Semi-Automated Segmentation of Bone Metastases from Whole-Body MRI: Reproducibility of Apparent Diffusion Coefficient Measurements
Abstract
1. Introduction
2. Materials and Methods
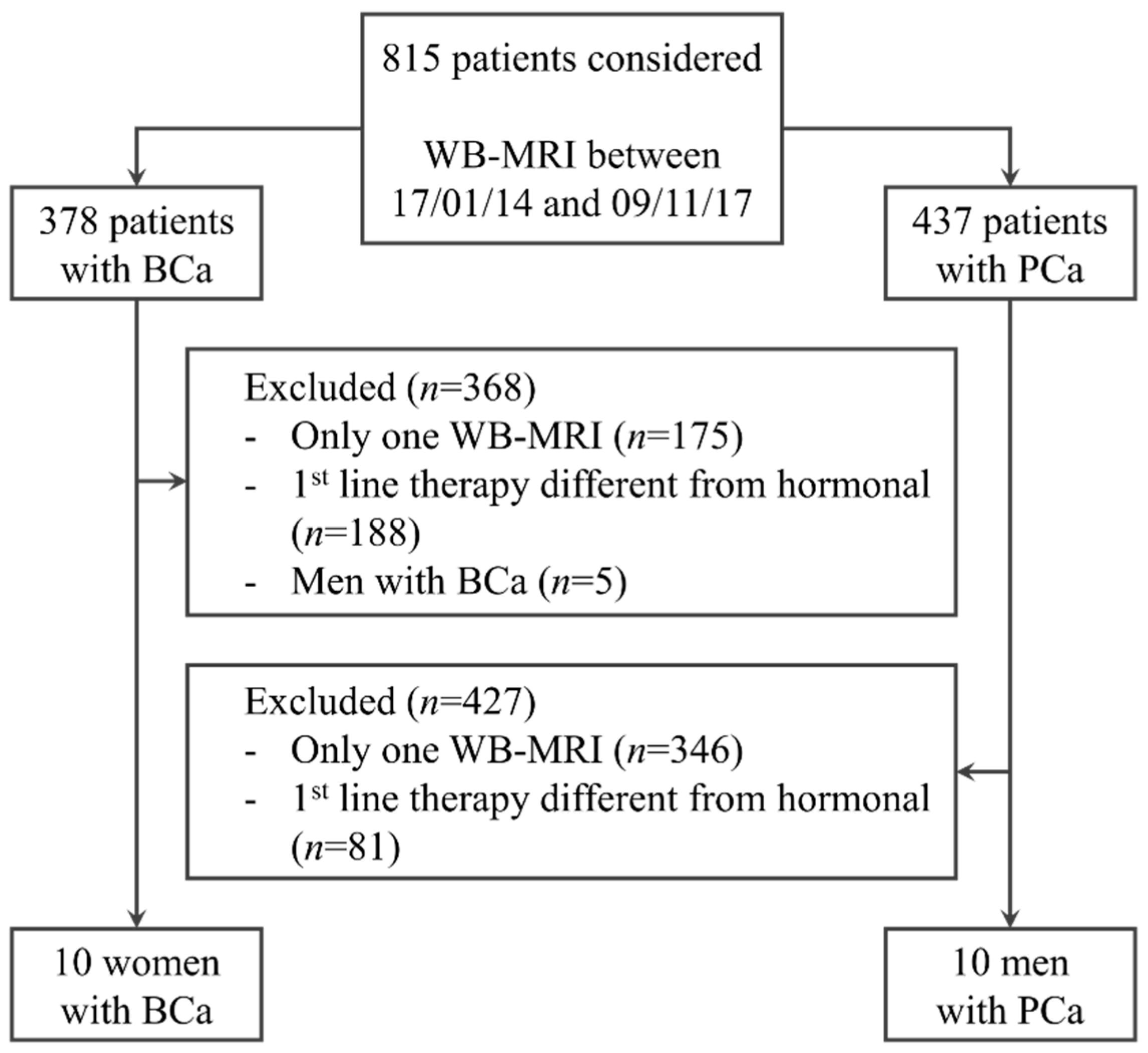
2.1. Population
2.2. WB-MRI Acquisition Protocol
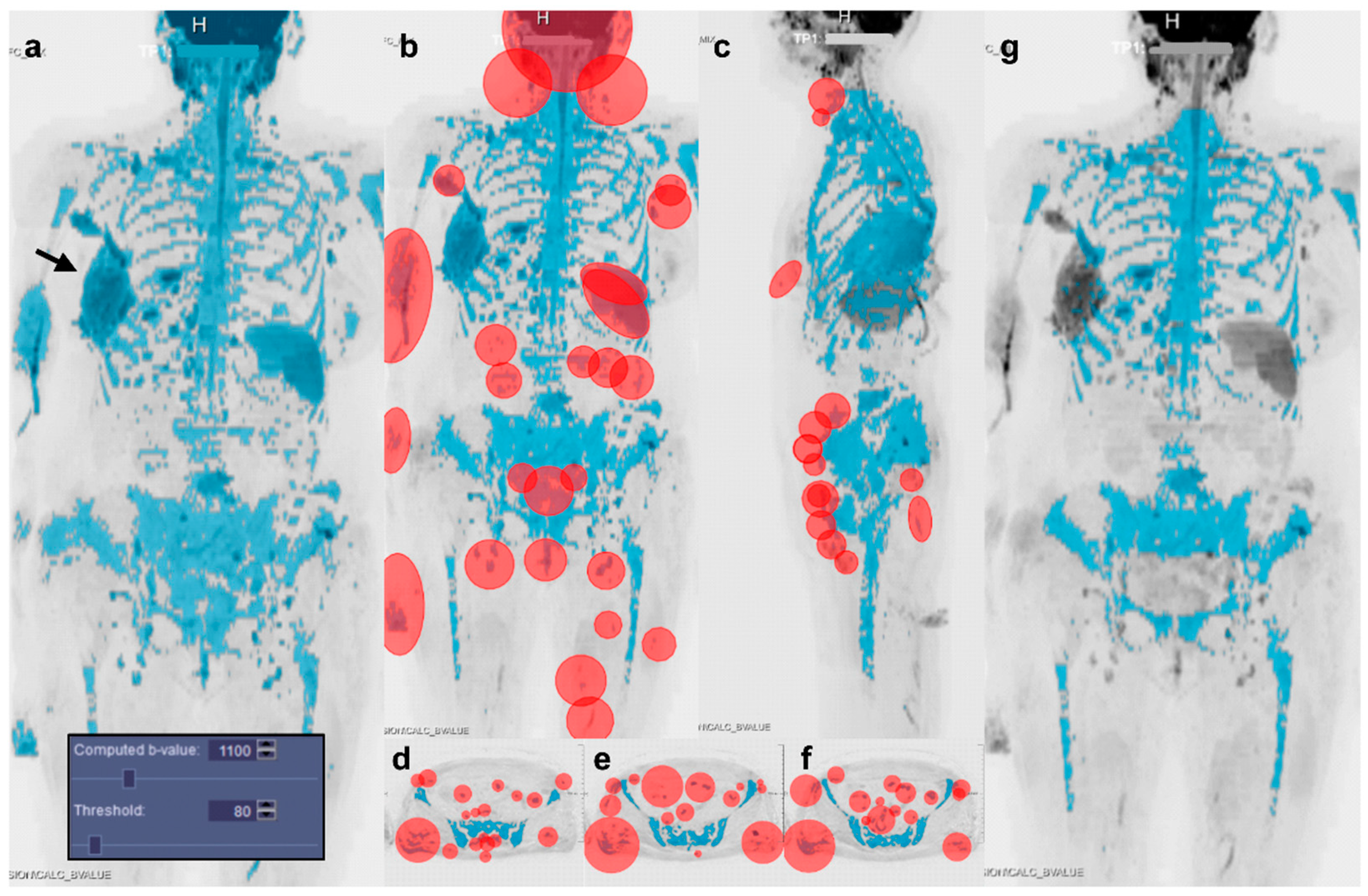
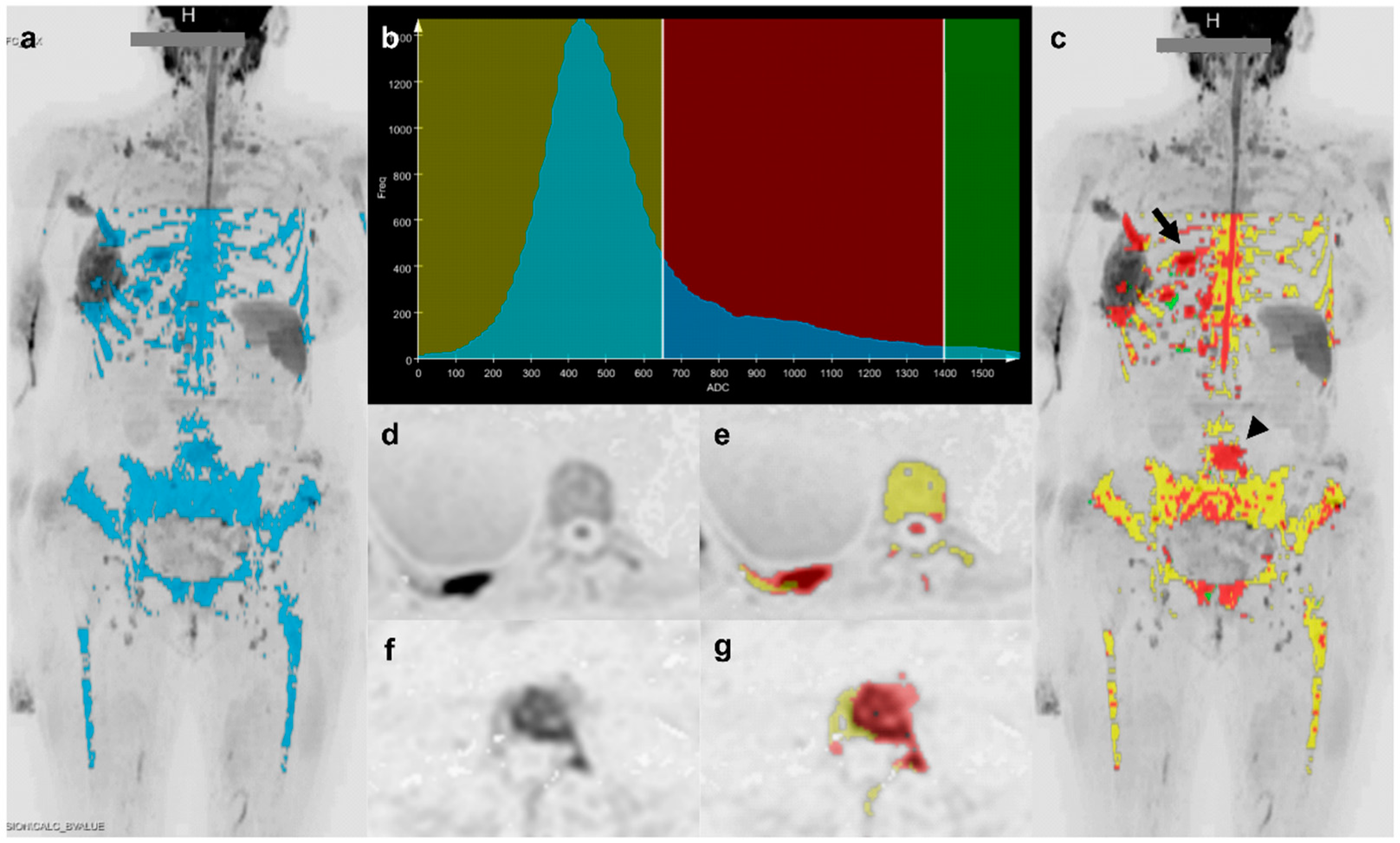
2.3. Image Segmentation
2.4. Observers
2.5. Statistical Analysis
3. Results
3.1. Population
3.2. Segmentation Settings and Duration
3.3. Factors Influencing Segmentation Similarity
3.4. Distribution of Quantitative Parameters Values
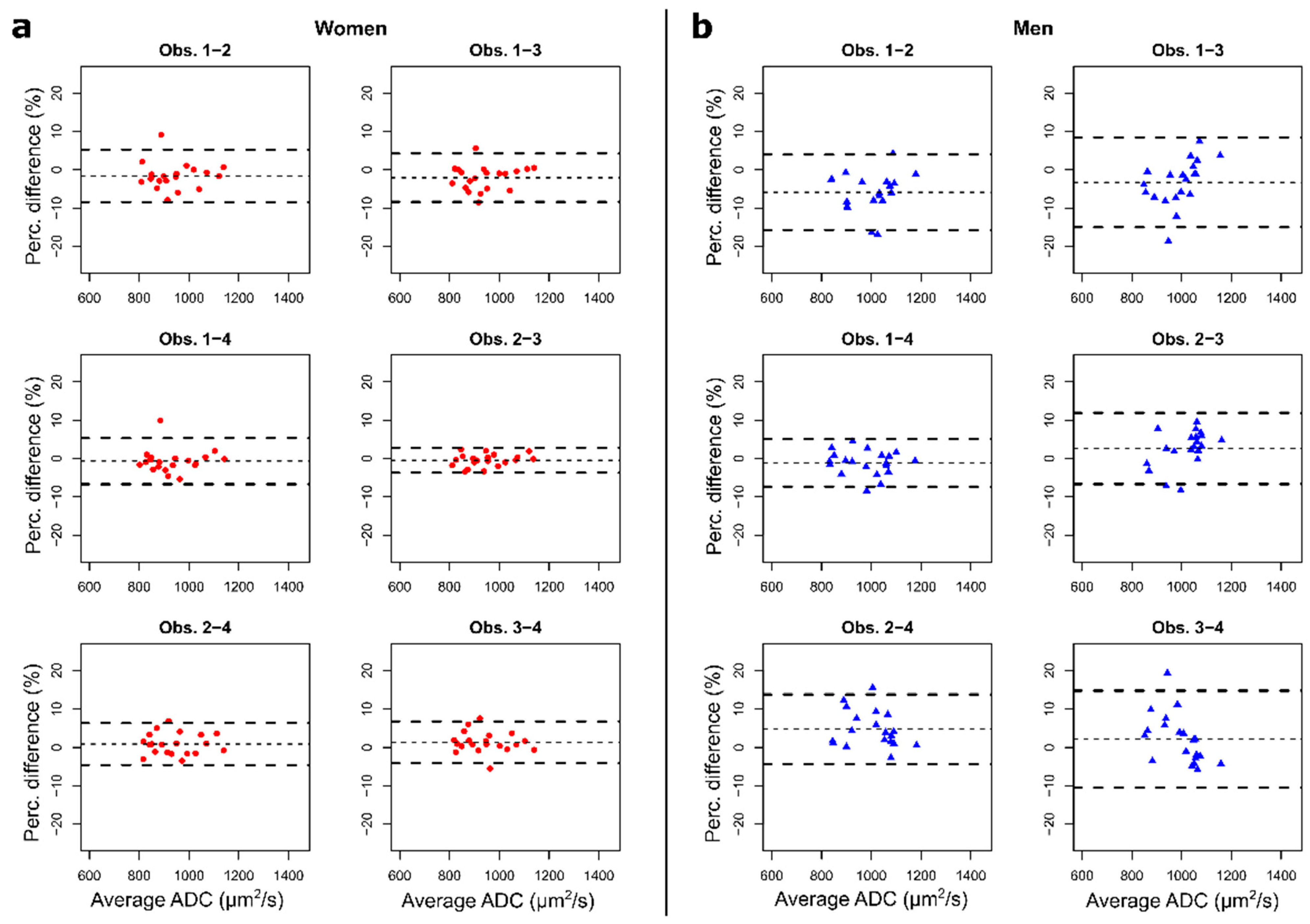
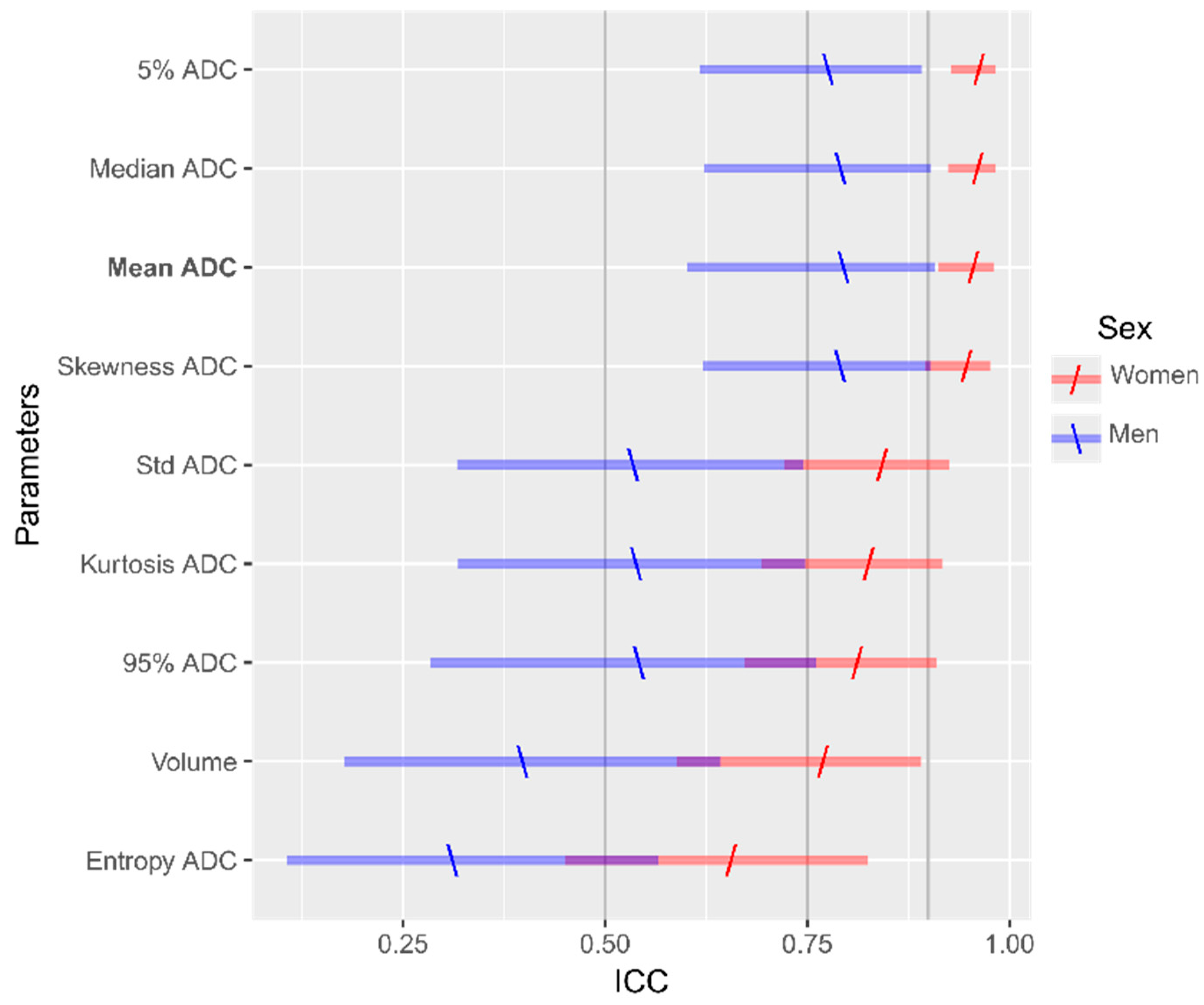
3.5. Intra- and Inter-Observer Reproducibility Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Roodman, G.D. Mechanisms of Bone Metastasis. N. Engl. J. Med. 2004, 350, 1655–1664. [Google Scholar] [CrossRef]
- Padhani, A.R.; Gogbashian, A. Bony metastases: Assessing response to therapy with whole-body diffusion MRI. Cancer Imaging 2011, 11, S129–S154. [Google Scholar] [CrossRef] [PubMed]
- Zugni, F.; Ruju, F.; Pricolo, P.; Alessi, S.; Iorfida, M.; Colleoni, M.A.; Bellomi, M.; Petralia, G. The added value of whole-body magnetic resonance imaging in the management of patients with advanced breast cancer. PLoS ONE 2018, 13, e0205251. [Google Scholar] [CrossRef] [PubMed]
- Eisenhauer, E.A.; Therasse, P.; Bogaerts, J.; Schwartz, L.H.; Sargent, D.; Ford, R.; Dancey, J.; Arbuck, S.; Gwyther, S.; Mooney, M.; et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur. J. Cancer 2009, 45, 228–247. [Google Scholar] [CrossRef] [PubMed]
- Lecouvet, F.E.; Larbi, A.; Pasoglou, V.; Omoumi, P.; Tombal, B.; Michoux, N.; Malghem, J.; Lhommel, R.; Vande Berg, B.C. MRI for response assessment in metastatic bone disease. Eur. Radiol. 2013, 23, 1986–1997. [Google Scholar] [CrossRef]
