrossRef] [PubMed]
- Dühring, S.; Germerodt, S.; Skerka, C.; Zipfel, P.F.; Dandekar, T.; Schuster, S. Host-pathogen interactions between the human innate immune system and Candida albicans-understanding and modeling defense and evasion strategies. Front. Microbiol. 2015, 6, 625. [Google Scholar] [CrossRef] [Green Version]
- Ganz, T.; Selsted, M.E.; Szklarek, D.; Harwig, S.S.; Daher, K.; Bainton, D.F.; Lehrer, R.I. Defensins. Natural peptide antibiotics of human neutrophils. J. Clin. Investig. 1985, 76, 1427–1435. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.L.; Li, J.X.; Huang, H.R.; Duan, J.L.; Dai, R.X.; Tao, R.J.; Yang, L.; Hou, J.Y.; Jia, X.M.; Xu, J.F. LL37 Inhibits Aspergillus fumigatus Infection via Directly Binding to the Fungus and Preventing Excessive Inflammation. Front. Immunol. 2019, 10, 283. [Google Scholar] [CrossRef] [PubMed]
- Koshlukova, S.E.; Lloyd, T.L.; Araujo, M.W.; Edgerton, M. Salivary histatin 5 induces non-lytic release of ATP from Candida albicans leading to cell death. J. Biol. Chem. 1999, 274, 18872–18879. [Google Scholar] [CrossRef] [Green Version]
- Luo, S.; Skerka, C.; Kurzai, O.; Zipfel, P.F. Complement and innate immune evasion strategies of the human pathogenic fungus Candida albicans. Mol. Immunol. 2013, 56, 161–169. [Google Scholar] [CrossRef]
- Erwig, L.P.; Gow, N.A. Interactions of fungal pathogens with phagocytes. Nat. Rev. Microbiol. 2016, 14, 163–176. [Google Scholar] [CrossRef] [PubMed]
- Plato, A.; Hardison, S.E.; Brown, G.D. Pattern recognition receptors in antifungal immunity. Semin. Immunopathol. 2015, 37, 97–106. [Google Scholar] [CrossRef] [Green Version]
- Haider, M.; Dambuza, I.M.; Asamaphan, P.; Stappers, M.; Reid, D.; Yamasaki, S.; Brown, G.D.; Gow, N.A.R.; Erwig, L.P. The pattern recognition receptors dectin-2, mincle, and FcRγ impact the dynamics of phagocytosis of Candida, Saccharomyces, Malassezia, and Mucor species. PLoS ONE 2019, 14, e0220867. [Google Scholar] [CrossRef] [Green Version]
- Rosowski, E.E.; Raffa, N.; Knox, B.P.; Golenberg, N.; Keller, N.P.; Huttenlocher, A. Macrophages inhibit Aspergillus fumigatus germination and neutrophil-mediated fungal killing. PLoS Pathog. 2018, 14, e1007229. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shao, X.; Mednick, A.; Alvarez, M.; van Rooijen, N.; Casadevall, A.; Goldman, D.L. An innate immune system cell is a major determinant of species-related susceptibility differences to fungal pneumonia. J. Immunol. 2005, 175, 3244–3251. [Google Scholar] [CrossRef] [PubMed]
- Taramelli, D.; Malabarba, M.G.; Sala, G.; Basilico, N.; Cocuzza, G. Production of cytokines by alveolar and peritoneal macrophages stimulated by Aspergillus fumigatus conidia or hyphae. J. Med. Vet. Mycol. 1996, 34, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Ristow, L.C.; Davis, J.M. The granuloma in cryptococcal disease. PLoS Pathog. 2021, 17, e1009342. [Google Scholar] [CrossRef]
- Netea, M.G.; Gijzen, K.; Coolen, N.; Verschueren, I.; Figdor, C.; Van der Meer, J.W.; Torensma, R.; Kullberg, B.J. Human dendritic cells are less potent at killing Candida albicans than both monocytes and macrophages. Microbes Infect. 2004, 6, 985–989. [Google Scholar] [CrossRef]
- Borghi, M.; Renga, G.; Puccetti, M.; Oikonomou, V.; Palmieri, M.; Galosi, C.; Bartoli, A.; Romani, L. Antifungal Th Immunity: Growing up in Family. Front. Immunol. 2014, 5, 506. [Google Scholar] [CrossRef] [Green Version]
- Newman, S.L.; Holly, A. Candida albicans is phagocytosed, killed, and processed for antigen presentation by human dendritic cells. Infect. Immun. 2001, 69, 6813–6822. [Google Scholar] [CrossRef] [Green Version]
- Castagnola, E.; Cesaro, S.; Giacchino, M.; Livadiotti, S.; Tucci, F.; Zanazzo, G.; Caselli, D.; Caviglia, I.; Parodi, S.; Rondelli, R.; et al. Fungal infections in children with cancer: A prospective, multicenter surveillance study. Pediatr. Infect. Dis. J. 2006, 25, 634–639. [Google Scholar] [CrossRef]
