The Prognostic Significance of Immune-Related Metabolic Enzyme MTHFD2 in Head and Neck Squamous Cell Carcinoma
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Source
2.2. Gene Ontology (GO) and Pathway Enrichment Analysis
2.3. Identification of Immune-Related Metabolic Enzymes
2.4. IRME-Based Prognostic Signature Generation and Prediction
2.5. Evaluation of Immune Cell Infiltration
2.6. Tissue Samples and IHC Analysis
2.7. Statistical Analysis
3. Results
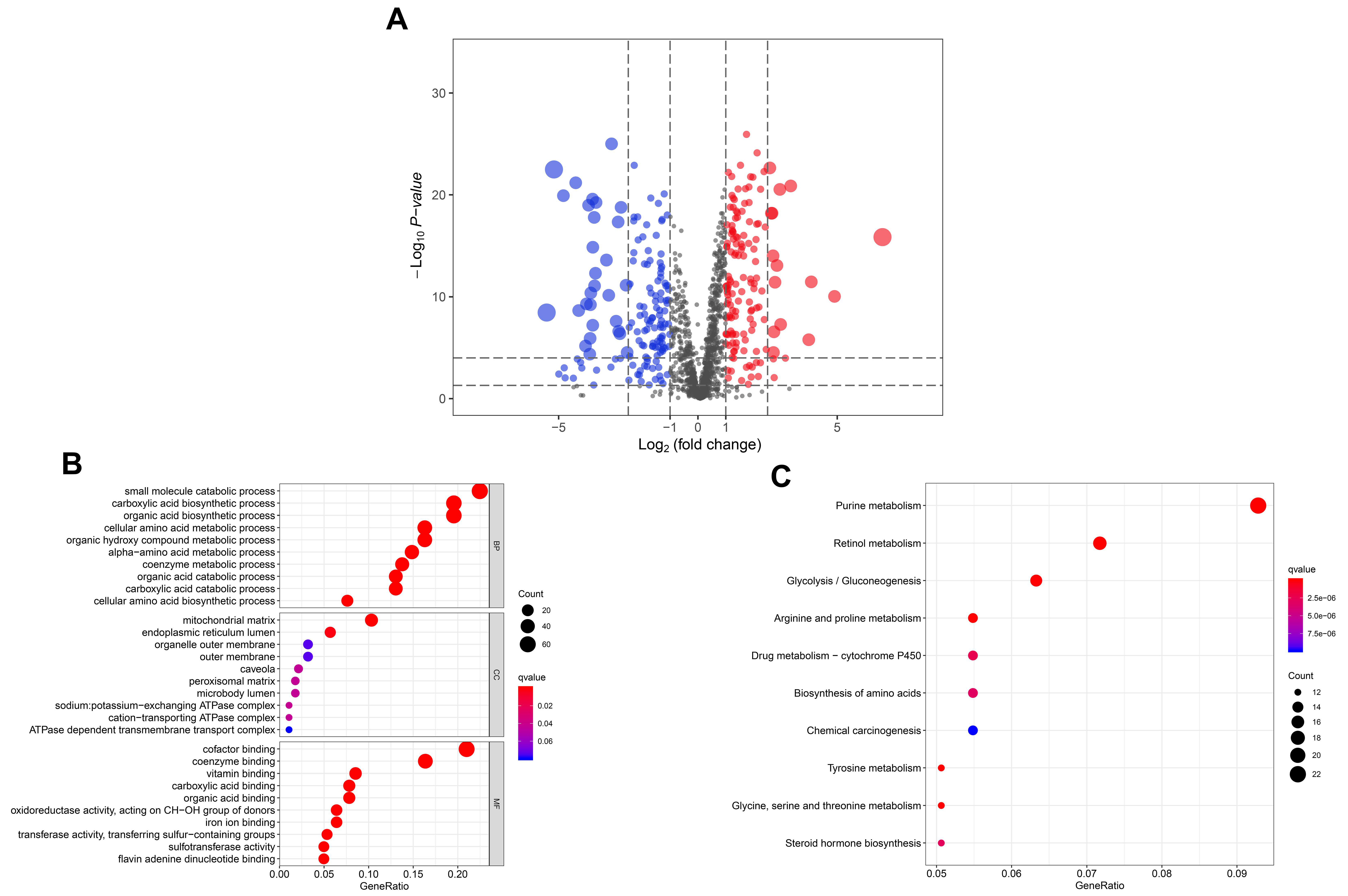
3.1. The Significantly Differentially Expressed Metabolic Enzymes in TCGA HNSCC Cohort
3.2. IRME-Based Prognostic Signature Generation and Prediction
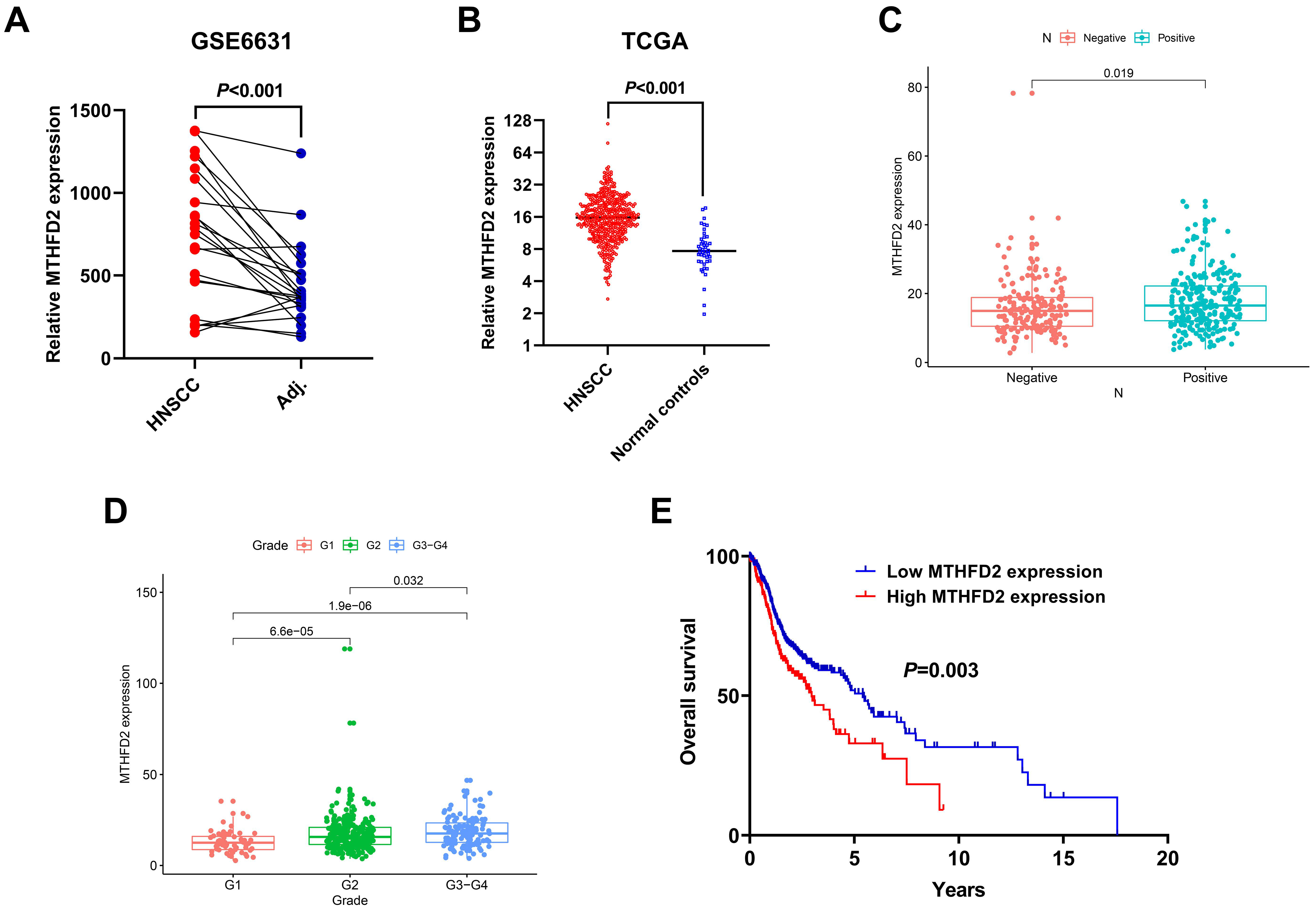
3.3. Upregulation of MTHFD2 Was Associated with Poor Clinical Outcome of HNSCC in the Public Datasets
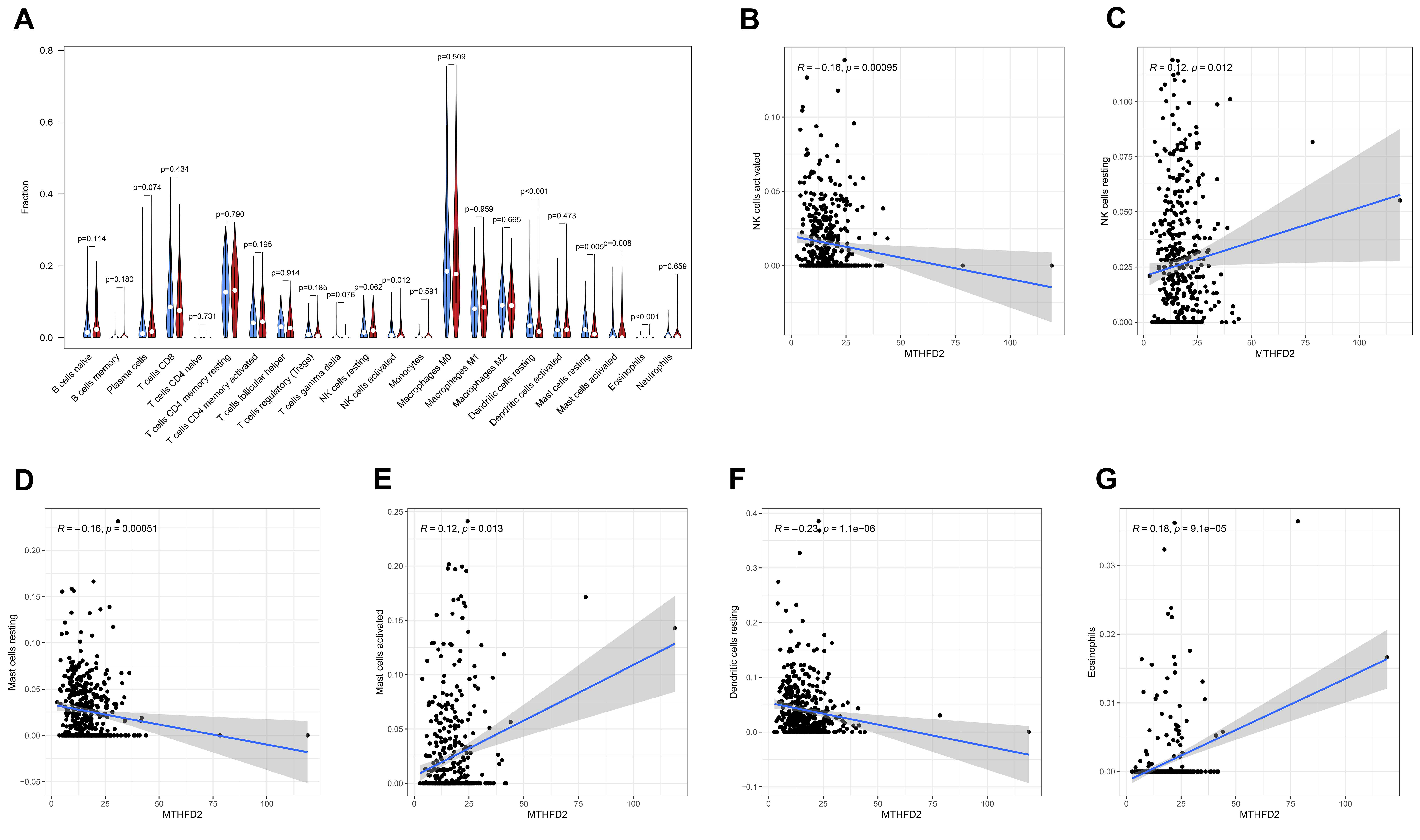
3.4. The Association between MTHFD2 and Immune Cell Infiltration in HNSCC
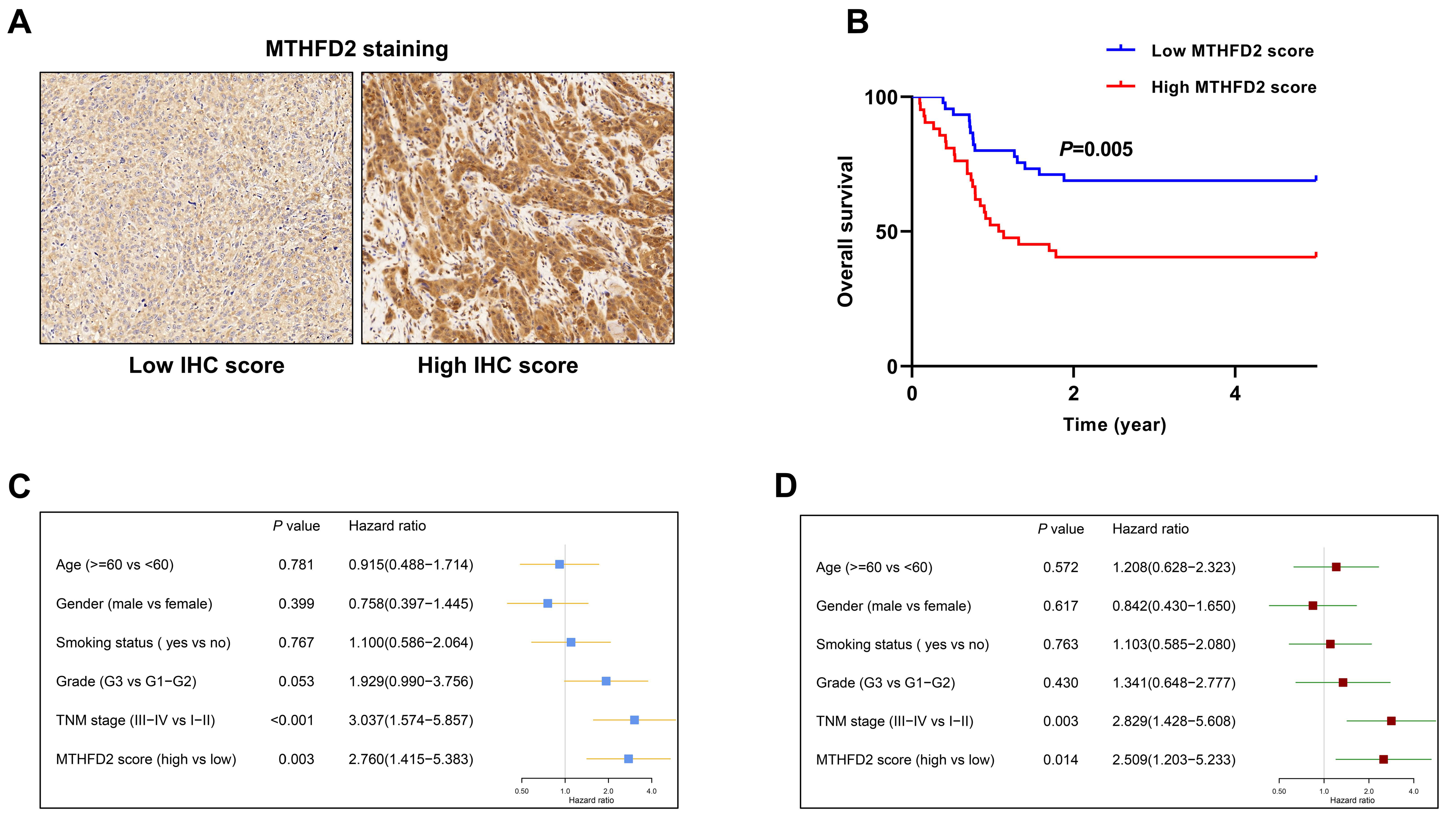
3.5. The Prognostic Value of MTHFD2 in HNSCC Was Validated in an Independent Cohort
4. Discussion
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Vigneswaran, N.; Williams, M. Epidemiologic Trends in Head and Neck Cancer and Aids in Diagnosis. Oral Maxillofac. Surg. Clin. N. Am. 2014, 26, 123–141. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, H.; Kulasinghe, A.; Allcock, R.J.N.; Tan, L.Y.; Mokany, E.; Kenny, L.; Punyadeera, C. A Pilot Study to Non-Invasively Track PIK3CA Mutation in Head and Neck Cancer. Diagn. 