- Padhani, A.R.; Lecouvet, F.E.; Tunariu, N.; Koh, D.-M.; De Keyzer, F.; Collins, D.J.; Sala, E.; Schlemmer, H.P.; Petralia, G.; Vargas, H.A.; et al. METastasis Reporting and Data System for Prostate Cancer: Practical Guidelines for Acquisition, Interpretation, and Reporting of Whole-body Magnetic Resonance Imaging-based Evaluations of Multiorgan Involvement in Advanced Prostate Cancer. Eur. Urol. 2017, 71, 81–92. [Google Scholar] [CrossRef]
- Petralia, G.; Padhani, A.R. Whole-Body Magnetic Resonance Imaging in Oncology: Uses and Indications. Magn. Reson. Imaging Clin. N. Am. 2018, 26, 495–507. [Google Scholar] [CrossRef]
- Pricolo, P.; Ancona, E.; Summers, P.; Abreu-Gomez, J.; Alessi, S.; Jereczek-Fossa, B.A.; De Cobelli, O.; Nolè, F.; Renne, G.; Bellomi, M.; et al. Whole-body magnetic resonance imaging (WB-MRI) reporting with the METastasis Reporting and Data System for Prostate Cancer (MET-RADS-P): Inter-observer agreement between readers of different expertise levels. Cancer Imaging 2020, 20, 77. [Google Scholar] [CrossRef]
- Pickles, M.D.; Gibbs, P.; Lowry, M.; Turnbull, L.W. Diffusion changes precede size reduction in neoadjuvant treatment of breast cancer. Magn. Reson. Imaging 2006, 24, 843–847. [Google Scholar] [CrossRef]
- Galbán, C.J.; Hoff, B.A.; Chenevert, T.L.; Ross, B.D. Diffusion MRI in early cancer therapeutic response assessment. NMR Biomed. 2017, 30, e3458. [Google Scholar] [CrossRef]
- Le Bihan, D. Apparent Diffusion Coefficient and Beyond: What Diffusion MR Imaging Can Tell Us about Tissue Structure. Radiology 2013, 268, 318–322. [Google Scholar] [CrossRef] [PubMed]
- Li, S.P.; Padhani, A.R. Tumor response assessments with diffusion and perfusion MRI. J. Magn. Reson. Imaging 2012, 35, 745–763. [Google Scholar] [CrossRef] [PubMed]