- Urban, C.F.; Reichard, U.; Brinkmann, V.; Zychlinsky, A. Neutrophil extracellular traps capture and kill Candida albicans yeast and hyphal forms. Cell Microbiol. 2006, 8, 668–676. [Google Scholar] [CrossRef]
- Kelly, M.N.; Zheng, M.; Ruan, S.; Kolls, J.; D’Souza, A.; Shellito, J.E. Memory CD4+ T cells are required for optimal NK cell effector functions against the opportunistic fungal pathogen Pneumocystis murina. J. Immunol. 2013, 190, 285–295. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schmidt, S.; Luckowwitsch, M.; Hogardt, M.; Lehrnbecher, T. Natural killer cell line NK-92-mediated damage of medically important fungi. J. Fungi 2021, 7, 144. [Google Scholar] [CrossRef]
- Huffnagle, G.B.; Lipscomb, M.F.; Lovchik, J.A.; Hoag, K.A.; Street, N.E. The role of CD4+ and CD8+ T cells in the protective inflammatory response to a pulmonary cryptococcal infection. J. Leukoc. Biol. 1994, 55, 35–42. [Google Scholar] [CrossRef]
- Read, K.A.; Powell, M.D.; Sreekumar, B.K.; Oestreich, K.J. In Vitro Differentiation of Effector CD4+ T Helper Cell Subsets. Methods Mol. Biol. 2019, 1960, 75–84. [Google Scholar]
- Moragues, M.D.; Omaetxebarria, M.J.; Elguezabal, N.; Sevilla, M.J.; Conti, S.; Polonelli, L.; Pontón, J. A monoclonal antibody directed against a Candida albicans cell wall mannoprotein exerts three anti-C. albicans activities. Infect. Immun. 2003, 71, 5273–5279. [Google Scholar] [CrossRef] [Green Version]
- Rayens, E.; Norris, K.A.; Cordero, J.F. Mortality Trends in Risk Conditions and Invasive Mycotic Disease in the United States, 1999–2018. Clin. Infect. Dis. 2021. Epub ahead of print. [Google Scholar] [CrossRef]
- Henriet, S.; Verweij, P.E.; Holland, S.M.; Warris, A. Invasive fungal infections in patients with chronic granulomatous disease. Adv. Exp. Med. Biol. 2013, 764, 27–55. [Google Scholar] [PubMed]
- Lee, P.P.; Lao-Araya, M.; Yang, J.; Chan, K.W.; Ma, H.; Pei, L.C.; Kui, L.; Mao, H.; Yang, W.; Zhao, X.; et al. Application of Flow Cytometry in the Diagnostics Pipeline of Primary Immunodeficiencies Underlying Disseminated Talaromyces marneffei Infection in HIV-Negative Children. Front. Immunol. 2019, 10, 2189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Antachopoulos, C. Invasive fungal infections in congenital immunodeficiencies. Clin. Microbiol. Infect. 2010, 16, 1335–1342. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ochoa, S.; Constantine, G.M.; Lionakis, M.S. Genetic susceptibility to fungal infection in children. Curr. Opin. Pediatr. 2020, 32, 780–789. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Vinh, D.C.; Casanova, J.L.; Puel, A. Inborn errors of immunity underlying fungal diseases in otherwise healthy individuals. Curr. Opin. Microbiol. 2017, 40, 46–57. [Google Scholar] [CrossRef]
- Zhou, L.H.; Jiang, Y.K.; Li, R.Y.; Huang, L.P.; Yip, C.W.; Denning, D.W.; Zhu, L.P. Risk-Based Estimate of Human Fungal Disease Burden, China. Emerg. Infect. Dis. 2020, 26, 2137–2147. [Google Scholar] [CrossRef]
- Borjian Boroujeni, Z.; Shamsaei, S.; Yarahmadi, M.; Getso, M.I.; Salimi Khorashad, A.; Haghighi, L.; Raissi, V.; Zareei, M.; Saleh Mohammadzade, A.; Moqarabzadeh, V.; et al. Distribution of invasive fungal infections: Molecular epidemiology, etiology, clinical conditions, diagnosis and risk factors: A 3-year experience with 490 patients under intensive care. Microb. Pathog. 2021, 152, 104616. [Google Scholar] [CrossRef]
- Balassa, K.; Danby, R.; Rocha, V. Haematopoietic stem cell transplants: Principles and indications. Br. J. Hosp. Med. 2019, 80, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Majhail, N.S.; Farnia, S.H.; Carpenter, P.A.; Champlin, R.E.; Crawford, S.; Marks, D.I.; Omel, J.L.; Orchard, P.J.; Palmer, J.; Saber, W.; et al. Indications for Autologous and Allogeneic Hematopoietic Cell Transplantation: Guidelines from the American Society for Blood and Marrow Transplantation. Biol. Blood Marrow Transplant. 2015, 21, 1863–1869. [Google Scholar] [CrossRef] [Green Version]