2018, 8, 79. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Sun, S.; Zeng, X.; Cui, L. Expression profiles analysis identifies a novel three-mRNA signature to predict overall survival in oral squamous cell carcinoma. Am. J. Cancer Res. 2018, 8, 450–461. [Google Scholar] [PubMed]
- Leemans, C.R.; Snijders, P.J.F.; Brakenhoff, R.H. The molecular landscape of head and neck cancer. Nat. Rev. Cancer 2018, 18, 269–282. [Google Scholar] [CrossRef] [PubMed]
- Ferris, R.L.; Blumenschein, G.; Fayette, J.; Guigay, J.; Colevas, A.D.; Licitra, L.; Harrington, K.; Kasper, S.; Vokes, E.E.; Even, C.; et al. Nivolumab for Recurrent Squamous-Cell Carcinoma of the Head and Neck. New Engl. J. Med. 2016, 375, 1856–1867. [Google Scholar] [CrossRef] [PubMed]
- Cramer, J.D.; Burtness, B.; Ferris, R.L. Immunotherapy for head and neck cancer: Recent advances and future directions. Oral Oncol. 2019, 99, 104460. [Google Scholar] [CrossRef] [PubMed]
- Seiwert, T.Y.; Burtness, B.; Mehra, R.; Weiss, J.; Berger, R.; Eder, J.P.; Heath, K.; McClanahan, T.; Lunceford, J.; Gause, C.; et al. Safety and clinical activity of pembrolizumab for treatment of recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-012): An open-label, multicentre, phase 1b trial. Lancet Oncol. 2016, 17, 956–965. [Google Scholar] [CrossRef]