- Ahlawat, S.; Fayad, L.M. Diffusion weighted imaging demystified: The technique and potential clinical applications for soft tissue imaging. Skelet. Radiol. 2018, 47, 313–328. [Google Scholar] [CrossRef] [PubMed]
- Dietrich, O.; Geith, T.; Reiser, M.F.; Baur-Melnyk, A. Diffusion imaging of the vertebral bone marrow. NMR Biomed. 2017, 30, e3333. [Google Scholar] [CrossRef] [PubMed]
- Park, G.E.; Jee, W.-H.; Lee, S.-Y.; Sung, J.-K.; Jung, J.-Y.; Grimm, R.; Son, Y.; Paek, M.Y.; Min, C.-K.; Ha, K.-Y. Differentiation of multiple myeloma and metastases: Use of axial diffusion-weighted MR imaging in addition to standard MR imaging at 3T. PLoS ONE 2018, 13, e0208860. [Google Scholar] [CrossRef] [PubMed]
- Padhani, A.R.; Koh, D.M.; Collins, D.J. Whole-body diffusion-weighted MR imaging in cancer: Current status and research directions. Radiology 2011, 261, 700–718. [Google Scholar] [CrossRef]
- Petralia, G.; Padhani, A.R.; Pricolo, P.; Zugni, F.; Martinetti, M.; Summers, P.E.; Grazioli, L.; Colagrande, S.; Giovagnoni, A.; Bellomi, M. Whole-body magnetic resonance imaging (WB-MRI) in oncology: Recommendations and key uses. Radiol. Med. 2019, 124, 218–233. [Google Scholar] [CrossRef]
- Barnes, A.; Alonzi, R.; Blackledge, M.; Charles-Edwards, G.; Collins, D.J.; Cook, G.; Coutts, G.; Goh, V.; Graves, M.; Kelly, C.; et al. UK quantitative WB-DWI technical workgroup: Consensus meeting recommendations on optimisation, quality control, processing and analysis of quantitative whole-body diffusion-weighted imaging for cancer. Br. J. Radiol. 2018, 91, 20170577. [Google Scholar] [CrossRef]
- Winfield, J.M.; Tunariu, N.; Rata, M.; Miyazaki, K.; Jerome, N.P.; Germuska, M.; Blackledge, M.D.; Collins, D.J.; de Bono, J.S.; Yap, T.A.; et al. Extracranial Soft-Tissue Tumors: Repeatability of Apparent Diffusion Coefficient Estimates from Diffusion-weighted MR Imaging. Radiology 2017, 284, 88–99. [Google Scholar] [CrossRef] [PubMed]