- Chu, S.; McCormick, T.S.; Lazarus, H.M.; Leal, L.O.; Ghannoum, M.A. Invasive fungal disease and the immunocompromised host including allogeneic hematopietic cell transplant recipients: Improved understanding and new strategic approach with sargramostim. Clin. Immunol. 2021, 238, 108731. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.C.; Chien, S.H.; Fan, N.W.; Hu, M.H.; Gau, J.P.; Liu, C.J.; Yu, Y.B.; Liu, C.Y.; Hsiao, L.T.; Liu, J.H.; et al. Incidence and risk factors of probable and proven invasive fungal infection in adult patients receiving allogeneic hematopoietic stem cell transplantation. J. Microbiol. Immunol. Infect. 2016, 49, 567–574. [Google Scholar] [CrossRef] [Green Version]
- Kontoyiannis, D.P.; Marr, K.A.; Park, B.J.; Alexander, B.D.; Anaissie, E.J.; Walsh, T.J.; Ito, J.; Andes, D.R.; Baddley, J.W.; Brown, J.M.; et al. Prospective surveillance for invasive fungal infections in hematopoietic stem cell transplant recipients, 2001–2006: Overview of the Transplant-Associated Infection Surveillance Network (TRANSNET) Database. Clin. Infect. Dis. 2010, 50, 1091–1100. [Google Scholar] [CrossRef]
- Girmenia, C.; Raiola, A.M.; Piciocchi, A.; Algarotti, A.; Stanzani, M.; Cudillo, L.; Pecoraro, C.; Guidi, S.; Iori, A.P.; Montante, B.; et al. Incidence and outcome of invasive fungal diseases after allogeneic stem cell transplantation: A prospective study of the Gruppo Italiano Trapianto Midollo Osseo (GITMO). Biol. Blood Marrow Transplant. 2014, 20, 872–880. [Google Scholar] [CrossRef]
- Gavaldà, J.; Meije, Y.; Fortún, J.; Roilides, E.; Saliba, F.; Lortholary, O.; Muñoz, P.; Grossi, P.; Cuenca-Estrella, M.; ESCMID Study Group for Infections in Compromised Hosts. Invasive fungal infections in solid organ transplant recipients. Clin. Microbiol. Infect. 2014, 20, 27–48. [Google Scholar] [CrossRef] [Green Version]
- Shoham, S.; Marr, K.A. Invasive fungal infections in solid organ transplant recipients. Future Microbiol. 2012, 7, 639–655. [Google Scholar] [CrossRef] [Green Version]
- Trotman, J.; Buske, C.; Tedeschi, A.; Matous, J.V.; MacDonald, D.; Tam, C.S.; Tournilhac, O.; Ma, S.; Treon, S.P.; Oriol, A.; et al. Single-Agent Ibrutinib for Rituximab-Refractory Waldenström’s Macroglobulinemia: Final Analysis of the Substudy of the Phase III iNNOVATETM Trial. Clin. Cancer Res. 2021. Epub ahead of print. [Google Scholar] [CrossRef]
- Lv, L.; Sun, X.; Wu, Y.; Cui, Q.; Chen, Y.; Liu, Y. Efficacy and Safety of Ibrutinib in Central Nervous System Lymphoma: A PRISMA-Compliant Single-Arm Meta-Analysis. Front. Oncol. 2021, 11, 707285. [Google Scholar] [CrossRef]
- Moore, D.C.; Thompson, D. A Review of the Bruton Tyrosine Kinase Inhibitors in B-Cell Malignancies. J. Adv. Pract. Oncol. 2021, 12, 439–447. [Google Scholar] [PubMed]
- Varughese, T.; Taur, Y.; Cohen, N.; Palomba, M.L.; Seo, S.K.; Hohl, T.M.; Redelman-Sidi, G. Serious Infections in Patients Receiving Ibrutinib for Treatment of Lymphoid Cancer. Clin. Infect. Dis. 2018, 67, 687–692. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chamilos, G.; Lionakis, M.S.; Kontoyiannis, D.P. Call for Action: Invasive Fungal Infections Associated With Ibrutinib and Other Small Molecule Kinase Inhibitors Targeting Immune Signaling Pathways. Clin. Infect. Dis. 2018, 66, 140–148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tillman, B.F.; Pauff, J.M.; Satyanarayana, G.; Talbott, M.; Werner, J.L. Systematic review of infectious events with the Bruton tyrosine inhibitor ibrutinib in the treatment of hematologic malignancies. Eur. J. Haematol. 2018, 100, 325–334. [Google Scholar] [CrossRef]
