Automatic Prediction and Assessment of Treatment Response in Patients with Hodgkin’s Lymphoma Using a Whole-Body DW-MRI Based Approach
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Cohort
2.2. Acquisition Protocol
2.3. PET Response Evaluation
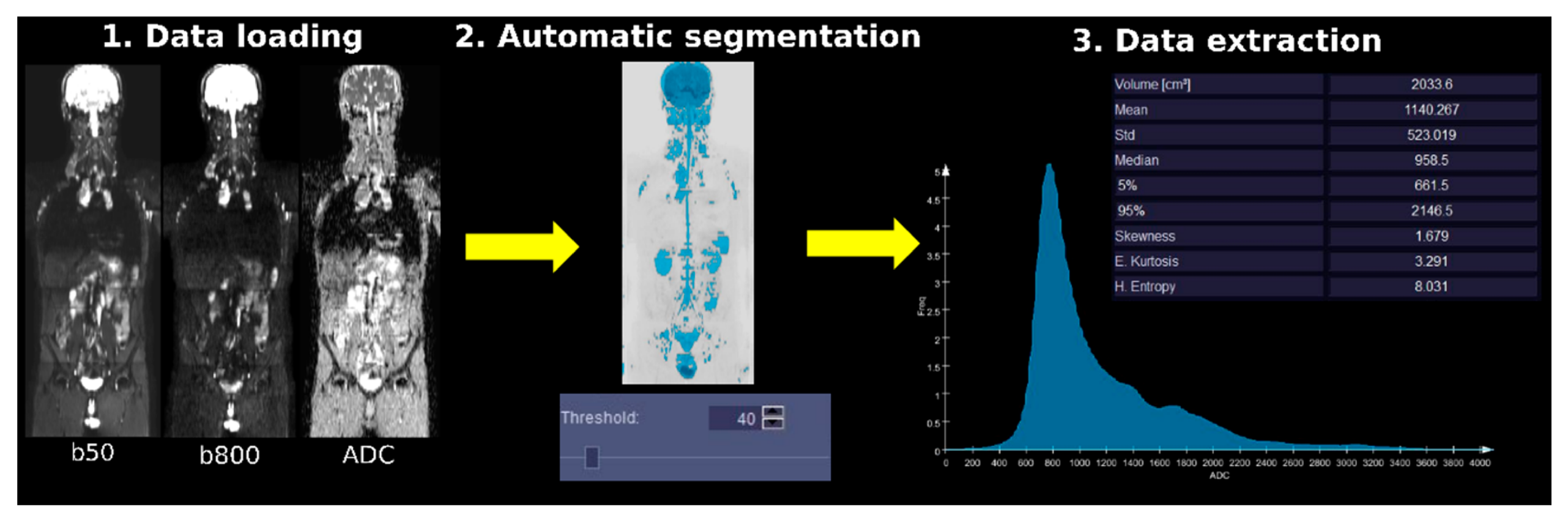
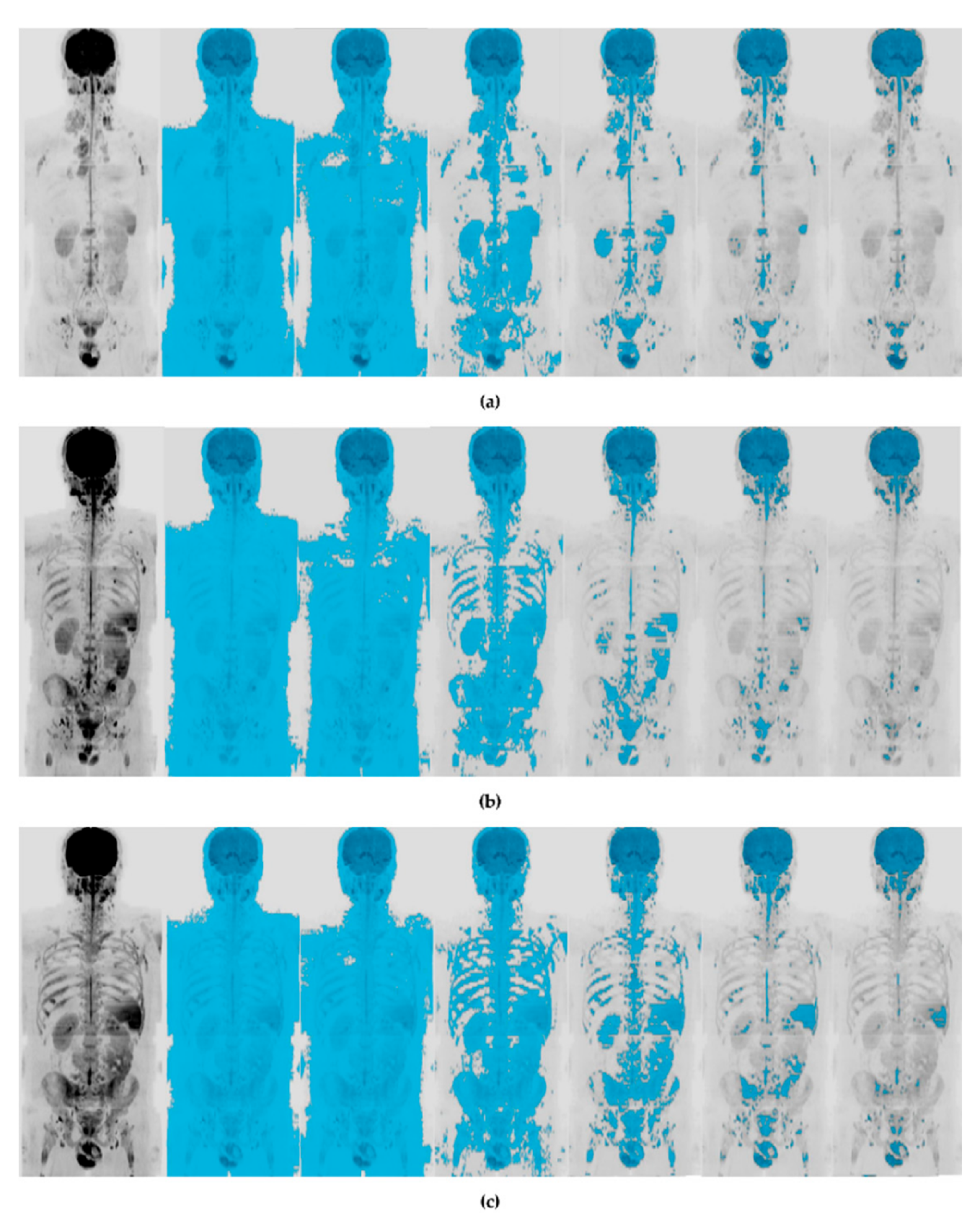
2.4. WB-DWI Image Analysis and Data Extraction
2.5. Statistical Analysis
3. Results
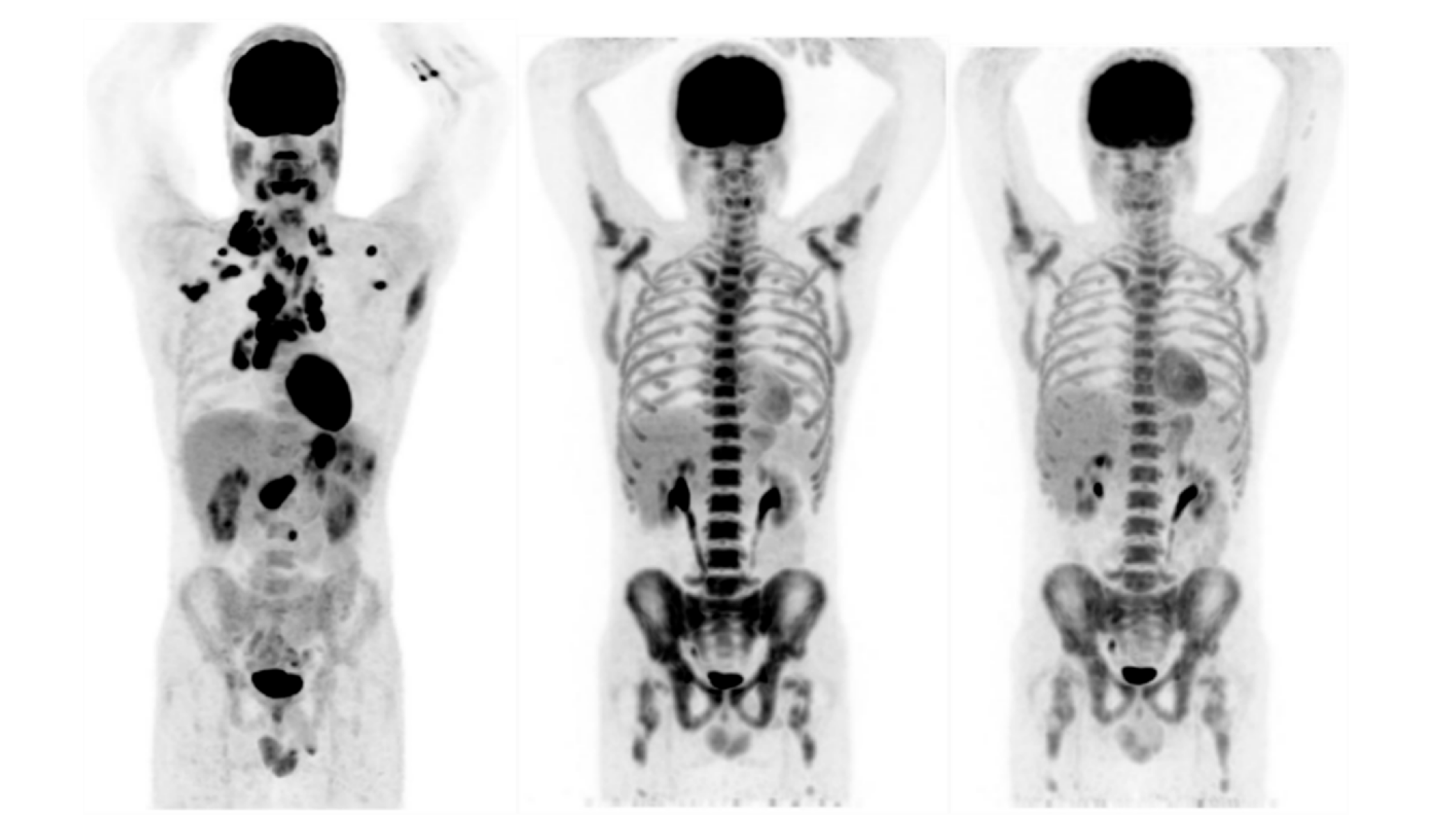
3.1. Response to Therapy (Lugano Assessment)
3.2. Image Analysis
3.3. Prediction of Response to Treatment
3.4. Assessment of Response to Treatment
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Vadakara, J.; Andrick, B. Current advances in Hodgkin’s lymphoma. Chronic Dis. Transl. Med. 2019, 5, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.-W.; Balakrishna, J.P.; Pittaluga, S.; Jaffe, E.S. Diagnosis of Hodgkin lymphoma in the modern era. Br. J. Haematol. 2018, 184, 45–59. [Google Scholar] [CrossRef] [PubMed]
- Bröckelmann, P.J.; Eichenauer, D.A.; Jakob, T.; Follmann, M.; Engert, A.; Skoetz, N. Hodgkin Lymphoma in Adults. Dtsch. Aerzteblatt Online 2018, 115, 535–540. [Google Scholar] [CrossRef]
- Cheson, B.D.; Fisher, R.I.; Barrington, S.; Cavalli, F.; Schwartz, L.H.; Zucca, E.; Lister, T.A. Recommendations for Initial Evaluation, Staging, and Response Assessment of Hodgkin and Non-Hodgkin Lymphoma: The Lugano Classification. J. Clin. Oncol. 2014, 32, 3059–3067. [Google Scholar] [CrossRef] [PubMed]
- Wang, X. PET/CT: Appropriate application in lymphoma. Chin. Clin. Oncol. 2015, 4, 4. [Google Scholar]
- Kulkarni, N.M.; Pinho, D.F.; Narayanan, S.; Kambadakone, A.R.; Abramson, J.S.; Sahani, D.V. Imaging for Oncologic Response Assessment in Lymphoma. Am. J. Roentgenol. 2017, 208, 18–31. [Google Scholar] [CrossRef]
- Cheson, B.D. Staging and response assessment in lymphomas: The new Lugano classification. Chin. Clin. Oncol. 2015, 4, 9. [Google Scholar]