- Blackledge, M.D.; Tunariu, N.; Orton, M.R.; Padhani, A.R.; Collins, D.J.; Leach, M.O.; Koh, D.-M. Inter- and Intra-Observer Repeatability of Quantitative Whole-Body, Diffusion-Weighted Imaging (WBDWI) in Metastatic Bone Disease. PLoS ONE 2016, 11, e0153840. [Google Scholar] [CrossRef] [PubMed]
- Reischauer, C.; Froehlich, J.M.; Koh, D.M.; Graf, N.; Padevit, C.; John, H.; Binkert, C.A.; Boesiger, P.; Gutzeit, A. Bone metastases from prostate cancer: Assessing treatment response by using diffusion-weighted imaging and functional diffusion maps - Initial observations. Radiology 2010, 257, 523–531. [Google Scholar] [CrossRef]
- Blackledge, M.D.; Collins, D.J.; Tunariu, N.; Orton, M.R.; Padhani, A.R.; Leach, M.O.; Koh, D.-M. Assessment of Treatment Response by Total Tumor Volume and Global Apparent Diffusion Coefficient Using Diffusion-Weighted MRI in Patients with Metastatic Bone Disease: A Feasibility Study. PLoS ONE 2014, 9, e91779. [Google Scholar] [CrossRef]
- Perez-Lopez, R.; Lorente, D.; Blackledge, M.D.; Collins, D.J.; Mateo, J.; Bianchini, D.; Omlin, A.; Zivi, A.; Leach, M.O.; De Bono, J.S.; et al. Volume of bone metastasis assessed with whole-Body Diffusion-weighted imaging is associated with overall survival in metastatic castration-resistant prostate cancer. Radiology 2016, 280, 151–160. [Google Scholar] [CrossRef]
- Perez-Lopez, R.; Mateo, J.; Mossop, H.; Blackledge, M.D.; Collins, D.J.; Rata, M.; Morgan, V.A.; Macdonald, A.; Sandhu, S.; Lorente, D.; et al. Diffusion-weighted imaging as a treatment response biomarker for evaluating bone metastases in prostate cancer: A pilot study. Radiology 2017, 283, 168–177. [Google Scholar] [CrossRef] [PubMed]