- Centers for Disease Control (CDC). Pneumocystis pneumonia—Los Angeles. MMWR Morb. Mortal Wkly Rep. 1981, 30, 250–252. [Google Scholar]
- Simon, V.; Ho, D.D.; Abdool Karim, Q. HIV/AIDS epidemiology, pathogenesis, prevention, and treatment. Lancet 2006, 368, 489–504. [Google Scholar] [CrossRef] [Green Version]
- Wang, R.J.; Miller, R.F.; Huang, L. Approach to fungal infections in human immunodeficiency virus-infected individuals: Pneumocystis and beyond. Clin. Chest Med. 2017, 38, 465–477. [Google Scholar] [CrossRef] [PubMed]
- Armstrong-James, D.; Meintjes, G.; Brown, G.D. A negelted epidermic: Fungal infections in HIV/AIDS. Trends Microbiol. 2014, 22, 122–127. [Google Scholar] [CrossRef]
- Chastain, D.B.; Henao-Martínez, A.F.; Franco-Paredes, C. Opportunistic invasive mycosis in AIDS. Cryptococcosis, histoplasmosis, coccidioidomycosis, and talaromycosis. Curr. Infect. Dis. Rep. 2017, 19, 36. [Google Scholar] [CrossRef]
- Shibuya, K.; Hirata, A.; Omuta, J.; Sugamata, M.; Katori, S.; Saito, N.; Murata, N.; Morita, A.; Takahashi, K.; Hasegawa, C.; et al. Granuloma and cryptococcosis. J. Infect. Chemother. 2005, 11, 115–122. [Google Scholar] [CrossRef]
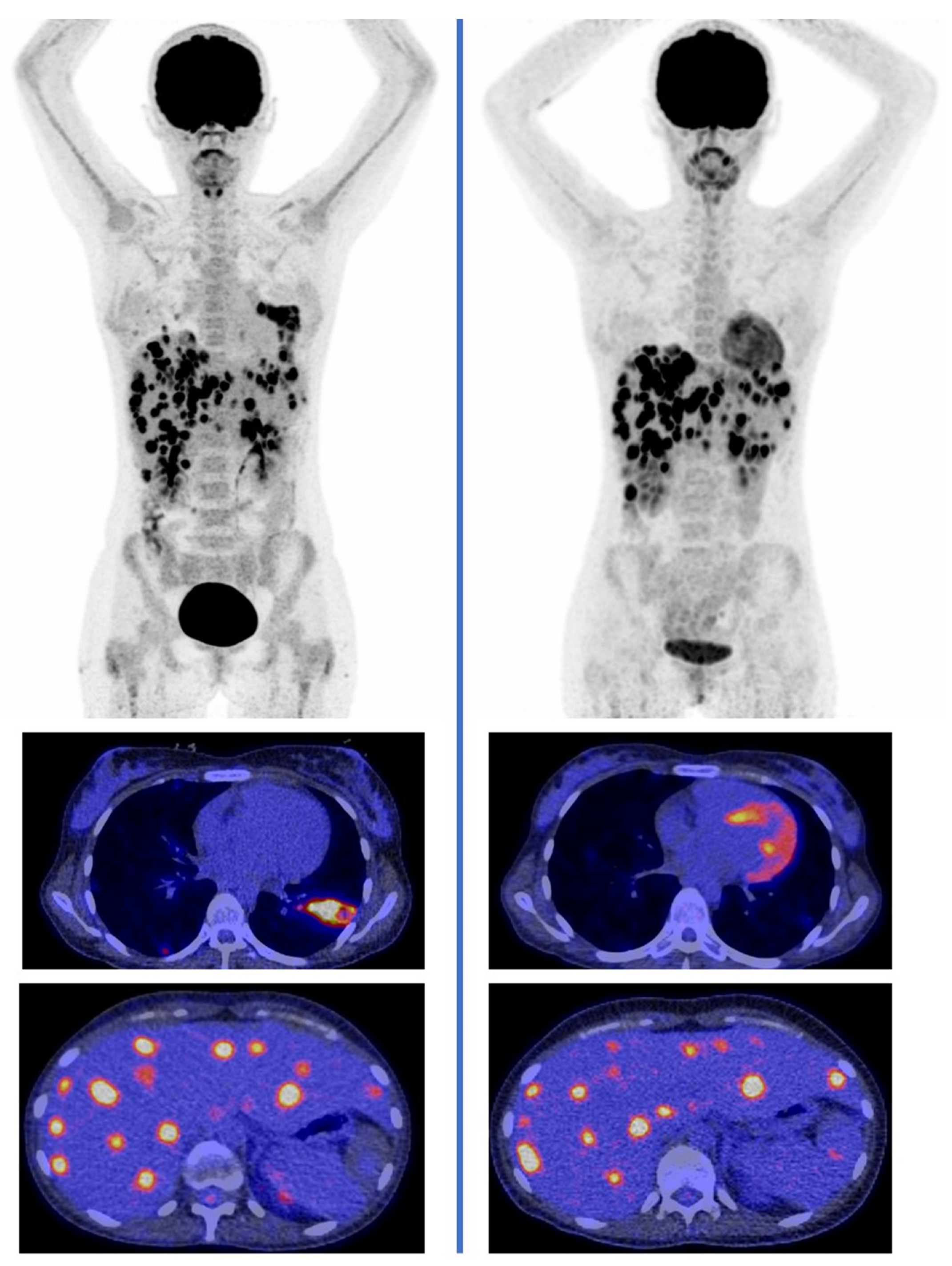
- Bleeker-Rovers, C.; Vos, F.J.; Wanten, G.J.; van der Meer, J.W.; Corstens, F.H.; Kullberg, B.J.; Oyen, W.J. 18F-FDG PET in detecting metastatic infectious disease. J. Nucl. Med. 2005, 46, 2014–2019. [Google Scholar]
- Huang, C.J.; You, D.L.; Lee, P.I.; Hsu, L.H.; Liu, C.C.; Shih, C.S.; Shih, C.C.; Tseng, H.C. Characteristics of integrated 18F-FDG PET/CT in Pulmonary Cryptococcosis. Acta Radiol. 2009, 50, 374–378. [Google Scholar] [CrossRef] [PubMed]
- Igai, H.; Gotoh, M.; Yokomise, H. Computed tomography (CT) and positron emission tomography with [18F]fluoro-2-deoxy-D-glucose (FDG-PET) images of pulmonary cryptococcosis mimicking lung cancer. Eur. J. Cardiothorac. Surg. 2006, 30, 837–839. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, J.Y.; Yoo, J.W.; Oh, M.; Park, S.H.; Shim, T.S.; Choi, Y.Y.; Ryu, J.S. (18)F-fluoro-2-deoxy-D-glucose positron emission tomography/computed tomography findings are different between invasive and noninvasive pulmonary aspergillosis. J. Comput. Assist. Tomogr. 2013, 37, 596–601. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Mukherjee, A.; Karunanithi, S.; Bal, C.; Kumar, R. Potential role of 18F-FDG PET/CT in patients with fungal infections. AJR Am. J. Roentgenol. 2014, 203, 180–189. [Google Scholar] [CrossRef]
- Hot, A.; Maunoury, C.; Poiree, S.; Lanternier, F.; Viard, J.P.; Loulergue, P.; Coignard, H.; Bougnoux, M.E.; Suarez, F.; Rubio, M.T.; et al. Diagnostic contribution of positron emission tomography with [18F]fluorodeoxyglucose for invasive fungal infections. Clin. Microbiol. Infect. 2011, 17, 409–417. [Google Scholar] [CrossRef] [Green Version]
- Signore, A.; Lauri, C.; Auletta, S.; Anzola, K.; Galli, F.; Casali, M.; Versari, A.; Glaudemans, A.W.J.M. Immuno-imaging to predict treatment response in infection, inflammation and oncology. J. Clin. Med. 2019, 8, 681. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ankrah, A.O.; Creemers-Schild, D.; de Keizer, B.; Klein, H.C.; Dierckx, R.A.J.O.; Kwee, T.C.; Span, L.F.R.; de Jong, P.A.; Glaudemans, A.W.J.M. The added value of [18F]FDG PET/CT in the management of invasive fungal infections. Diagnostics 2021, 11, 137. [Google Scholar] [CrossRef] [PubMed]
- Douglas, A.P.; Thursky, K.A.; Worth, L.J.; Drummond, E.; Hogg, A.; Hicks, R.J.; Slavin, M.A. FDG PET/CT imaging in detecting and guiding management of invasive fungal infections: A retrospective comparison to conventional CT imaging. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 166–173. [Google Scholar] [CrossRef]