- Fitzpatrick, J.J.; Ryan, M.A.; Bruzzi, J.F. Diagnostic accuracy of diffusion-weighted imaging- magnetic resonance imaging compared to positron emission tomography/computed tomography in evaluating and assessing pathological response to treatment in adult patients with lymphoma: A systematic review. J. Med. Imaging Radiat. Oncol. 2018, 62, 530–539. [Google Scholar] [CrossRef]
- Van Heertum, R.L.; Scarimbolo, R.; Wolodzko, J.G.; Klencke, B.; Messmann, R.; Tunc, F.; Sokol, L.; Agarwal, R.; Strafaci, J.A.; O’Neal, M. Lugano 2014 criteria for assessing FDG-PET/CT in lymphoma: An operational approach for clinical trials. Drug Des. Dev. Ther. 2017, 11, 1719–1728. [Google Scholar] [CrossRef]
- Grimm, R.; Padhani, A.R. Whole-body Diffusion-weighted MR Image Analysis with syngo.via Frontier MR Total Tumor. Magn. Flash 2017, 68, 73–75. [Google Scholar]
- Padhani, A.R. Observing Endocrine Therapy Resistance in Metastatic Breast Cancer with Whole-body MRI. Magn. Flash 2017, 68, 80–83. [Google Scholar]
- Dalili, D.; Padhani, A.R.; Grimm, R. Quantitative WB-MRI with ADC Histogram Analysis for Response Assessment in Diffuse Bone Disease. Magn. Flash 2017, 69, 32–37. [Google Scholar]
- Padhani, A.R.; Tunariu, N. Metastatic Prostate Cancer in Practice—The MET-RADS-P Imaging Response System Using Whole-body MRI. Magn. Flash 2017, 68, 64–72. [Google Scholar]
- Tsiflikas, I. MR Total Tumor Load—First Clinical Experience in Pediatric Oncology Patients. Magn. Flash 2019, 73, 72–76. [Google Scholar]
- Martinez-Möller, A.; Souvatzoglou, M.; Delso, G.; Bundschuh, R.A.; Chefd’Hotel, C.; Ziegler, S.; Navab, N.; Schwaiger, M.; Nekolla, S.G. Tissue Classification as a Potential Approach for Attenuation Correction in Whole-Body PET/MRI: Evaluation with PET/CT Data. J. Nucl. Med. 2009, 50, 520–526. [Google Scholar] [CrossRef] [PubMed]