- Rosario, S.R.; Long, M.D.; Affronti, H.C.; Rowsam, A.M.; Eng, K.H.; Smiraglia, D.J. Pan-cancer analysis of transcriptional metabolic dysregulation using The Cancer Genome Atlas. Nat. Commun. 2018, 9, 5330. [Google Scholar] [CrossRef]
- Hirschey, M.D.; DeBerardinis, R.J.; Diehl, A.M.E.; Drew, J.E.; Frezza, C.; Green, M.F.; Jones, L.W.; Ko, Y.H.; Le, A.; Lea, M.A.; et al. Dysregulated metabolism contributes to oncogenesis. Semin. Cancer Boil. 2015, 35, S129–S150. [Google Scholar] [CrossRef]
- Li, X.; Wenes, M.; Romero, P.; Huang, S.C.; Fendt, S.-M.; Ho, P.-C. Navigating metabolic pathways to enhance antitumour immunity and immunotherapy. Nat. Rev. Clin. Oncol. 2019, 16, 425–441. [Google Scholar] [CrossRef]
- Zhao, X.; Cui, L. Development and validation of a m6A RNA methylation regulators-based signature for predicting the prognosis of head and neck squamous cell carcinoma. Am. J. Cancer Res 2019, 9, 2156–2169. [Google Scholar] [PubMed]
- Pedley, A.M.; Benkovic, S.J. A New View into the Regulation of Purine Metabolism: The Purinosome. Trends Biochem. Sci. 2016, 42, 141–154. [Google Scholar] [CrossRef] [PubMed]
- Vettore, L.; Westbrook, R.L.; Tennant, D.A. New aspects of amino acid metabolism in cancer. Br. J. Cancer 2019, 122, 150–156. [Google Scholar] [CrossRef] [PubMed]