- Leroy-Freschini, B.; Treglia, G.; Argemi, X.; Bund, C.; Kessler, R.; Herbrecht, R.; Imperiale, A. 18F-FDG PET/CT for invasive fungal infection in immunocompromised patients. QJM 2018, 111, 613–622. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ankrah, A.O.; Span, L.F.R.; Klein, H.C.; de Jong, P.A.; Dierckx, R.A.J.O.; Kwee, T.C.; Sathekge, M.M.; Glaudemans, A.W.J.M. Role of FDG PET/CT in monitoring treatment response in patients with invasive fungal infections. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 174–183. [Google Scholar] [CrossRef] [Green Version]
- Lawal, I.O.; Nyakale, N.E.; Harry, L.M.; Modiselle, M.R.; Ankrah, A.O.; Msomi, A.P.; Mokgoro, N.P.; Boshomane, T.G.; de Wiele, C.V.; Sathekge, M.M. The role of F-18 FDG PET/CT in evaluating the impact of HIV infection on tumor burden and therapy outcome in patients with Hodgkin lymphoma. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 2025–2033. [Google Scholar] [CrossRef]
- Lawal, I.O.; Ankrah, A.O.; Popoola, G.O.; Nyakale, N.E.; Boshomane, T.G.; Reyneke, F.; Lengana, T.; Vorster, M.; Sathekge, M.M. 18F-FDG-PET metabolic metrics and International Prognostic Score for risk assessment in HIV-infected patients with Hodgkin lymphoma. Nucl. Med. Commun. 2018, 39, 1005–1012. [Google Scholar] [CrossRef]
- Mokoala, K.M.G.; Lawal, I.O.; Lengana, T.; Popoola, G.O.; Boshomane, T.M.G.; Mokgoro, N.P.; Vorster, M.; Sathekge, M.M. The Association of Tumor Burden by 18F-FDG PET/CT and Survival in Vulvar Carcinoma. Clin. Nucl. Med. 2021, 46, 375–381. [Google Scholar] [CrossRef]
- Lawal, I.O.; Lengana, T.; Janse van Rensburg, C.; Reyneke, F.; Popoola, G.O.; Ankrah, A.O.; Sathekge, M.M. Fluorodeoxyglucose Positron Emission Tomography integrated with computed tomography in carcinoma of the cervix: Its impact on accurate staging and the predictive role of its metabolic parameters. PLoS ONE 2019, 14, e0215412. [Google Scholar] [CrossRef]
- Lawal, I.O.; Ankrah, A.O.; Mokoala, K.M.G.; Popoola, G.O.; Kaoma, C.A.; Maes, A.; Mokgoro, N.P.; Van de Wiele, C.; Sathekge, M.M. Prognostic Value of Pre-treatment F-18 FDG PET Metabolic Metrics in Patients with Locally Advanced Carcinoma of the Anus with and without HIV Infection. Nuklearmedizin 2018, 57, 190–197. [Google Scholar] [CrossRef]
- Van de Wiele, C.; Kruse, V.; Smeets, P.; Sathekge, M.; Maes, A. Predictive and prognostic value of metabolic tumour volume and total lesion glycolysis in solid tumours. Eur. J. Nucl. Med. Mol. Imaging 2013, 40, 290–301. [Google Scholar] [CrossRef]
- Lawal, I.O.; Popoola, G.O.; Lengana, T.; Ankrah, A.O.; Ebenhan, T.; Sathekge, M.M. Diagnostic utility of 18F-FDG PET/CT in fever of unknown origin among patients with end-stage renal disease treated with renal replacement therapy. Hell. J. Nucl. Med. 2019, 22, 70–75. [Google Scholar]
- Malherbe, S.T.; Shenai, S.; Ronacher, K.; Loxton, A.G.; Dolganov, G.; Kriel, M.; Van, T.; Chen, R.Y.; Warwick, J.; Via, L.E.; et al. Persisting positron emission tomography lesion activity and Mycobacterium tuberculosis mRNA after tuberculosis cure. Nat. Med. 2016, 22, 1094–1100. [Google Scholar] [CrossRef]
- Lawal, I.O.; Fourie, B.P.; Mathebula, M.; Moagi, I.; Lengana, T.; Moeketsi, N.; Nchabeleng, M.; Hatherill, M.; Sathekge, M.M. 18F-FDG PET/CT as a Noninvasive Biomarker for Assessing Adequacy of Treatment and Predicting Relapse in Patients Treated for Pulmonary Tuberculosis. J. Nucl. Med. 2020, 61, 412–417. [Google Scholar] [CrossRef] [PubMed]
- Nevitt, T. War-Fe-re: Iron at the core of fungal virulence and host immunity. Biometals 2011, 24, 547–558. [Google Scholar] [CrossRef] [PubMed]
- Ding, C.; Festa, R.A.; Sun, T.S.; Wang, Z.Y. Iron and copper as virulence modulators in human fungal pathogens. Mol. Microbiol. 2014, 93, 10–23. [Google Scholar] [CrossRef] [PubMed]
- Schrettl, M.; Kim, H.S.; Eisendle, M.; Kragl, C.; Nierman, W.C.; Heinekamp, T.; Werner, E.R.; Jacobsen, I.; Illmer, P.; Yi, H.; et al. SreA-mediated iron regulation in Aspergillus fumigatus. Mol. Microbiol. 2008, 70, 27–43. [Google Scholar] [CrossRef] [Green Version]