- Blackledge, M.; Collins, D.J.; Tunariu, N.; Orton, M.R.; Padhani, A.R.; Leach, M.O.; Koh, D.-M. Assessment of Treatment Response by Total Tumor Volume and Global Apparent Diffusion Coefficient Using Diffusion-Weighted MRI in Patients with Metastatic Bone Disease: A Feasibility Study. PLoS ONE 2014, 9, e91779. [Google Scholar] [CrossRef]
- Toledano-Massiah, S.; Luciani, A.; Itti, E.; Zerbib, P.; Vignaud, A.; Belhadj, K.; Baranes, L.; Haioun, C.; Lin, C.; Rahmouni, A. Whole-Body Diffusion-weighted Imaging in Hodgkin Lymphoma and Diffuse Large B-Cell Lymphoma. Radiographics 2015, 35, 747–764. [Google Scholar] [CrossRef] [PubMed]
- Littooij, A.S.; Kwee, T.C.; De Keizer, B.; Bruin, M.C.; Coma, A.; Beek, F.J.; Fijnheer, R.; Nievelstein, R.A.J. Whole-body MRI-DWI for assessment of residual disease after completion of therapy in lymphoma: A prospective multicenter study. J. Magn. Reson. Imaging 2015, 42, 1646–1655. [Google Scholar] [CrossRef]
- Stecco, A.; Buemi, F.; Iannessi, A.; Carriero, A.; Gallamini, A. Current concepts in tumor imaging with whole-body MRI with diffusion imaging (WB-MRI-DWI) in multiple myeloma and lymphoma. Leuk. Lymphoma 2018, 59, 2546–2556. [Google Scholar] [CrossRef] [PubMed]
- Kwee, T.C.; Basu, S.; Torigian, E.A.; Nievelstein, R.A.J.; Alavi, A. Evolving Importance of Diffusion-Weighted Magnetic Resonance Imaging in Lymphoma. PET Clin. 2012, 7, 73–82. [Google Scholar] [CrossRef]
- Koh, D.-M.; Collins, D.J. Diffusion-Weighted MRI in the Body: Applications and Challenges in Oncology. Am. J. Roentgenol. 2007, 188, 1622–1635. [Google Scholar] [CrossRef] [PubMed]
- Kwee, T.C.; Takahara, T.; Ochiai, R.; Koh, D.-M.; Ohno, Y.; Nakanishi, K.; Niwa, T.; Chenevert, T.L.; Luijten, P.R.; Alavi, A. Complementary Roles of Whole-Body Diffusion-Weighted MRI and 18F-FDG PET: The State of the Art and Potential Applications. J. Nucl. Med. 2010, 51, 1549–1558. [Google Scholar] [CrossRef] [PubMed]