- Blackledge, M.D.; Leach, M.O.; Collins, D.J.; Koh, D.M. Computed diffusion-weighted MR imaging may improve tumor detection. Radiology 2011, 261, 573–581. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Xue, H.; Alto, S.; Hui, L.; Kannengiesser, S.; Berthold, K.; Jin, Z. Integrated Shimming Improves Lesion Detection in Whole-Body Diffusion-Weighted Examinations of Patients With Plasma Disorder at 3 T. Investig. Radiol. 2016, 51, 297–305. [Google Scholar] [CrossRef][Green Version]
- Padhani, A.R.; Van Ree, K.; Collins, D.J.; D’Sa, S.; Makris, A. Assessing the relation between bone marrow signal intensity and apparent diffusion coefficient in diffusion-weighted MRI. Am. J. Roentgenol. 2013, 200, 163–170. [Google Scholar] [CrossRef] [PubMed]
- Messiou, C.; Collins, D.J.; Morgan, V.A.; Desouza, N.M. Optimising diffusion weighted MRI for imaging metastatic and myeloma bone disease and assessing reproducibility. Eur. Radiol. 2011, 21, 1713–1718. [Google Scholar] [CrossRef]
- Lavdas, I.; Rockall, A.G.; Castelli, F.; Sandhu, R.S.; Papadaki, A.; Honeyfield, L.; Waldman, A.D.; Aboagye, E.O. Apparent Diffusion Coefficient of Normal Abdominal Organs and Bone Marrow from Whole-Body DWI at 1.5 T: The Effect of Sex and Age. Am. J. Roentgenol. 2015, 205, 242–250. [Google Scholar] [CrossRef]
- Messiou, C.; Collins, D.J.; Giles, S.; de Bono, J.S.; Bianchini, D.; de Souza, N.M. Assessing response in bone metastases in prostate cancer with diffusion weighted MRI. Eur. Radiol. 2011, 21, 2169–2177. [Google Scholar] [CrossRef]
- Zou, K.H.; Warfield, S.K.; Bharatha, A.; Tempany, C.M.C.; Kaus, M.R.; Haker, S.J.; Wells, W.M.; Jolesz, F.A.; Kikinis, R. Statistical Validation of Image Segmentation Quality Based on a Spatial Overlap Index. Acad. Radiol. 2004, 11, 178–189. [Google Scholar] [CrossRef]
- Bland, J.M.; Altman, D.G. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1986, 1, 307–310. [Google Scholar] [CrossRef]