- Petrik, M.; Pfister, J.; Misslinger, M.; Decristoforo, C.; Haas, H. Siderophore-Based Molecular Imaging of Fungal and Bacterial Infections-Current Status and Future Perspectives. J. Fungi 2020, 6, 73. [Google Scholar] [CrossRef]
- Petrik, M.; Zhai, C.; Haas, H.; Decristoforo, C. Siderophores for molecular imaging applications. Clin. Transl. Imaging 2017, 5, 15–27. [Google Scholar] [CrossRef] [Green Version]
- Kramer, E.L.; Sanger, J.J.; Garay, S.M.; Greene, J.B.; Tiu, S.; Banner, H.; McCauley, D.I. Gallium-67 scans of the chest in patients with acquired immunodeficiency syndrome. J. Nucl. Med. 1987, 28, 1107–1114. [Google Scholar]
- Woolfenden, J.M.; Carrasquillo, J.A.; Larson, S.M.; Simmons, J.T.; Masur, H.; Smith, P.D.; Shelhamer, J.H.; Ognibene, F.P. Acquired immunodeficiency syndrome: Ga-67 citrate imaging. Radiology 1987, 162, 383–387. [Google Scholar] [CrossRef] [PubMed]
- Schuster, D.M.; Alazraki, N. Gallium and Other Agents in Diseases of the Lung. Semin. Nucl. Med. 2002, 32, 193–211. [Google Scholar] [CrossRef] [PubMed]
- Ankrah, A.O.; Lawal, I.O.; Boshomane, T.M.G.; Klein, H.C.; Ebenhan, T.; Dierckx, R.A.J.O.; Vorster, M.; Glaudemans, A.W.J.M.; Sathekge, M.M. Comparison of Fluorine(18)-fluorodeoxyglucose and Gallium(68)-citrate PET/CT in patients with tuberculosis. Nuklearmedizin 2019, 58, 371–378. [Google Scholar] [CrossRef] [PubMed]
- Petrik, M.; Haas, H.; Dobrozemsky, G.; Lass-Flörl, C.; Helbok, A.; Blatzer, M.; Dietrich, H.; Decristoforo, C. 68Ga-siderophores for PET imaging of invasive pulmonary aspergillosis: Proof of principle. J. Nucl. Med. 2010, 51, 639–645. [Google Scholar] [CrossRef] [Green Version]
- Petrik, M.; Haas, H.; Schrettl, M.; Helbok, A.; Blatzer, M.; Decristoforo, C. In vitro and in vivo evaluation of selected 68Ga-siderophores for infection imaging. Nucl. Med. Biol. 2012, 39, 361–369. [Google Scholar] [CrossRef] [Green Version]
- Petrik, M.; Haas, H.; Laverman, P.; Schrettl, M.; Franssen, G.M.; Blatzer, M.; Decristoforo, C. 68Ga-triacetylfusarinine C and 68Ga-ferrioxamine E for Aspergillus infection imaging: Uptake specificity in various microorganisms. Mol. Imaging Biol. 2014, 16, 102–108. [Google Scholar] [CrossRef] [Green Version]
- Petrik, M.; Franssen, G.M.; Haas, H.; Laverman, P.; Hörtnagl, C.; Schrettl, M.; Helbok, A.; Lass-Flörl, C.; Decristoforo, C. Preclinical evaluation of two 68Ga-siderophores as potential radiopharmaceuticals for Aspergillus fumigatus infection imaging. Eur. J. Nucl. Med. Mol. Imaging 2012, 39, 1175–1183. [Google Scholar] [CrossRef] [Green Version]
- Kaeopookum, P.; Summer, D.; Pfister, J.; Orasch, T.; Lechner, B.E.; Petrik, M.; Novy, Z.; Matuszczak, B.; Rangger, C.; Haas, H.; et al. Modifying the Siderophore Triacetylfusarinine C for Molecular Imaging of Fungal Infection. Mol. Imaging Biol. 2019, 21, 1097–1106. [Google Scholar] [CrossRef] [Green Version]
- Pfister, J.; Summer, D.; Petrik, M.; Khoylou, M.; Lichius, A.; Kaeopookum, P.; Kochinke, L.; Orasch, T.; Haas, H.; Decristoforo, C. Hybrid imaging of Aspergillus fumigatus pulmonary infection with florescent, 68Ga-labelled siderophores. Biomolecules 2020, 10, 168. [Google Scholar] [CrossRef] [Green Version]
- Summer, D.; Petrik, M.; Mayr, S.; Hermann, M.; Kaeopookum, P.; Pfister, J.; Klingler, M.; Rangger, C.; Haas, H.; Decristoforo, C. Hybrid imaging agents for pretargeting applications based on fusarinine C—Proof of concept. Molecules 2020, 25, 2123. [Google Scholar] [CrossRef]
- Thornton, C.R. Molecular imaging of invasive pulmonary aspergillosis using immunoPET/MRI: The future looks bright. Front. Microbiol. 2018, 9, 691. [Google Scholar] [CrossRef]
- Lupetti, A.; De Boer, M.G.J.; Erba, P.; Campa, M.; Nibbering, P.H. Radiotracers for fungal infection imaging. Med. Mycol. 2011, 49, S62–S69. [Google Scholar] [CrossRef] [Green Version]