- Sadik, M.; Lind, E.; Polymeri, E.; Enqvist, O.; Ulén, J.; Trägårdh, E. Automated quantification of reference levels in liver and mediastinal blood pool for the Deauville therapy response classification using FDG-PET/CT in Hodgkin and non-Hodgkin lymphomas. Clin. Physiol. Funct. Imaging 2018, 39, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Norvell, D.C. Study types and bias—Don’t judge a study by the abstract’s conclusion alone. Evid. Based Spine-Care J. 2010, 1, 7–10. [Google Scholar] [CrossRef] [PubMed]
- Cottereau, A.-S.; El-Galaly, T.C.; Becker, S.; Broussais, F.; Petersen, L.J.; Bonnet, C.; Prior, J.O.; Tilly, H.; Hutchings, M.; Casasnovas, O.; et al. Predictive Value of PET Response Combined with Baseline Metabolic Tumor Volume in Peripheral T-Cell Lymphoma Patients. J. Nucl. Med. 2017, 59, 589–595. [Google Scholar] [CrossRef]
- Cottereau, A.-S.; Hapdey, S.; Chartier, L.; Modzelewski, R.; Casasnovas, O.; Itti, E.; Tilly, H.; Vera, P.; Meignan, M.; Becker, S. Baseline Total Metabolic Tumor Volume Measured with Fixed or Different Adaptive Thresholding Methods Equally Predicts Outcome in Peripheral T Cell Lymphoma. J. Nucl. Med. 2016, 58, 276–281. [Google Scholar] [CrossRef]
- Moia, R.; Favini, C.; Rasi, S.; Deambrogi, C.; Ferri, V.; Schipani, M.; Sagiraju, S.; Mahmoud, A.M.; Kodipad, A.A.; Adhinaveni, R.; et al. Liquid biopsy in lymphomas: A potential tool for refining diagnosis and disease monitoring. J. Cancer Metastasis Treat. 2019, 2019. [Google Scholar] [CrossRef]
- Rossi, D.; Spina, V.; Bruscaggin, A.; Gaidano, G. Liquid biopsy in lymphoma. Haematologica 2019, 104, 648–652. [Google Scholar] [CrossRef]
- Zanfardino, M.; Franzese, M.; Pane, K.; Cavaliere, C.; Monti, S.; Esposito, G.; Salvatore, M.; Aiello, M. Bringing radiomics into a multi-omics framework for a comprehensive genotype-phenotype characterization of oncological diseases. J. Transl. Med. 2019, 17, 337. [Google Scholar] [CrossRef]
| Variable | Value |