- Bland, J.M.; Altman, D.G. Measuring agreement in method comparison studies. Stat. Methods Med. Res. 1999, 8, 135–160. [Google Scholar] [CrossRef]
- Shrout, P.E.; Fleiss, J.L. Intraclass correlations: Uses in assessing rater reliability. Psychol. Bull. 1979, 86, 420–428. [Google Scholar] [CrossRef]
- Messiou, C.; Hillengass, J.; Delorme, S.; Lecouvet, F.E.; Moulopoulos, L.A.; Collins, D.J.; Blackledge, M.D.; Abildgaard, N.; Østergaard, B.; Schlemmer, H.-P.; et al. Guidelines for Acquisition, Interpretation, and Reporting of Whole-Body MRI in Myeloma: Myeloma Response Assessment and Diagnosis System (MY-RADS). Radiology 2019, 291, 5–13. [Google Scholar] [CrossRef] [PubMed]
- Cui, F.-Z.; Cui, J.-L.; Wang, S.-L.; Yu, H.; Sun, Y.-C.; Zhao, N.; Cui, S.-J. Signal characteristics of normal adult bone marrow in whole-body diffusion-weighted imaging. Acta Radiol. 2016, 57, 1230–1237. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-Y.; Wu, C.-L.; Shen, S.-H. High Signal in Bone Marrow on Diffusion-Weighted Imaging of Female Pelvis: Correlation With Anemia and Fibroid-Associated Symptoms. J. Magn. Reson. Imaging 2018, 48, 1024–1033. [Google Scholar] [CrossRef] [PubMed]
- Dionísio, F.C.F.; Oliveira, L.S.; Hernandes, M.A.; Engel, E.E.; Rangayyan, R.M.; Azevedo-Marques, P.M.; Nogueira-Barbosa, M.H. Manual and semiautomatic segmentation of bone sarcomas on MRI have high similarity. Braz. J. Med. Biol. Res. 2020, 53, e8962. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Sun, P.; Hao, Q.; Yin, W.; Xu, B.; Ma, C.; Stemmer, A.; Fu, C.; Wang, M.; Lu, J. Diffusion-weighted MRI in the evaluation of the thyroid nodule: Comparison between integrated-shimming EPI and conventional 3D-shimming EPI techniques. Oncotarget 2018, 9, 26209–26216. [Google Scholar] [CrossRef] [PubMed][Green Version]
| Station-Specific Shim | Slice-Specific Shim | |
|---|---|---|