- Jiang, B. Aerobic glycolysis and high level of lactate in cancer metabolism and microenvironment. Gene Funct. Dis. 2017, 4, 25–27. [Google Scholar] [CrossRef] [PubMed]
- Kappler, M.; Kotrba, J.; Kaune, T.; Bache, M.; Rot, S.; Bethmann, D.; Wichmann, H.; Güttler, A.; Bilkenroth, U.; Horter, S.; et al. P4HA1: A single-gene surrogate of hypoxia signatures in oral squamous cell carcinoma patients. Clin. Transl. Radiat. Oncol. 2017, 5, 6–11. [Google Scholar] [CrossRef]
- Pikman, Y.; Puissant, A.; Alexe, G.; Furman, A.; Chen, L.M.; Frumm, S.M.; Ross, L.; Fenouille, N.; Bassil, C.F.; Lewis, C.A.; et al. Targeting MTHFD2 in acute myeloid leukemia. J. Exp. Med. 2016, 213, 1285–1306. [Google Scholar] [CrossRef]
- Koufaris, C.; Gallage, S.; Yang, T.; Lau, C.-H.E.; Valbuena, G.N.; Keun, H. Suppression of MTHFD2 in MCF-7 Breast Cancer Cells Increases Glycolysis, Dependency on Exogenous Glycine, and Sensitivity to Folate Depletion. J. Proteome Res. 2016, 15, 2618–2625. [Google Scholar] [CrossRef]
- Green, N.H.; Galvan, D.L.; Badal, S.S.; Chang, B.H.; LeBleu, V.S.; Long, J.; Jonasch, E.; Danesh, F.R. MTHFD2 links RNA methylation to metabolic reprogramming in renal cell carcinoma. Oncogene 2019, 38, 6211–6225. [Google Scholar] [CrossRef]
- Sheppard, N.G.; Jarl, L.; Mahadessian, D.; Strittmatter, L.; Schmidt, A.; Madhusudan, N.; Tegnér, J.; Lundberg, E.K.; Asplund, A.; Jain, M.; et al. The folate-coupled enzyme MTHFD2 is a nuclear protein and promotes cell proliferation. Sci. Rep. 2015, 5, 15029. [Google Scholar] [CrossRef]