- Zeng, H.; Wu, Z.; Yu, B.; Wang, B.; Wu, C.; Wu, J.; Lai, J.; Gao, X.; Chen, J. Network meta-analysis of triazole, polyene, and echinocandin antifungal agents in invasive fungal infection prophylaxis in patients with hematological malignancies. BMC Cancer 2021, 21, 404. [Google Scholar] [CrossRef]
- Lupetti, A.; Welling, M.; Mazzi, U.; Nibbering, P.H.; Pauwels, E.K. Technetium-99m labelled fluconazole and antimicrobial peptides for imaging of Candida albicans and Aspergillus fumigatus infections. Eur. J. Nucl. Med. 2002, 29, 674–679. [Google Scholar] [CrossRef] [PubMed]
- Livni, E.; Fischman, A.J.; Ray, S.; Sinclair, I.; Elmaleh, D.R.; Alpert, N.M.; Weiss, S.; Correia, J.A.; Webb, D.; Dahl, R. Synthesis of 18F-labeled fluconazole and positron emission tomography studies in rabbits. Int. J. Rad. Appl. Instrum. B 1992, 19, 191–199. [Google Scholar] [CrossRef]
- Fischman, A.J.; Alpert, N.M.; Livni, E.; Ray, S.; Sinclair, I.; Elmaleh, D.R.; Weiss, S.; Correia, J.A.; Webb, D.; Liss, R. Pharmacokinetics of 18F-labeled fluconazole in rabbits with candidal infections studied with positron emission tomography. J. Pharmacol. Exp. Ther. 1991, 259, 1351–1359. [Google Scholar] [PubMed]
- Fischman, A.J.; Alpert, N.M.; Livni, E.; Ray, S.; Sinclair, I.; Callahan, R.J.; Correia, J.A.; Webb, D.; Strauss, H.W.; Rubin, R.H. Pharmacokinetics of 18F-labeled fluconazole in healthy human subjects by positron emission tomography. Antimicrob. Agents Chemother. 1993, 37, 1270–1277. [Google Scholar] [CrossRef] [Green Version]
- Ordonez, A.A.; Tucker, E.W.; Anderson, C.J.; Carter, C.L.; Ganatra, S.; Kaushal, D.; Kramnik, I.; Lin, P.L.; Madigan, C.A.; Mendez, S.; et al. Visualizing the dynamics of tuberculosis pathology using molecular imaging. J. Clin. Investig. 2021, 13, e145107. [Google Scholar] [CrossRef]
- Tucker, E.W.; Guglieri-Lopez, B.; Ordonez, A.A.; Ritchie, B.; Klunk, M.H.; Sharma, R.; Chang, Y.S.; Sanchez-Bautista, J.; Frey, S.; Lodge, M.A.; et al. Noninvasive 11C-rifampicin positron emission tomography reveals drug biodistribution in tuberculous meningitis. Sci. Transl. Med. 2018, 10, eaau0965. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ordonez, A.A.; Wang, H.; Magombedze, G.; Ruiz-Bedoya, C.; Srivastava, S.; Chen, A.; Tucker, E.W.; Urbanowski, M.E.; Pieterse, L.; Cardozo, E.F.; et al. Dynamic imaging in patients with tuberculosis reveals heterogeneous drug exposures in pulmonary lesions. Nat. Med. 2020, 26, 529–534. [Google Scholar] [CrossRef]
- Reyes, A.L.; Fernandez, L.; Rey, A.; Teran, M. Development and evaluation of Tc-Tricarbonyl-Caspofungin as potential diagnostic agent of fungal infections. Curr. Radiopharm. 2014, 7, 144–150. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, L.; Teran, M. Development and evaluation of 99mTc-amphotericin complexes as potential diagnostic agents in nuclear medicine. Int. J. Infect. 2017, 4, e62150. [Google Scholar] [CrossRef] [Green Version]
- Page, L.; Ullmann, A.J.; Schadt, F.; Wurster, S.; Samnick, S. In vitro evaluation of radiolabeled amphotericin B for molecular imaging of mold infections. Antimicrob. Agents Chemother. 2020, 64, e02377-19. [Google Scholar] [CrossRef] [PubMed]
- Lewis, R.E. Current concepts in antifungal pharmacology. Mayo Clin. Proc. 2011, 86, 805–817. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rolle, A.M.; Hasenberg, M.; Thornton, C.R.; Solouk-Saran, D.; Männ, L.; Weski, J.; Maurer, A.; Fischer, E.; Spycher, P.R.; Schibli, R.; et al. ImmunoPET/MR imaging allows specific detection of Aspergillus fumigatus lung infection in vivo. Proc. Natl. Acad. Sci. USA 2016, 113, E1026–E1033. [Google Scholar] [CrossRef] [Green Version]