| Sequence | Diffusion-Weighted SS-EPI | Diffusion-Weighted SS-EPI |
| Orientation | Transversal | Transversal |
| b-value (s/mm2) | 50, 900 | 50, 900 |
| Encoding mode | 3-scan trace | 3D-diagonal |
| Averages per b-value | 6, 6 | 5, 15 |
| Repetition Time (ms) | 9000 | 6550 |
| Echo Time (ms) | 67 | 62 |
| Fat Saturation | STIR | STIR |
| Inversion Time (ms) | 180 | 180 |
| Field of View (mm) | 337 × 450 | 390 × 429 |
| Slice thickness (mm) | 5.0 | 5.0 |
| Gap between slices (mm) | 0.0 | 0.0 |
| Voxel size (mm3) | 1.8 × 1.8 × 5.0 | 1.6 × 1.6 × 5.0 |
| Acquisition time (min) | 22:00 | 15:02 |
| Men with PCa | Women with BCa | ||
|---|---|---|---|
| Patients | Number | 10 | 10 |
| Age at baseline (years) 1 | 67.4 (51–77) | 62.3 (31–76) | |
| No. of skeletal regions with metastasis 1 | 3.7 (2–6) | 5.0 (3–6) | |
| Type of primary tumour | Prostatic adenocarcinoma | 10 | 0 |
| Invasive ductal breast cancer | 0 | 3 | |
| Invasive lobular breast cancer | 0 | 7 | |
| No. of patients with metastasis for each skeletal region | Skull | 0 | 2 |
| Spine (cervical) | 3 | 9 | |
| Spine (thorax) | 6 | 7 | |
| Spine (lumbosacral) | 8 | 9 | |
| Thorax | 7 | 8 | |
| Pelvis | 9 | 10 | |
| Limbs | 4 | 7 | |
| Other sites of disease | Lymph nodes | 60% (6/10) | 50% (5/10) |
| Visceral | 0% (0/10) | 40% (4/10) | |
| Local disease | 50% (5/10) | 10% (1/10) | |
| Other (muscles) | 0% (0/10) | 10% (1/10) | |
| WB-MRI examinations | Observation period | 17/01/14–29/05/17 | 03/09/15–09/11/17 |
| No. baseline/follow-up | 10/10 | 10/10 | |
| Days between baseline and follow-up 1 | 213.8 (90–373) | 197.9 (109–291) | |
| No. with station-/slice-specific shim | 8/12 | 15/5 |
| Women with BCa (n = 20) | Men with PCa (n = 20) | ||
|---|---|---|---|
| Intra-observer | |||
| DSC | mean ± std dev | 0.78 ± 0.14 | 0.55 ± 0.21 |
| BA (Mean_ADC) | bias (LoA) | 0.5% (−5.2%, 6.0%) | 0.5% (−9.0%, 9.9%) |