- Koufaris, C.; Nilsson, R. Protein interaction and functional data indicate MTHFD2 involvement in RNA processing and translation. Cancer Metab. 2018, 6, 12. [Google Scholar] [CrossRef]
- Hu, W.; Wang, G.; Huang, D.; Sui, M.; Xu, Y. Cancer Immunotherapy Based on Natural Killer Cells: Current Progress and New Opportunities. Front. Immunol. 2019, 10, 10. [Google Scholar] [CrossRef] [PubMed]
- Bachanova, V.; Miller, J.S. NK cells in therapy of cancer. Crit. Rev. Oncog. 2014, 19, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Oldford, S.A.; Marshall, J.S. Mast cells as targets for immunotherapy of solid tumors. Mol. Immunol. 2015, 63, 113–124. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhang, Y.; Zhao, J.; Yang, Z.; Li, D.; Katirai, F.; Huang, B. Mast cell: Insight into remodeling a tumor microenvironment. Cancer Metastasis Rev. 2011, 30, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Perri, F.; Ionna, F.; Longo, F.; Scarpati, G.D.V.; De Angelis, C.; Ottaiano, A.; Botti, G.; Caponigro, F. Immune Response Against Head and Neck Cancer: Biological Mechanisms and Implication on Therapy. Transl. Oncol. 2020, 13, 262–274. [Google Scholar] [CrossRef] [PubMed]

| Variables | Staining Score | Min/Max | p |
|---|---|---|---|
| Age | 0.801 | ||