|---|---|
| No. of patients (n) | 20 |
| Age (y) | |
| Mean ± SD | 35.7 ± 11.7 |
| Range | 19–63 |
| Gender (n (%)) | |
| Male | 11 (55) |
| Female | 9 (45) |
| HL Subtype (WHO classification) (n (%)) | |
| Nodular sclerosis | 13 (65) |
| Mixed cellularity | 4 (20) |
| Lymphocyte rich | 2 (10) |
| Lymphocyte depleted | 1 (5) |
| B symptoms (n (%)) | |
| Fever | 14 (70) |
| Sweats | 9 (45) |
| Weight loss ≥ 10% | 6 (30) |
| Histology (n (%)) | |
| Stage | |
| I | 2 (10) |
| II | 11 (55) |
| III | 4 (20) |
| IV | 3 (15) |
| Erythrocyte sedimentation rate ≥ 50 mm | 2 (10) |
| Sequence | Orientation | TR (ms) | TE (ms) | ST (mm) | TI (mm) | b-Values (s/mm2) |
|---|---|---|---|---|---|---|
| T2 TIRM | Coronal | 4500 | 84 | 5 | 220 | |
| DWI | Axial | 78 | 6 | 220 | 50,800 | |
| T2 HASTE | Axial and Coronal | 1400 | 89 | 6 | ||
| T1 GRE | Axial | 94 | 2.46 | 5 |
| Lugano Assessment | Response to Therapy at Interim (T1) (n (%)) | Response to Therapy at the EOT (T2) (n (%)) |
|---|---|---|
| CMR | 14 (70) | 15 (75) |
| PMR | 6 (30) | 3 (15) |
| SMD | 0 (0) | 0 (0) |
| PMD | 0 (0) | 2 (10) |
| Parameters a | Median (IQR)—CMR | Median (IQR)—PMR | p | AUC | T | Sen (%) | Spec (%) | Acc (%) |
|---|---|---|---|---|---|---|---|---|
(cm3) | 2033.6 (1907.5–2087.75) | 2446.1 (2439.88–2542.55) | 0.017 | 0.93 | 2342.15 | 100 | 93 | 94 |
(cm3) | 1525.7 (1440.48–1556.23) | 1667.4 (1626.23–1733.4) | 0.01 | 0.96 | 1604.6 | 100 | 93 | 94 |
(×10−6 mm2/s) | 634.5 (607.5–634.5) | 580.5 (540–583.88) | 0.037 | 0.89 | 596.25 | 80 | 100 | 83 |
(×10−6 mm2/s) | 2686.5 (2416.5–2902.5) | 2335.5 (2254.5–2376) | 0.032 | 0.91 | 2403 | 85 | 100 | 88 |
| Parameters a | Median (IQR)—CMR | Median (IQR)—PMR | p | AUC | T | Sen (%) | Spec (%) | Acc (%) |
|---|---|---|---|---|---|---|---|---|
(cm3) | 1916.35 (1846.85–2084.55) | 2311.45 (2235.5–2446.7) | 0.005 | 0.90 | 2116.5 | 100 | 83 | 89 |