| ICC (Mean_ADC) | estimate (95% CI) | 0.96 (0.90–0.98) | 0.82 (0.63–0.92) |
| Inter-observer (2nd reading) | |||
| DSC | mean ± std dev | 0.71 ± 0.16 | 0.40 ± 0.19 |
| BA (Mean_ADC) | bias (LoA) | 1.2% (−6.0%, 5.1%) | 3.3% (−9.9%, 9.6%) |
| ICC (Mean_ADC) | estimate (95% CI) | 0.96 (0.91–0.98) | 0.79 (0.60–0.91) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Colombo, A.; Saia, G.; Azzena, A.A.; Rossi, A.; Zugni, F.; Pricolo, P.; Summers, P.E.; Marvaso, G.; Grimm, R.; Bellomi, M.; et al. Semi-Automated Segmentation of Bone Metastases from Whole-Body MRI: Reproducibility of Apparent Diffusion Coefficient Measurements. Diagnostics 2021, 11, 499. https://doi.org/10.3390/diagnostics11030499
Colombo A, Saia G, Azzena AA, Rossi A, Zugni F, Pricolo P, Summers PE, Marvaso G, Grimm R, Bellomi M, et al. Semi-Automated Segmentation of Bone Metastases from Whole-Body MRI: Reproducibility of Apparent Diffusion Coefficient Measurements. Diagnostics. 2021; 11(3):499. https://doi.org/10.3390/diagnostics11030499
Chicago/Turabian StyleColombo, Alberto, Giulia Saia, Alcide A. Azzena, Alice Rossi, Fabio Zugni, Paola Pricolo, Paul E. Summers, Giulia Marvaso, Robert Grimm, Massimo Bellomi, and et al. 2021. "Semi-Automated Segmentation of Bone Metastases from Whole-Body MRI: Reproducibility of Apparent Diffusion Coefficient Measurements" Diagnostics 11, no. 3: 499. https://doi.org/10.3390/diagnostics11030499
APA StyleColombo, A., Saia, G., Azzena, A. A., Rossi, A., Zugni, F., Pricolo, P., Summers, P. E., Marvaso, G., Grimm, R., Bellomi, M., Jereczek-Fossa, B. A., Padhani, A. R., & Petralia, G. (2021). Semi-Automated Segmentation of Bone Metastases from Whole-Body MRI: Reproducibility of Apparent Diffusion Coefficient Measurements. Diagnostics, 11(3), 499. https://doi.org/10.3390/diagnostics11030499