| ≥60 | 7.222 ± 3.819 | 0/12 | |
| <60 | 7.429 ± 3.781 | 2/12 | |
| Gender | 0.460 | ||
| Male | 7.508 ± 3.856 | 0/12 | |
| Female | 6.833 ± 3.608 | 2/12 | |
| Smoking status | 0.199 | ||
| No | 7.944 ± 3.561 | 2/12 | |
| Yes | 6.882 ± 3.902 | 0/12 | |
| Grade | 0.007 | ||
| G1–G2 | 6.576 ± 3.979 | 0/12 | |
| G3 | 8.893 ± 2.780 | 3/12 | |
| TNM stage | 0.002 | ||
| I–II | 6.174 ± 3.641 | 0/12 | |
| III–IV | 8.610 ± 3.549 | 2/12 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cui, L.; Chen, H.; Zhao, X. The Prognostic Significance of Immune-Related Metabolic Enzyme MTHFD2 in Head and Neck Squamous Cell Carcinoma. Diagnostics 2020, 10, 689. https://doi.org/10.3390/diagnostics10090689
Cui L, Chen H, Zhao X. The Prognostic Significance of Immune-Related Metabolic Enzyme MTHFD2 in Head and Neck Squamous Cell Carcinoma. Diagnostics. 2020; 10(9):689. https://doi.org/10.3390/diagnostics10090689
Chicago/Turabian StyleCui, Li, Huan Chen, and Xinyuan Zhao. 2020. "The Prognostic Significance of Immune-Related Metabolic Enzyme MTHFD2 in Head and Neck Squamous Cell Carcinoma" Diagnostics 10, no. 9: 689. https://doi.org/10.3390/diagnostics10090689
APA StyleCui, L., Chen, H., & Zhao, X. (2020). The Prognostic Significance of Immune-Related Metabolic Enzyme MTHFD2 in Head and Neck Squamous Cell Carcinoma. Diagnostics, 10(9), 689. https://doi.org/10.3390/diagnostics10090689