(×10−6 mm2/s) | 1311.58 (1252.74–1356.32) | 1221.34 (1111.06–1240.01) | 0.009 | 0.88 | 1243.85 | 83 | 83 | 83 |
(×10−6 mm2/s) | 695.49 (644.57–737.93) | 609.96 (577.22–635.21) | 0.009 | 0.88 | 656.93 | 75 | 100 | 83 |
(×10−6 mm2/s) | 2686.5 (2497.5–2902.5) | 2281.5 (2227.5–2389.5) | 0.001 | 0.93 | 2403 | 92 | 100 | 94 |
(×10−6 mm2/s) | 2646 (2349–2796.75) | 2214 (2093–2335.5) | 0.013 | 0.86 | 2362.5 | 75 | 83 | 78 |
(×10−6 mm2/s) | 8.49 (8.4–8.54) | 8.37 (8.3–8.47) | 0.024 | 0.83 | 8.48 | 67 | 100 | 78 |
(%) | 4.85 (1.87–5.66) | −4.79 (−9.43 to −2.67) | 0.006 | 0.92 | 0.81 | 83 | 100 | 88 |
(%) | 2.69 (−0.08 to 5.12) | −7.38 (−10.08 to −0.04) | 0.036 | 0.83 | 0.1 | 75 | 80 | 76 |
(%) | 8.47 (−2.99 to 12.54) | −10.37 (−14.92 to −5.93) | 0.013 | 0.88 | −6.79 | 83 | 80 | 82 |
(%) | 7.76 (−0.09 to 12) | −9.52 (−21.81 to −2.84) | 0.019 | 0.87 | −2.05 | 83 | 100 | 88 |
(%) | 0.44 (−1.41 to 3.49) | −2.72 (−8.86 to −2.36) | 0.045 | 0.82 | −2.09 | 0.83 | 0.8 | 0.82 |
(%) | 2.8 (−5.56 to 6.43) | −18.43 (−20.71 to −6.46) | 0.013 | 0.88 | −5.52 | 75 | 80 | 76 |
(%) | 4.65 (−3.64 to 13.14) | −10.33 (−24.45 to −1.82) | 0.02 | 0.86 | −1.21 | 75 | 80 | 76 |
(%) | 1.95 (−4.34 to 6.81) | −3.85 (−15.68 to 0.31) | 0.048 | 0.82 | 1.28 | 0.75 | 1 | 0.82 |
(%) | 0.32 (−0.04 to 0.56) | −0.53 (−1.24 to −0.19) | 0.013 | 0.88 | −0.27 | 92 | 8 | 88 |
(%) | 0.27 (0.16–0.56) | −0.51 (−1.16 to −0.03) | 0.019 | 0.87 | 0.02 | 83 | 80 | 82 |
(%) | 0.71 (−0.27 to 1.21) | –0.83 (−2.16 to −0.39) | 0.009 | 0.90 | −0.03 | 75 | 100 | 82 |
(%) | 0.88 (−0.18 to 1.36) | –1.42 (−1.65 to −0.92) | 0.013 | 0.88 | −1.15 | 92 | 80 | 88 |
| Parameters a | Median (IQR)—CMR | Median (IQR)—PMR | p | AUC | T | Sen (%) | Spec (%) | Acc (%) |
|---|---|---|---|---|---|---|---|---|
(×10−6 mm2/s) | 1103.54 (1061.37–1159.35) | 1211.08 (1180.53–1227.21) | 0.014 | 0.95 | 1169.09 | 100 | 85 | 88 |
(×10−6 mm2/s) | 1066.5 (1034.44–1174.5) | 1228.5 (1208.25–1289.25) | 0.01 | 0.96 | 1188 | 100 | 92 | 94 |