- Henneberg, S.; Hasenberg, A.; Maurer, A.; Neumann, F.; Bornemann, L.; Gonzalez-Menendez, I.; Kraus, A.; Hasenberg, M.; Thornton, C.R.; Pichler, B.J.; et al. Antibody-guided in vivo imaging of Aspergillus fumigatus lung infections during antifungal azole treatment. Nat. Commun. 2021, 12, 1707. [Google Scholar] [CrossRef]
- Siaens, R.; Eijsink, V.G.; Dierckx, R.; Slegers, G. 123I-Labeled chitinase as specific radioligand for in vivo detection of fungal infections in mice. J. Nucl. Med. 2004, 45, 1209–1216. [Google Scholar] [PubMed]
- Siaens, R.; Eijsink, V.G.; Vaaje-Kolstad, G.; Vandenbulcke, K.; Cornelissen, B.; Cuvelier, C.; Dierckx, R.; Slegers, G. Synthesis and evaluation of a 99mTechnetium labeled chitin-binding protein as potential specific radioligand for the detection of fungal infections in mice. Q. J. Nucl. Med. Mol. Imaging 2006, 50, 155–166. [Google Scholar]
- Kempf, V.A.J.; Trebesius, K.; Autenrieth, I.B. Floresecent in situ hybridization allows rapid identification of microorganisms in blood cultures. J. Clin. Microbiol. 2000, 38, 830–838. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Chen, L.; Liu, X.; Cheng, D.; Liu, G.; Liu, Y.; Dou, S.; Hnatowich, D.J.; Rusckowski, M. Detection of Aspergillus fumigatus pulmonary fungal infections in mice with 99mTc-labeled MORF oligomers targeting ribosomal RNA. Nucl. Med. Biol. 2013, 40, 89–96. [Google Scholar] [CrossRef] [Green Version]
- Gunzer, M.; Thornton, C.R.; Beziere, N. Advances in the in vivo molecular imaging of invasive aspergillosis. J. Fungi 2020, 6, 338. [Google Scholar] [CrossRef]
- Paez, D.; Sathekge, M.M.; Douis, H.; Giammarile, F.; Fatima, S.; Dhal, A.; Puri, S.K.; Erba, P.A.; Lazzeri, E.; Ferrando, R.; et al. Comparison of MRI, [18F]FDG PET/CT, and 99mTc-UBI 29-41 scintigraphy for postoperative spondylodiscitis-a prospective multicenter study. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 1864–1875. [Google Scholar] [CrossRef] [PubMed]
- Sathekge, M.; Garcia-Perez, O.; Paez, D.; El-Haj, N.; Kain-Godoy, T.; Lawal, I.; Estrada-Lobato, E. Molecular imaging in musculoskeletal infections with 99mTc-UBI 29-41 SPECT/CT. Ann. Nucl. Med. 2018, 32, 54–59. [Google Scholar] [CrossRef] [Green Version]
- Ebenhan, T.; Sathekge, M.M.; Lengana, T.; Koole, M.; Gheysens, O.; Govender, T.; Zeevaart, J.R. 68Ga-NOTA-Functionalized Ubiquicidin: Cytotoxicity, Biodistribution, Radiation Dosimetry, and First-in-Human PET/CT Imaging of Infections. J. Nucl. Med. 2018, 59, 334–339. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Welling, M.M.; Lupetti, A.; Balter, H.S.; Lazzeri, S.; Souto, B.; Rey, A.M.; Savio, E.O.; Paulusma-Annema, A.; Pauwels, E.K.; Nibbering, P.H. 99mTc-labeled antimicrobial peptides for detection of bacterial and Candida albicans infections. J. Nucl. Med. 2001, 42, 788–794. [Google Scholar] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lawal, I.O.; Mokoala, K.M.G.; Kgatle, M.M.; Dierckx, R.A.J.O.; Glaudemans, A.W.J.M.; Sathekge, M.M.; Ankrah, A.O. Radionuclide Imaging of Invasive Fungal Disease in Immunocompromised Hosts. Diagnostics 2021, 11, 2057. https://doi.org/10.3390/diagnostics11112057
Lawal IO, Mokoala KMG, Kgatle MM, Dierckx RAJO, Glaudemans AWJM, Sathekge MM, Ankrah AO. Radionuclide Imaging of Invasive Fungal Disease in Immunocompromised Hosts. Diagnostics. 2021; 11(11):2057. https://doi.org/10.3390/diagnostics11112057
Chicago/Turabian StyleLawal, Ismaheel O., Kgomotso M. G. Mokoala, Mankgopo M. Kgatle, Rudi A. J. O. Dierckx, Andor W. J. M. Glaudemans, Mike M. Sathekge, and Alfred O. Ankrah. 2021. "Radionuclide Imaging of Invasive Fungal Disease in Immunocompromised Hosts" Diagnostics 11, no. 11: 2057. https://doi.org/10.3390/diagnostics11112057
APA StyleLawal, I. O., Mokoala, K. M. G., Kgatle, M. M., Dierckx, R. A. J. O., Glaudemans, A. W. J. M., Sathekge, M. M., & Ankrah, A. O. (2021). Radionuclide Imaging of Invasive Fungal Disease in Immunocompromised Hosts. Diagnostics, 11(11), 2057. https://doi.org/10.3390/diagnostics11112057