(%) | 15.04 (4.49–34.57) | −10.05 (−22.72 to −1.69) | 0.025 | 0.92 | 4.06 | 77 | 100 | 81 |
(%) | −2.09 (−4.8 to 2.38) | 4.6 (2.99–4.91) | 0.039 | 0.9 | 2.36 | 100 | 77 | 81 |
(%) | −0.84 (−5.82 to 4.66) | –23.12 (−28.21 to −10.25) | 0.025 | 0.92 | −5.79 | 77 | 100 | 81 |
(%) | −4.26 (−6.69 to 0.91) | 4.26 (4.26–7.26) | 0.014 | 0.95 | 4.17 | 100 | 92 | 94 |
(%) | −1.79 (−4.5 to 1.22) | 6.35 (5.09–10.09) | 0.017 | 0.94 | 4.21 | 100 | 92 | 93 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brancato, V.; Aiello, M.; Della Pepa, R.; Basso, L.; Garbino, N.; Nicolai, E.; Picardi, M.; Salvatore, M.; Cavaliere, C. Automatic Prediction and Assessment of Treatment Response in Patients with Hodgkin’s Lymphoma Using a Whole-Body DW-MRI Based Approach. Diagnostics 2020, 10, 702. https://doi.org/10.3390/diagnostics10090702
Brancato V, Aiello M, Della Pepa R, Basso L, Garbino N, Nicolai E, Picardi M, Salvatore M, Cavaliere C. Automatic Prediction and Assessment of Treatment Response in Patients with Hodgkin’s Lymphoma Using a Whole-Body DW-MRI Based Approach. Diagnostics. 2020; 10(9):702. https://doi.org/10.3390/diagnostics10090702
Chicago/Turabian StyleBrancato, Valentina, Marco Aiello, Roberta Della Pepa, Luca Basso, Nunzia Garbino, Emanuele Nicolai, Marco Picardi, Marco Salvatore, and Carlo Cavaliere. 2020. "Automatic Prediction and Assessment of Treatment Response in Patients with Hodgkin’s Lymphoma Using a Whole-Body DW-MRI Based Approach" Diagnostics 10, no. 9: 702. https://doi.org/10.3390/diagnostics10090702
APA StyleBrancato, V., Aiello, M., Della Pepa, R., Basso, L., Garbino, N., Nicolai, E., Picardi, M., Salvatore, M., & Cavaliere, C. (2020). Automatic Prediction and Assessment of Treatment Response in Patients with Hodgkin’s Lymphoma Using a Whole-Body DW-MRI Based Approach. Diagnostics, 10(9), 702. https://doi.org/10.3390/diagnostics10090702








